Abstract
Background
Over the last 2 decades, cord blood (CB) has become an important source of blood stem cells. Clinical experience has shown that CB is a viable source for blood stem cells in the field of unrelated hematopoietic blood stem cell transplantation.
Methods
Studies of CB units (CBUs) stored and ordered from the US (National Marrow Donor Program (NMDP) and Swiss (Swiss Blood Stem Cells (SBSQ)) CB registries were conducted to assess whether these CBUs met the needs of transplantation patients, as evidenced by units being selected for transplantation. These data were compared to international banking and selection data (Bone Marrow Donors Worldwide (BMDW), World Marrow Donor Association (WMDA)). Further analysis was conducted on whether current CB banking practices were economically viable given the units being selected from the registries for transplant. It should be mentioned that our analysis focused on usage, deliberately omitting any information about clinical outcomes of CB transplantation.
Results
A disproportionate number of units with high total nucleated cell (TNC) counts are selected, compared to the distribution of units by TNC available. Therefore, the decision to use a low threshold for banking purposes cannot be supported by economic analysis and may limit the economic viability of future public CB banking.
Conclusions
We suggest significantly raising the TNC level used to determine a bankable unit. A level of 125 × 107 TNCs, maybe even 150 × 107 TNCs, might be a viable banking threshold. This would improve the return on inventory investments while meeting transplantation needs based on current selection criteria.
Keywords: Blood products, CD34+ cells, Collection efficiency, Cord blood, Cost-benefit analysis, Cost-effectiveness analysis, Cryopreservation, Health economics, Hematopoietic cell transplantation, Hematopoietic stem cells
Introduction
The transplantation of bone marrow has developed considerably since the first publication of results in 1957 by Thomas et al. [1]. In the year 2010, almost 17,000 products were provided for allogeneic transplantation worldwide for unrelated patients with oncologic, genetic, hematologic, and immunodeficiency disorders (3,574 bone marrow units, 9,248 peripheral blood stem cell (PBSC) units, 4,054 cord blood units (CBUs)) [2, 3].
Over the last 2 decades, umbilical cord blood (CB) has become an increasingly important source of blood stem cells. Clinical experience has shown that CB is a viable source for blood stem cells in the field of unrelated hematopoietic blood stem cell transplantation [4]. In 1989, the first CB transplantation was reported in a boy suffering from Fanconi anemia; the first two series of CB transplantation from related and unrelated donors were reported in 1994 and 1995 [for review see 5]. The first unrelated CB banking programs were started in 1992 at the Eurocord/Netcord Bank in Düsseldorf [6] and in 1993 at the New York Blood Center [7].
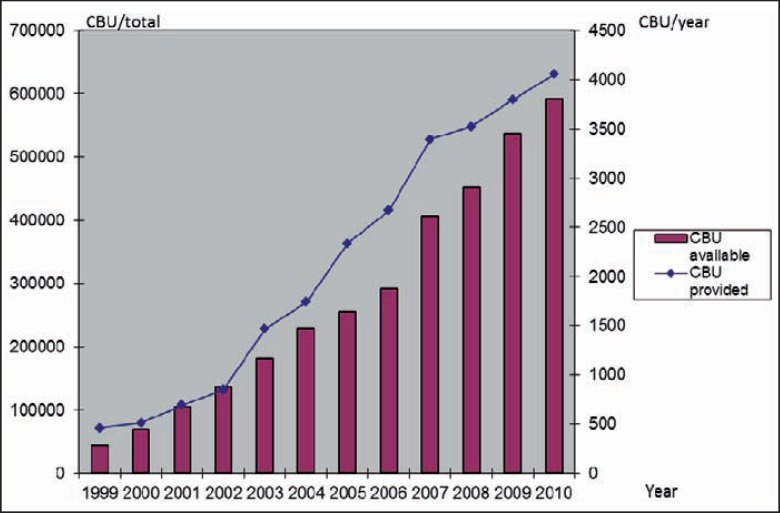
The global inventory of CBUs available grew from less than 100,000 CBUs in 2000 to almost 600,000 at the end of 2010, stored in more than 140 CB banks worldwide (fig. 1) [2, 3]. Worldwide, CB has become the second most important source of blood stem cells after PBSCs and before bone marrow (2010 World Marrow Donor Association (WMDA) data). Some 25% of all hematopoietic blood stem cell donations worldwide are performed using CB [2, 3].
