Abstract
Background
Interleukin 1 beta (IL-1β) contributes to the development of inflammatory bowel disease (IBD) and is correlated with the severity of intestinal inflammation. However, the precise source of IL-1β producing cells in DSS colitis is currently not known.
Methods
To determine IL-1β activity during intestinal inflammation in real time, an IL-1β transgenic mouse has been generated by incorporating the firefly luciferase gene driven by a 4.5-kb fragment of human IL-1β gene promoter (named cHS4I-hIL-1βP-Luc transgenic mice). Dextran sodium sulfate (DSS) induced colitis was confirmed with clinical presentation and histopathology.
Results
A substantial increase in luciferase activity (reflecting IL-1β production) in the region of inflamed colon was observed in a time dependent manner, followed by additional activity in the region of the mesenteric lymph node. The up-regulated luciferase activity was suppressed by dexamethasone (steroids) during DSS challenge, consistent with reduced severity of colitis, confirming the specificity of luciferase activity.
Conclusions
Our data suggests that bioluminescence is an interesting technology, which may be used to evaluate transcription of various genes in real time in experimental colitis.
Background
Inflammatory bowel disease (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is recognized as a widespread, debilitating condition with increasing incidence in Western societies in both children and adults [1-3]. The natural history of IBD is characterized by relapse and remission, with several factors known to trigger relapses including infection, ingestion of non-steroidal anti-inflammatory drugs and changes in smoking habits [4]. The aetiology of IBD is still not fully understood, despite decades of extensive research. It is believed that the imbalance of pro-inflammatory and anti-inflammatory cytokines contributes to the development of colitis [5-7].
Interleukin-1β, primarily secreted by monocytes and macrophages upon activation is one of the main drivers of inflammation. Macrophages are recruited and activated from peripheral blood into the inflamed colon [8,9]. IL-1β stimulates the production of inflammatory eicosanoids that subsequently induce neutrophil - chemoattractant and neutrophil-stimulating [10]. Released mature IL-1β protein resulting from inflammatory stimulus at the injured tissue, together with other cytokines and mediators (e.g. oxygen radicals) cause a cascade of inflammatory responses and tissue damage [11,12]. The binding between IL-1 and IL-1 receptor activates the NF-κB signal-transduction pathway [13], resulting in the up-regulation of other pro-inflammatory mediators such as TNF, IL-6 and IL-12 [14]. IL-1β is one of the key mediators of intestinal inflammation in IBD with a role in amplifying mucosal inflammation [15,16], consistent with the finding that IL-1β is up-regulated in IBD patients [17] and animal models [18,19]. On the other hand, IL-1β receptor antagonist reduces inflammation in an animal model of colitis [18,19].
IL-1β in inflamed intestine is mainly produced by infiltrating lamina properia monocytes including macrophages in the IBD mucosa [16]. However, IL-1β can also be produced by intestinal smooth muscle cells and fibroblasts [20]. The precise source of IL-1β producing cells in our animal model will be investigated in our future experiment.
Animal models of experimental colitis have been useful in confirmation of these clinical observations [11,21,22]. Furthermore, developing a method to monitor real time IL-1β activity in vivo would provide a unique opportunity to assess the precise progression of intestinal inflammation, using a DSS induced colitis model.
In this paper, colitis was induced using dextran sodium sulfate (DSS) in a cHS4I-hIL-1βP-Luc transgenic mouse, in which the expression of luciferase reporter gene was under the control of the human IL-1β gene promoter [23,24]. A “biophotonic” imaging system equipped with a highly light-sensitive camera allows non-invasive study of the transcriptional activity of IL-1β gene promoter in real time during the development of IBD, which could be used to evaluate the effects of anti-inflammatory compounds on IL-1β gene induction in vivo.