Fig. 1.
Development of worldwide unrelated CB banking (data source: WMDA).
The most relevant advantages of CB as a source of hematopoietic stem cells are the relative ease of procurement, the absence of risks for mothers and donors, the reduced likelihood of transmitting infections, particularly cytomegaloviruses, and the ability to cryopreserve fully tested and human leukocyte antigen (HLA)-typed stem cells, available at short notice [8]. Disadvantages include: slower engraftment, more frequent graft failure, less graft-versus-host disease and possibly less graft-versus-leukemia effect, and more infections [4, 5, 9].
Economic Analysis
Evaluating the costs of handling different blood stem cell sources is complex. Majhail et al. [10] found in 2010 that costs of matched unrelated donors and CBUs are similar when costs of graft acquisition were considered within the total costs of transplantation.
However, other studies show significant economic differences between CB on the one hand and bone marrow and PBSCs on the other. Costs of procuring CBUs are possibly more than 10 times higher than the costs of bone marrow or PBSCs, mainly due to the fact that the overwhelming majority of the more than 500,000 CBUs in the past 10 years have not been used and are unlikely ever to be used. As such, the costs for processing and storing these CBUs could be regarded economically as ‘sunk costs’ [11]. These units may have some value in the future for research or should expansion technologies become clinically available.
Another reason is the high discard rate of CBUs before cryopreservation [12]. Lauber et al. [13] reported in 2010 for the Mannheim Cord Blood Bank that only about 25% of all collected CBUs met storage criteria. The main reasons for exclusion were insufficient volume, delayed time to processing, and low cell count.
In 2005, Kurtzberg et al. [12] described data from the Cord Blood Transplantation Study (COBLT). From a total of 17,207 units that were collected between 1998 and 2001, 11,077 units were cryopreserved and quarantined. Of these, only 8,750 units (50%) met eligibility criteria, were HLAtyped and entered into the search registry. Other authors report high deferral rates as well: Lecchi et al. [14] with Italian data, Stanworth et al. [15] in an international and Troeger et al. in a Swiss survey [16], ranging from 20 to 90%.
One of the more important criteria for CBU selection is the total nucleated cell (TNC) count of the unit. CBUs with an increased TNC count and those more recently collected were more likely to be ordered for transplantation. One explanation for this is the increasing use of CBU transplantation in adults. (The average adult in the USA weighs approximately 75 kg. Assuming a minimum dose of 2.5 × 107 TNCs/kg, a unit of approximately 188 × 107 TNCs is necessary as a minimum for transplantation.) Studies report a direct correlation between patient size and CBU TNCs, emphasizing the need to maximize the TNC count, especially when a mismatched unit is selected [17].
Data from Australia, Germany, and Korea support this finding, showing that transplanted CBUs have higher mean numbers of TNCs than stored CBUs [13, 18, 19]. Gragert et al. [20] concluded in 2011 that CB banks may want to focus more on adding CBUs with a high TNC rate rather than aiming for large overall increases in CBU inventory. Querol [21] came to a similar conclusion, noting that a focus on adding more units alone may not be a sustainable strategy for CB banking. Clark et al. [18], in 2011, identified these facts as risks for the CB inventory.
Based on data from the Mannheim Cord Blood Bank [13], the median TNC count of CBUs shipped was 119 × 107, while the median TNC count of CBUs in inventory was 65 × 107.
Medical and Economic Implications
The high cost of CB handling has medical and economic implications. To overcome the limitation of cell dose per CBU, infusion of 2 CBUs was adopted as a new transplantation strategy a few years ago. However, one limitation of double CB transplantation is the high costs of the 2 CBUs [22].
The high costs have consequences for global public CB banking, mostly because public or external funding is scarcer in today's economic climate and a continuation of the previous banking strategy is not financially sustainable in the medium term. In the long term, a collapse of individual banks and maybe even the whole system is possible.
In light of these concerns, this paper examines 2 questions: First, are public banks using the right criteria for selecting CBUs to bank and, second, is the current model of banking financially sustainable?
Material and Methods
Selection of CBUs
An analysis of the unrelated CB registries maintained by the National Marrow Donor Program (NMDP) in the USA and the Swiss Blood Stem Cells (SBSC), a unit of Transfusion SRC Switzerland, was performed to identify which CBUs were being selected for transplantation from these registries and whether the selection practice has changed over time. The study looked at the total number of CBUs being added to the inventory from 2006 through 2010, divided into subsets by TNC count.
The analysis then looked at the selection of CBUs for transplant each year and divided them into comparable TNC cohorts. This was compared to the same data reported by the Bone Marrow Donors Worldwide (BMDW).
Financial Viability of Banking
Separately, the NMDP surveyed 4 CB banks in the USA on the costs of operating a CB bank. Information obtained included cost of supplies, equipment and labor associated with the recruitment, collection, transportation, processing and storage of CBUs for public use. Indirect expenses were also estimated.
The CB banks represented a variety of settings, including academic medical centers, blood banks, and free-standing centers, as well as a variety of collection methods including collections by obstetrical volunteers, by paid staff, and by a mix of the two. When all costs were included, the average cost of recruitment, collection, banking, and storage for each unit actually stored was USD 1,830.00, including costs of units that were collected but not banked due to a variety of reasons, including failure of testing, below bankable size, etc. These data were averaged across all banks, and an estimate of indirect expenses and overhead was included based upon additional data provided by the banks.
The data collected by the NMDP were then combined and reformatted to create a generic CB banking operation. Cost elements were confirmed through interviews with the banks to establish as best as possible that all costs were identified. The NMDP categorized the costs based on its own model.
A similar analysis was conducted by SBSC in the year 2005. This analysis showed costs for collection, processing and banking for CBUs of approximately CHF 2,500.00 per banked unit. Taking inflation rate and the recent variability of currency exchange rates into account, the US and the Swiss costs appear comparable, adjusting for differences in purchasing power parity of the 2 countries, which showed in 2010 an over-valuation of the Swiss franc of 66% in comparison to the US dollar [23].
The banking model developed by the NMDP was then used to assess the business of CB banking in the USA based on the current demand from the US market and the current inventory available in the USA. The US market demand was determined by comparing NMDP data with the data available through the WMDA, to estimate the number of CBUs used in the USA in 2010. Total available inventory was assumed to be inventory listed on the NMDP registry plus an estimate of inventory available outside the NMDP registry, but located within the USA. For purposes of the analysis, it was assumed that all units used in the USA for unrelated CB transplant were selected from this national inventory. Based upon data reported from the banks, it assumed the price of each unit used in transplant to be USD 30,358.00. Data for the year 2010 can be seen in table 1.
Table 1.
Financial implications of public CB banking
| Industry year 1 | |
|---|---|
| Beginning inventory | 145,000 |
| Number of cord units recruited | 89,187 |
| Cost to recruit per unit, USD | 206 |
| % Banked (processed as % of recruited) | 33 |
| Number of cord units processed | 29,234 |
| Cost to process per unit, USD | 886 |
| Cost to recruit and process, USD | 1,524 |
| % Cord units distributed | 1.16 |
| Number of cord units distributed | 1,682 |
| Cost to distribute per unit, USD | 616 |
| Total average overhead cost per unit processed, USD | 505 |
| Cost to store 1 CBU for 1 year, USD | 27 |
| Average distribution price, USD | 30,358 |
| Cost to recruit, USD | 18,398,924 |
| Cost to process, USD | 25,993,499 |
| Cost to distribute, USD | 1,036,716 |
| Cost of overhead (fixed at baseline levels), USD | 14,798,094 |
| % Overhead of all cost | 23.08 |
| Cost to store, USD | 3,902,943 |
| Total cost, USD | 64,130,176 |
| Total revenue, USD | 51,062,396 |
| Net cash flow – excluding subsidies, USD | 13,067,780 |
Results and Discussion
Medical Aspects
Selection of CB units for transplant is based on 2 primary characteristics of a CBU. It must meet the HLA matching requirements of the transplant center (typically no less than 4 of 6 based upon antigen level matching for A and B and allele level matching for DRB1), and it must meet minimum cell dose requirements (TNC count).
NMDP data shows that, in selection of a CBU for an adult, a 4-of-6 unit will be selected 57% of the time. Therefore, dose becomes a critical factor. Wagner et al. [17] even suggest that the choice of a CBU should be based primarily on cell dose and only secondly on HLA matching.