Methods
Genotyping of cHS4I-hIL-1βP-Luc transgene in mice
cHS4I-hIL-1βP-Luc transgenic mice, generated in the C57/B6 × CBA background [23,24], were backcrossed to C57/B6 for 3 generations before the experiment. Transgenic founders and their offsprings were identified by PCR using the forward-luc (5′ TTCCGCCCTTCTTGGCCTTTATGA 3′) and reverse-luc (5′ CAGCTATTCTGATTACACCCGAGG 3′) primers specific for the luciferase gene. All animals were housed under conventional laboratory conditions with food and water ad libitum. Experiments adhered to the guidelines of the local institutional animal care and use committee.
Induction of colitis
Adult (10 week old, male) cHS4I-hIL-1βP-Luc transgenic mice were given ad libitum 3% w/v dextran sulphate sodium (DSS, MW 36 000–44 000; MP Biomedicals, CA, USA) dissolved in tap water for four consecutive days, as described [11,12,22], while control groups received tap water only. On day five, the DSS solution was replaced with water, allowing some degree of colonic epithelial recovery. To confirm that the luciferin activity was inflammation specific, the mice were challenged with 3% DSS in drinking water and also dexamethasone (St. Louis, MO, USA,1.5 mg/mg) i.p. daily for five days. The luciferase signal was imaged and compared with that of the control group, which was injected with saline.
Assessment of the extent of experimental colitis
To confirm the severity of the colitis model, DSS-induced colitis was evaluated by body-weight and stool score daily. Weight loss on each day was calculated as the percentage of the baseline of bodyweight. Blood loss and stool consistency were scored, based on our previous description [11,12,22]. Scores were defined as follows: Blood loss: 0 = negative, 2 = positive, 4 = gross bleeding. Stool consistency: 0 = normal, 2 = loose stools, 4 = diarrhea.
Histopathologic analysis
For histopathologic analysis at day 6 (2 days after the end of DSS challenge), transverse colon was collected and fixed in 10% buffered formalin phosphate, embedded in sucrose, frozen in dry ice using optimal cutting temperature (OCT) compound and cryosectioned. Cross sections were stained with hematoxylin/eosin (H&E, Lerner, New Haven, CT). Histopathological scores were used to quantify the intestinal inflammation, as described previously [11,12,22].
In vivo and ex vivo imaging
In vivo bioluminescent imaging was performed using an IVIS imaging system (Bio-Real, QuickView3000, Austria). At the selected time points, the mice were anesthetized with isoflurane/oxygen, then were injected i.p. with substrate luciferin (Biosyth, Basel, Switzerland) dissolved in PBS (15 mg/ml) at a dose of 150 mg/kg. After 12 min of luciferin injection, images were taken on the imaging stage for 1–5 min. Photons emitted from specific regions were quantified using a LivingImage software (Bio-Real, QuickView3000, Austria). In vivo luciferase activity was presented in photons emitted per second.
For ex vivo imaging, the experimental mice were scarified at the days 4, 6 and 8 post DSS challenge. The colon, MLN and spleen were collected and were imaged with the same settings used for the in vivo studies on a heated stage in the IVIS system.
Statistics
All data are expressed as means ± SE. The data were analysed by one-way ANOVA. A P value of < 0.05 was considered significant.
Results
DSS induced acute colitis
Mice body weight dropped gradually from day 4, to a low at day 7 (17.5%) (p<0.001) post DSS challenge, before slowly gaining again 3 days after cessation of DSS (Figure 1a). The body weight loss was consistent with faecal blood scores and faecal scores, showing progressive diarrhoea and bloody stools starting from day 2 and peaking at day 5 post DSS challenge (Figure 1b and c). These scores plateaued from day 5 till 10 and gradually dropped on day 13. The large intestine was collected immediately on day 6 following DSS. Macroscopic gross bleeding was observed in some areas of the colon from all DSS treated animals. Furthermore, colon weight was increased by about 30%, but colon length was reduced about by 30%, compared to water treated controls (data not shown). Histological examination of the distal colon of DSS treated mice showed transmural inflammation involving all layers of the bowel wall, with a marked increase in the thickness of the muscular layer, adherence to surrounding tissues, mucosal ulceration, pronounced depletion of goblet cells, reduction of the density of the tubular glands, disseminate fibrosis, and focal loss of crypts (data not shown) with substantial leucocyte infiltration. In contrast, no abnormality was observed in the colon of mice without DSS challenge (data not shown).
Figure 1.
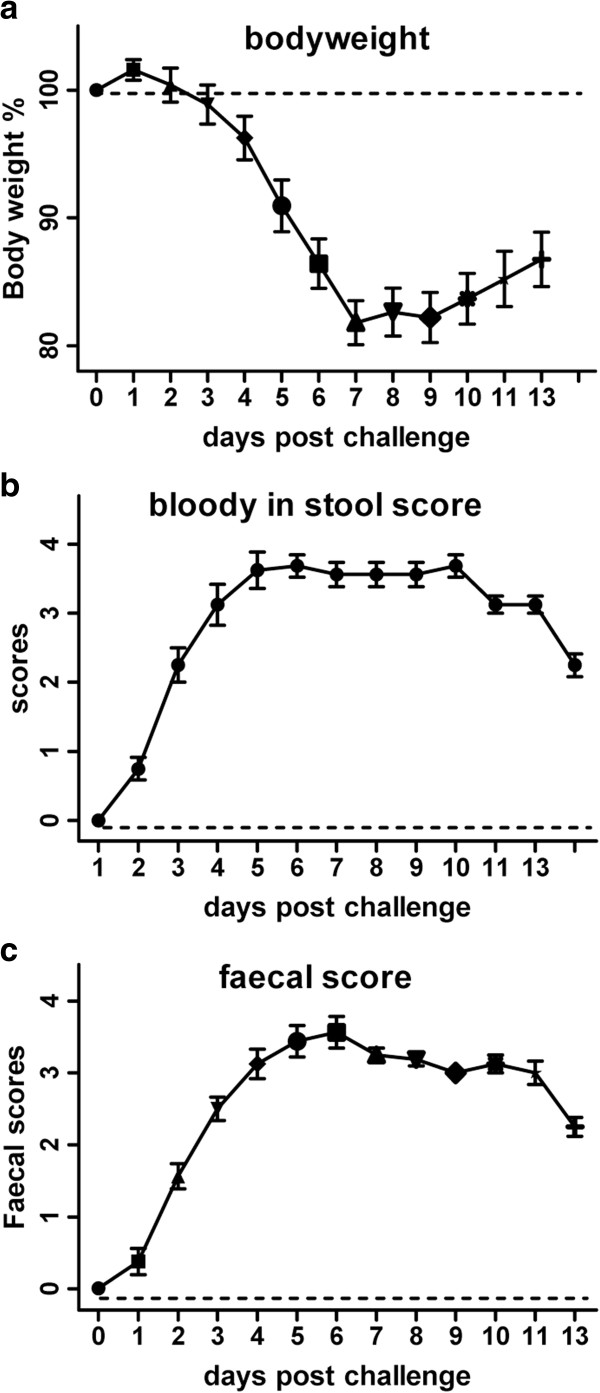
Clinical and faecal scores. Body weight changes from 100% baseline (a), blood appearance in faeces (b) and faecal score (c) over 5 d of DSS oral challenge commenced at the period of 13 days in cHS4I-hIL-1βP-Luc transgenic mice. Mean ± SEM. * P<0.05, ** P< 0.01, *** P< 0.001.