The increasing use of 2 CBUs in a single transplant has likely contributed to the increase in the number of CB transplants performed in adults. Selection of units for double transplant from the NMDP inventory shows that units selected tended to be the larger units in the inventory and have been relatively equal in size. This may be due to the need to obtain at least the minimum dose or the uncertainty of which unit will engraft. As a result, in the majority of double-CBUs shipped through the NMDP in 2010, the difference between the 2 units selected is less than 20 × 107 TNC.
Units Banked
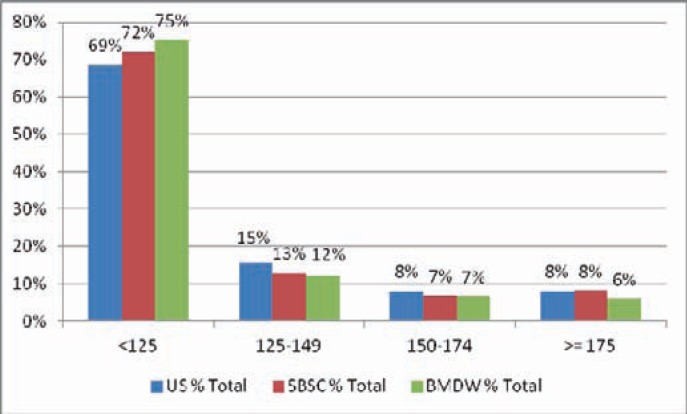
Figure 2 and table 2 show the current units banked in the NMDP and SBSC registries, arranged by TNC cohorts, alongside similar data from the BMDW inventory. The number of banked units is significantly weighted to the smaller end of the scale.
Fig. 2.
Inventory distribution by TNC count (US/NMDP, Swiss/SBSC and international/BMDW data 2010).
Table 2.
Comparison of US and Swiss CB inventories, distribution by TNC counta
| USA |
Switzerland |
||||||
|---|---|---|---|---|---|---|---|
| TNC | CBU | % total | % cumulated | TNC | CBU | % total | % cumulated |
| < 125 | 98,126 | 68.54 | 69 | < 125 | 2,248 | 71.94 | 72 |
| 125–149 | 22,146 | 15.47 | 84 | 125–149 | 406 | 12.99 | 85 |
| 150–174 | 11,517 | 8.04 | 92 | 150–174 | 217 | 6.94 | 92 |
| ≥ 175 | 11,371 | 7.94 | 100 | ≥ 175 | 254 | 8.13 | 100 |
| Total | 143,160 | 100.00 | Total | 3,125 | 100.00 | ||
| < 125 | 98,126 | 68.54 | 69 | < 125 | 2,248 | 71.94 | 72 |
| ≥ 125 | 45,034 | 31.46 | 100 | ≥ 125 | 877 | 28.06 | 100 |
| Total | 143,160 | 100.00 | Total | 3,125 | 100.00 | ||
2010 data.
Units Selected
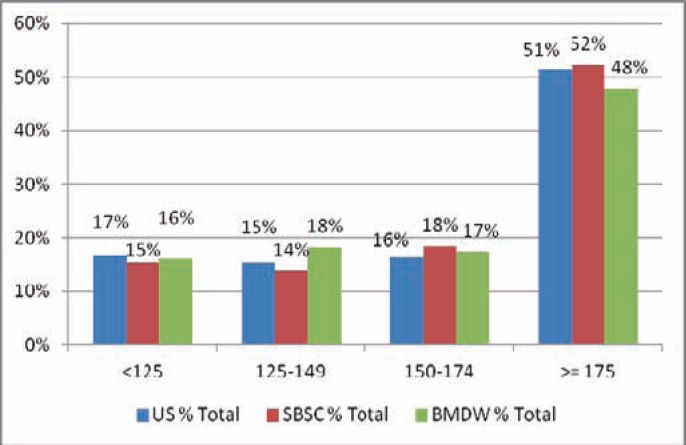
While banking inventories are heavily weighted to smaller CBUs, units selected for transplant are weighted to larger CBUs. For instance, in the NMDP inventory, the median TNC count of the inventory is 104 × 107 and the median of units shipped is 176 × 107. Figure 3 and table 3 show the selection by transplant centers of units from the NMDP and SBSC registries and the units provided globally reported to the BMDW.