Luciferase expression induced by DSS in cHS4I-hIL-1βP-Luc transgenic mice
Constitutive levels of IL-1β production (luciferase expression) in the abdominal region of cHS4I-hIL-1βP-Luc transgenic mice were determined at day 0. IL-1β (luciferase expression) production was up-regulated gradually following DSS challenge, starting on day 1 and peaking on day 9. The magnitude of the increase was 3.8, 6.9, 7.3, 9.8, 10.3 and 11.1-fold on days 1, 2, 4, 6, 7, and 9 days post challenge, respectively (Figure 2). IL-1β (luciferase expression) was observed throughout the abdominal region prior to challenge. Following DSS challenge, the distribution was denser and localised near the centre/left lower quadrant of the abdomen on day 1. The density was up-regulated gradually from day 2 and appeared close to plateau by day 6, with the distribution seemingly localised to the proximal colon. Condensed but strong distribution was observed on days 7 to 9, but then slowly decreased on days 11 to 13, accompanied by more diffuse distribution (Figure 2). The kinetics and magnitude of IL-1β signals (luciferase expression) were consistent with previously published expression patterns of endogenous IL-1β mRNA [25].
Figure 2.
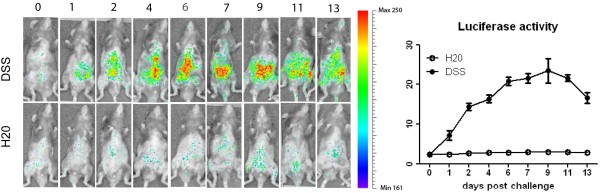
Luciferase activity in the animals ± DSS challenge in vivo. Luciferase activity in the animals ± DSS challenge was quantified using an IVIS imaging system. The distribution and intensity were recorded at the different day following DSS or H20 challenge.
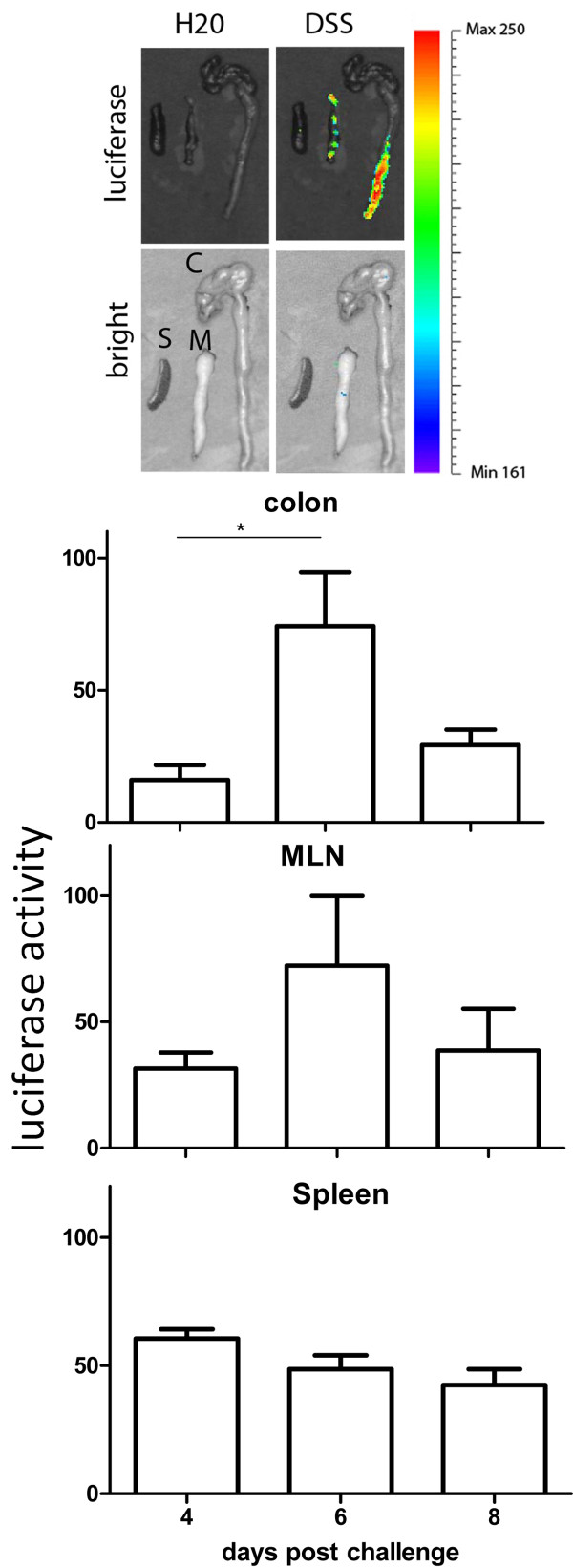
To confirm the luciferase activity detected in vivo was mainly from the inflamed colon, as well as clarify the kinetics of IL-1β, ex vivo luciferase activity from the colon, MLN and spleen were evaluated by live imaging on days 4, 6 and 8 post DSS challenge. IL-1β expression was ~2-, 8- and ~3-fold greater in the colon from DSS challenged animals compared to that from mock challenged (Figure 3). This difference was significant at days 4 and 6 (P<0.05), but not at day 8. A similar pattern of IL-1β expression was observed in the MLN from DSS challenged individuals, but no significant difference was detected among the days 4, 6 and 8. No significant difference in IL-1β expression was detected in the spleen with DSS challenge at various time-points, despite showing the highest level on day 4 post challenge (Figure 3).
Figure 3.
Luciferase activity in the colon, MLN and spleen following DSS challenge ex vivo. The level of luciferase activity driven by the promoter of IL-1β was quantified. Luciferase activity was confirmed ex vivo in the colon, MLN and spleen following DSS challenge at the days 4, 6 and 8 with quantification. Mean ± SEM. * P<0.05, ** P< 0.01, *** P< 0.001.
Dexamethasone, a synthetic glucocorticoid, has been well characterized for its ability to inhibit inflammation. To confirm that up-regulated luciferase activity is IL-1β specific, dexamethasone was used alone and as a co-treatment with DSS (1.5 mg/kg). There was a 20% or ~60% reduction in luciferase activity in the dexamethasone and DSS co-treated mice as compared with DSS treated mice on day 4, or day 5 (Figure 4).
Figure 4.
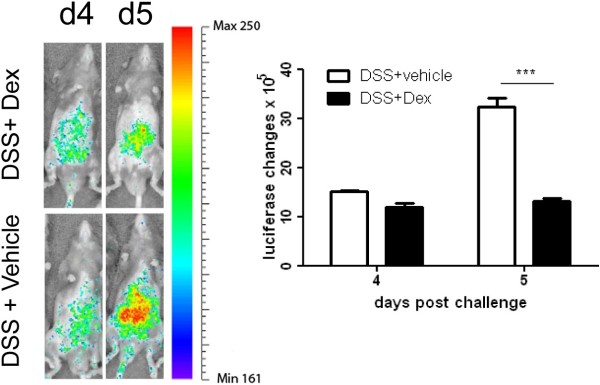
Luciferase activity in the animals with DSS challenge ± Dex treatment in vivo. Luciferase activity in the animals with DSS challenge ± Dex treatment was quantified using an IVIS imaging system. The distribution and intensity were recorded at the days 4 and 5 following DSS challenge. Luciferase activity was suppressed in the mice with Dex treatment. Mean ± SEM. * P<0.05, ** P< 0.01, *** P< 0.001.
Discussion
Our DSS-induced model of colonic inflammation displays pathological and clinical similarities to human colitis and has been widely used for pharmacological and pathophysiological studies [11,21,25,26]. DSS induced colitis results from the influx of bacteria into the lamina propria, due to an alteration of the colonic inner mucus layer [11]. The inflammatory cytokine, IL-1β, plays a major role in intestinal inflammation development and increased dramatically in the colonic mucosa during disease [27,28]. Our results are in agreement with these findings, showing an increase in luciferase expression driven by IL-1β gene promoter in the inflamed colon. The luciferase activity was significantly higher in the intestine, and relatively higher expression levels were also seen at the position of the mesenteric lymph node. These ex vivo data were consistent with previous studies.