Fig. 3.
Selection distribution by TNC count (2010 BMDW data).
Table 3.
Comparison of US and Swiss CBUs selected, distribution by TNC counta
| USA |
Switzerland |
||||||
|---|---|---|---|---|---|---|---|
| TNC | CBU | % total | % cumulated | TNC | CBU | % total | % cumulated |
| < 125 | 200 | 16.75 | 17 | < 125 | 10 | 15.38 | 15 |
| 125–149 | 184 | 15.41 | 32 | 125–149 | 9 | 13.85 | 29 |
| 150–174 | 197 | 16.50 | 49 | 150–174 | 12 | 18.46 | 48 |
| ≥ 175 | 613 | 51.34 | 100 | ≥ 175 | 34 | 52.31 | 100 |
| Total | 1,194 | 100.00 | Totalb | 65 | 100.00 | ||
| < 125 | 200 | 16.75 | 17 | < 125 | 10 | 15.38 | 15 |
| ≥ 125 | 994 | 83.25 | 100 | ≥ 125 | 55 | 84.62 | 100 |
| Total | 1,194 | 100.00 | Totalb | 65 | 100.00 | ||
2010 data.
Cumulated.
In 2010, 1,194 CBUs were shipped out of the available US inventory of 143,160 CBUs, resulting in a yearly distribution rate of 0.8%. The 65 Swiss units that were cumulatively shipped out of the 3125 available Swiss CBUs in inventory at the time resulted in a cumulated distribution rate of 2.1%. These shipments served both pediatric and adult patients. Based on this banking and distribution pattern, the vast majority of the CB inventory would remain unused, based on current indications.
Data from the NMDP shows that 68% of all CBU shipments for adults in 2010 had a TNC count of over 150, compared to 65% in 2005. Furthermore, data shows that 28% of all CBUs had a TNC count of less than 90, but only 3% of units shipped were from that group. 40% of the inventory was in the 90–124 group compared to 13% of all units shipped.
Thus, 69% of the inventory had a TNC count of less than 125, yet only 17% of all units shipped came from that group. 83% of all CBUs shipped in 2010 had a TNC count of greater than 125 and came from 31% of the available inventory.
At SBSC, the numbers are similar: More than 50% of shipped CBUs have a TNC of more than 175, but only 8% of the currently stored CBUs have this value. 85% of the shipped CBUs show a TNC of above 125, but only 28% of the currently stored CBUs have this value.
Altogether, the comparison of US, Swiss and international data shows a striking resemblance in the relationship between CBU inventory and shipments as well as TNC count.
Likelihood of a Unit to Be Used
The NMDP examined its records to determine the likelihood that a unit would be used for transplant over a 5-year period based upon the TNC count of that unit. Looking at the base year of 2005 and examining the actual selection from the inventory by each TNC cohort, table 4 shows the likelihood that a unit will be used out of each cohort over the 5-year period.
Table 4.
Likelihood of CBUs to be used over a 5-year perioda,b
| Cumulative % shipped | Years in inventory |
|||||
|---|---|---|---|---|---|---|
| year 0C | year ld | year 2 | year 3 | year 4 | year 5 | |
| Total % shipped | 1 | 2 | 4 | 6 | 7 | 8 |
| TNC 125 or greater, % | 1 | 4 | 9 | 12 | 15 | 16 |
| TNC 150 or greater, % | 2 | 7 | 13 | 17 | 20 | 22 |
| TNC 175 or greater, % | 2 | 9 | 16 | 22 | 27 | 29 |
NMDP data.
Distribution rates by TNC count for inventory banked in calendar year 2005.
Year in which the unit was added to the inventory.
First year after the unit was added to the inventory.
Actual selection data demonstrates that a unit with a TNC count of 125 or greater will likely be selected at 16% of the time over a 5-year period, while a unit of at least 175 or greater will be selected at 29% of the time over a 5-year period, both these compared to a likelihood of 8% of the total.
Financial Implications
The average cost of each unit banked increases with the use of a higher TNC count, because the discard rate of units collected but not banked increases with the higher TNC count. On average, 3 units will be collected for each unit processed at a cut-off at TNC 90, while 13 units will be collected for each unit processed at a cut-off of TNC 150 (NMDP data based on statistics collected from the 4 CB banks interviewed for the model). The average costs to collect a unit for processing at a cut-off of 90 is: 3 × USD 206.00 (= USD 618.00), while the costs to collect a unit at a cut-off of 150 is: 13 × USD 206.00 (= USD 2,678.00).
Translating this into an industry model, the increase in TNC threshold on an industry-wide basis will significantly reduce the growth rate in the available public CB bank inventories, but will not significantly impact the utilization of CBUs out of those inventories, firstly because the units preferred by the transplant centers will continue to be banked and secondly because there remains a significant inventory of smaller units that still retains potential clinical utility.
Table 5 summarizes all units that have been added to the NMDP inventory by TNC cohort, as well as all shipments by TNC cohort.
Table 5.
NMDP CB inventory: Cost of banking (total) and shipment (per CBU), distributed by TNCa
| TNC count (× 107) | Recruited | Estimated cost to collect, process and bank, USD | Shipments | Cost per shipment, USD |
|---|---|---|---|---|
| <90 | 47,730 | 71,595,000 | 323 | 221,656 |
| 90–124 | 67,720 | 101,580,000 | 1,069 | 95,023 |
| 125–149 | 26,902 | 40,353,000 | 1,044 | 38,652 |
| 150–174 | 14,704 | 22,056,000 | 1,080 | 20,422 |
| 175–199 | 7,789 | 11,683,500 | 922 | 12,672 |
| 200–224 | 4,050 | 6,075,000 | 775 | 7,839 |
| 225–249 | 2,159 | 3,238,500 | 532 | 6,087 |
| 250–274 | 1,169 | 1,753,500 | 372 | 4,714 |
| 275–300 | 624 | 936,000 | 200 | 4,680 |
| >300 | 794 | 1,191,000 | 348 | 3,422 |
| Total | 173,641 | 260,461,500 | 6,665 | 39,079 |
Variable cost to process and bank estimated at USD 1500 per unit, historical shipments through February 2011, all CBUs ever recruited, through February 28, 2011.
Assuming an average variable cost to collect and process of USD 1,500.00 per unit, the table shows the excessive actual cost of banking units below 150 × 107 TNC based on utilization. The cost of banking those units can never be recovered based on the current usage patterns. The table clearly illustrates that, for units below 125 TNC, there is a disproportionate relationship between a high inventory and a low utilization rate. The average cost of these units is in the range of USD 95,023.00–221,656.00 per CBU to collect, process and cryopreserve. The processing cost of those CBUs with very low utilization rates is mainly responsible for the current high cost of public CB banking. Even units with a TNC count between 125 and 149 are prohibitively expensive to bank as their cost to collect, process and cryopreserve ranges at USD 38,652.00, which is significantly higher than most CB banks charge for a unit.
A concern often expressed is that expansion might bring higher utilization rates to smaller units. If expansion proves clinically effective, indeed:
– banks have many units in inventory that can now become useful and that is truly where the HLA diversity of the CB registry is, and
– if there is an increase in demand, banks will be able to ramp up banking activity almost immediately.
Conclusions
The experience of the US and Swiss CB registries shows a similar pattern of banking and use when comparing TNC counts. The inventories of these registries are disproportionately weighted to smaller CBUs when compared to units actually selected. This has significant economic consequences on the public CB banks, which have expended substantial resources on units unlikely to be selected. The global database, BMDW, and the WMDA annual reports confirm this observation.
Current banking practices using comparatively low minimum TNC numbers as a threshold for banking appear not to be sustainable. Increasing the threshold rate will result in a sustainable banking model, assuming the commitment of current resources to collection and banking, while at the same time assuring that the units preferred for transplant will continue to be banked at the same or higher rates. At the present level of knowledge, a level of at least 125 × 107 TNC, maybe even 150 × 107 TNC, might be a viable banking threshold. For a successful implementation of this fundamental change in banking strategy, it is necessary, in addition to purely technical adjustments of locally relevant banking regulations, to include all key stakeholders (especially harvesting/collection centers, gynecology departments, but also expectant mothers) in integrated communication and information measures, to explain the rationale and the background of the changes to come.
Disclosure Statement
The authors declare to have no financial or non-financial conflict of interest to disclose.
References
- 1.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 2.World Marrow Donor Association : Stem Cell Donor Registries Annual Report 2010, ed 14. Leiden, WMDA, 2011.