Conventional methods for monitoring IL-1β gene expression rely on either measuring circulating levels of IL-1β in the serum or mRNA expression in tissues. Compared with these methods, the approach reported in this study is convenient and sensitive, while allowing less animals to be used. Moreover, this approach offers kinetic quantification and information on the anatomical distribution of IL-1β gene expression.
IL-1β, scarcely distributed throughout the abdominal region prior to challenge, was up-regulated and broadly distributed, but most densely observed near the centre/left lower quadrant of the abdomen on day 1, which supports that DSS gradually induced intestinal inflammation. The increased IL-1β appeared close to plateau by day 6 with the distribution seemingly shifted to the proximal colon, which is line with induced colitis present after 5 days of DSS challenge. Condensed but strong distribution was observed on days 7 to 9, but then slowly decreased on days 11 to 13, accompanied by more diffuse distribution (Figure 2). Our ex-vivo data showed that IL-1β was highest in the colon at day 6, but declined at day 8 (Figure 3), supporting that the main source of induced IL-1β is in the inflamed colon in vivo. A similar pattern of IL-1β in MLN ex vivo suggests IL-1β producing leucocytes migrated into the draining lymph nodes. No significant increase present in the spleen suggests that DSS induced acute colitis focuses on the gut rather than systemic inflammation.
Despite DSS challenge ceasing on day 5, IL-1β activity continued to increase, peaking at day 9 following DSS challenge, consistent with faecal and blood scores over this time frame. This data suggests that IL-1β activity correlates with the severity of colitis, confirmed with histopathological findings, making the bioluminescence model a reliable method for monitoring inflammation.
Despite the robustness of our bioluminescence model, we acknowledge that there remain a number of limitations. Currently, this model has limited resolution and cannot pinpoint the exact source of IL-1β at the cellular level. Furthermore, it doesn’t provide information about IL-1β translation and whether this correlates with observed transcriptional changes. In future experiments tissues will be collected at different time points for detection of IL-1β protein, using Western blot and immunohistochemistry to confirm the relationship between transcription and translation.
The observed dexamethasone dependant reduction of IL-1β expression suggests that this model can be used to evaluate the efficacy of medical therapy by showing decreased expression of inflammatory mediators. The bioluminescence/IL-1β model could be also used to study experimental therapies in IBD [29] such as faecal transplantation [30], which is an alternative therapy in treatment of IBD with promising outcomes.
Conclusions
Compared with the traditional method to monitor IL-1β gene expression in DSS induced experimental colitis, bioluminescence is an interesting technology that may be used to evaluate transcription of various genes in real time in experimental colitis.
Competing interests
All the authors declare that there is non-financial competing interest.
Authors’ contributions
LL and ZL performed the major part of the experiment. XY and HY performed partial live imaging experiments. SB contributed intellectual input to the experiments and wrote manuscript. JF designed the experiments and coordinated the project. All authors read and approved the final manuscript.
Contributor Information
Limei Li, Email: lmli@sibs.ac.cn.
Zhenzhe Liu, Email: liu_zhenze@hotmail.com.
Xinyu Yang, Email: xingyu456987@sohu.com.
Huimin Yan, Email: yanhuimin123@hotmail.com.
Shisan Bao, Email: bob.bao@sydney.edu.au.
Jian Fei, Email: jfei124@126.com.
Acknowledgements
We thank Geneway International Trading Co. Limited for technical support. This work was supported by grants from the National High Technology Research and Development Program (2008AA02Z126), National Key Project (2010CB945501), Science and Technology Commission of Shanghai Municipality (10410703800, 10DZ2251500, 09ZR1422600) E-Institutes of Shanghai Municipal Education Commission (E03003).