- 3.World Marrow Donor Association : Unrelated Cord Blood Banks/Registries Annual Report 2010, ed 12. Leiden, WMDA, 2011.
- 4.Cornetta K, Laughlin M, Carter S, Wall D, Weinthal J, Delaney C, et al. Umbilical cord blood transplantation in adults: Results of the prospective cord blood transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 6.Kögler G, Somvilale T, Göbel U, Hakenberg P, Knipper A, Fischer J, Adams O, Krempe C, McKenzie C, Rüttgers H, Meier W, Bellmann O, Streng H, Ring A, Rosseck U, Rocha V, Wernet P. Haematopoietic transplant potential of unrelated and related cord blood: the first six years of the EUROCORD/NETCORD Bank Germany. Klin Padiatr. 1999;211:224–232. doi: 10.1055/s-2008-1043793. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gluckman E, Rocha V. History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy. 2005;7:219–227. doi: 10.1080/14653240510027136. [DOI] [PubMed] [Google Scholar]
- 9.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 10.Majhail NS, Mothukuri JM, MacMillan ML, Verneris MR, Orchard PJ, Wagner JE, et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr Blood Cancer. 2010;54:138–143. doi: 10.1002/pbc.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bart T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. Clinicoecon Outcomes Res. 2010;141–147 doi: 10.2147/CEOR.S11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzberg J, Cairo MS, Fraser JK, Baxter-Lowe L, Cohen G, Carter SL, et al. Results of the cord blood transplantation (COBLT) study unrelated donor banking program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 13.Lauber S, Latta M, Klüter H, Müller-Steinhardt M. The Mannheim Cord Blood Bank: Experiences and perspectives for the future. Transfus Med Hemother. 2010;37:90–97. doi: 10.1159/000289589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecchi L, Ratti I, Lazzari L, Rebulla P, Sirchia G. Reasons for discard of umbilical cord blood units before cryopreservation. Transfusion. 2000;40:122–124. doi: 10.1046/j.1537-2995.2000.40010122.x. author's reply 124–125. [DOI] [PubMed] [Google Scholar]
- 15.Stanworth S, Warwick R, Fehily D, Persaud C, Armitage S, Navarrete C, et al. An international survey of unrelated umbilical cord blood banking. Vox Sang. 2001;80:236–243. doi: 10.1046/j.1423-0410.2001.00039.x. [DOI] [PubMed] [Google Scholar]
- 16.Troeger C, Meyer-Monard S, Tichelli A, Manegold G, Pauli D, Surbek D, et al. Problems in umbilical cord blood collection. Transfus Med Hemother. 2007;34:95–98. [Google Scholar]
- 17.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 18.Clark P, Elwood N, Rodwell R, Holdsworth R, Montague A: Analysis of the Australian cord blood (CB) inventory and collection and banking strategies. Abstract presented at the 9th Annual International Cord Blood Transplantation Symposium, San Francisco 2011;abstr 105.
- 19.Lee YH, Park YR, Jun HJ, Lee MA, Jang HI, Nah JH, Chon YS, Koo HH: Comparative analysis of stored and transplanted cord blood units from KoreaCORD – For strategy in banking and optimal selection of cord blood. Abstract presented at the 9th Annual International Cord Blood Transplantation Symposium, San Francisco 2011;abstr 110.
- 20.Gragert L, Maiers M, Williams E, Confer D, Klitz W: Simulation of cord blood unit (CBU) inventories shows lower match rates than population-based models. www.ashi-hla.org/abstracts/2010/view/5297/, last accessed 14 July 2011.
- 21.Querol S. Towards more rational allogeneic CB inventories. Abstract presented at the World Cord Blood Congress III, Rome 2011; Published in: Cord blood banking: current status. Hematology. 2012;17(suppl 1):187–188. doi: 10.1179/102453312X13336169157013. [DOI] [PubMed] [Google Scholar]
- 22.Rocha V, Crotta A, Ruggeri A, Purtill D, Boudjedir K, Herr A, et al. Double cord blood transplantation: extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best practice & research. Clin Haematol. 2010;23:223–229. doi: 10.1016/j.beha.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Burgernomics. When the chips are down. The Economist, 22 July 2010, www.economist.com/node/16646178?story_id=16646178, last accessed 22 December 2011.