References
- Bousvaros A, Sylvester F, Kugathasan S, Szigethy E, Fiocchi C, Colletti R, Otley A, Amre D, Ferry G, Czinn SJ. Challenges in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:885–913. doi: 10.1097/01.mib.0000228358.25364.8b. [DOI] [PubMed] [Google Scholar]
- Frangos CC, Frangos CC. Inflammatory bowel disease: reviewing an old study under a new perspective. Gut. 2007;56:1638–1639. [PMC free article] [PubMed] [Google Scholar]
- Kirsner JB. Inflammatory bowel diseases: from the mystical to the cellular and now the molecular. World J Gastroenterol. 2005;11:4127–4128. [PubMed] [Google Scholar]
- Qiu BS, Vallance BA, Blennerhassett PA, Collins SM. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med. 1999;5:1178–1182. doi: 10.1038/13503. [DOI] [PubMed] [Google Scholar]
- Fort M, Lesley R, Davidson N, Menon S, Brombacher F, Leach M, Rennick D. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2001;166:2793–2800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. Am J Gastroenterol. 2002;97:2820–2828. doi: 10.1111/j.1572-0241.2002.07029.x. [DOI] [PubMed] [Google Scholar]
- Palmen MJ, Dieleman LA, van der Ende MB, Uyterlinde A, Pena AS, Meuwissen SG, van Rees EP. Non-lymphoid and lymphoid cells in acute, chronic and relapsing experimental colitis. Clin Exp Immunol. 1995;99:226–232. doi: 10.1111/j.1365-2249.1995.tb05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Bao S, Carr ED, Xu YH, Hunt NH. Gp91(phox) contributes to the development of experimental inflammatory bowel disease. Immunol Cell Biol. 2011;89:853–860. doi: 10.1038/icb.2011.4. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hunt NH, Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18:1220–1229. doi: 10.1038/cr.2008.310. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- Roebuck KA, Jacobs JJ, Glant TT. New horizons in orthopaedic research: elucidation of cellular signal transduction pathways. J Bone Joint Surg Am. 1999;81:599–602. doi: 10.2106/00004623-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Ko JK, Chik CW. The protective action of radix Astragalus membranaceus against hapten-induced colitis through modulation of cytokines. Cytokine. 2009;47:85–90. doi: 10.1016/j.cyto.2009.05.014. [DOI] [PubMed] [Google Scholar]
- McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42:214–219. doi: 10.1136/gut.42.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, Tilg H. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138:323–329. doi: 10.1111/j.1365-2249.2004.02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F, Nast CC, Clark BD, Schindler R, Lierena R, Eysselein VE, Thompson RC, Dinarello CA. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86:972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Strong SA, Pizarro TT, Klein JS, Cominelli F, Fiocchi C. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology. 1998;114:1244–1256. doi: 10.1016/S0016-5085(98)70431-7. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hunt NH, Bao S. The correlation between proinflammatory cytokines, MAdCAM-1 and cellular infiltration in the inflamed colon from TNF-alpha gene knockout mice. Immunol Cell Biol. 2007;85:633–639. doi: 10.1038/sj.icb.7100112. [DOI] [PubMed] [Google Scholar]
- Bian MJ, Li LM, Yu M, Fei J, Huang F. Elevated interleukin-1beta induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine aggravating dopaminergic neurodegeneration in old male mice. Brain Res. 2009;1302:256–264. doi: 10.1016/j.brainres.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Li L, Fei Z, Ren J, Sun R, Liu Z, Sheng Z, Wang L, Sun X, Yu J, Wang Z, Fei J. Functional imaging of interleukin 1 beta expression in inflammatory process using bioluminescence imaging in transgenic mice. BMC Immunol. 2008;9:49. doi: 10.1186/1471-2172-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hunt NH, Bao S. The effect of restraint stress on experimental colitis is IFN-gamma independent. J Neuroimmunol. 2008;200:53–61. doi: 10.1016/j.jneuroim.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Martinez-Augustin O, Merlos M, Zarzuelo A, Suarez MD, de Medina FS. Disturbances in metabolic, transport and structural genes in experimental colonic inflammation in the rat: a longitudinal genomic analysis. BMC Genomics. 2008;9:490. doi: 10.1186/1471-2164-9-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease–enhanced production during active disease. Gut. 1990;31:686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]