Abstract
Adipocytes constitute a major part of the bone marrow stroma in vivo and may play an active role in lymphohematopoiesis. Earlier studies had shown that the bone marrow stromal cell clone BMS2 was capable of adipocyte differentiation in vitro, in addition to its well-defined ability to support B lymphopoiesis. We now demonstrate that the process of adipogenesis in this functional bone marrow stromal cell clone can be inhibited by the cytokines interleukin-1 alpha, tumor necrosis factor, and transforming growth factor beta. Exposure of preadipocyte BMS2 cells to these agents blocked the induction of adipocyte differentiation as assessed by morphologic criteria and analysis of the neutral lipid content. Both interleukin-1 alpha and tumor necrosis factor elicited a rapid transient elevation in the steady-state mRNA levels of c-fos, c-jun, and JE. When added to differentiated adipocytes, the three cytokines continued to act as adipogenic antagonists. This was indicated by concentration- and time-dependent decreases in the activity of an adipocyte-specific enzyme, lipoprotein lipase. These changes in enzyme activity correlated directly with a decrease in steady-state levels of lipoprotein lipase mRNA. Another RNA marker of adipocyte differentiation (adipsin) was less influenced by the adipogenic antagonists. This may reflect the longer half-life of this mRNA transcript compared with those of lipoprotein lipase. Our results dramatically demonstrate that the differentiation state of bone marrow stromal cells can be modulated by exogenous factors in vitro. It is also the first report that transformation growth factor beta regulates the activity of lipoprotein lipase. These data suggest potential physiologic actions for these cytokines in vivo within the overall context of lymphohematopoiesis.
Full text
PDF

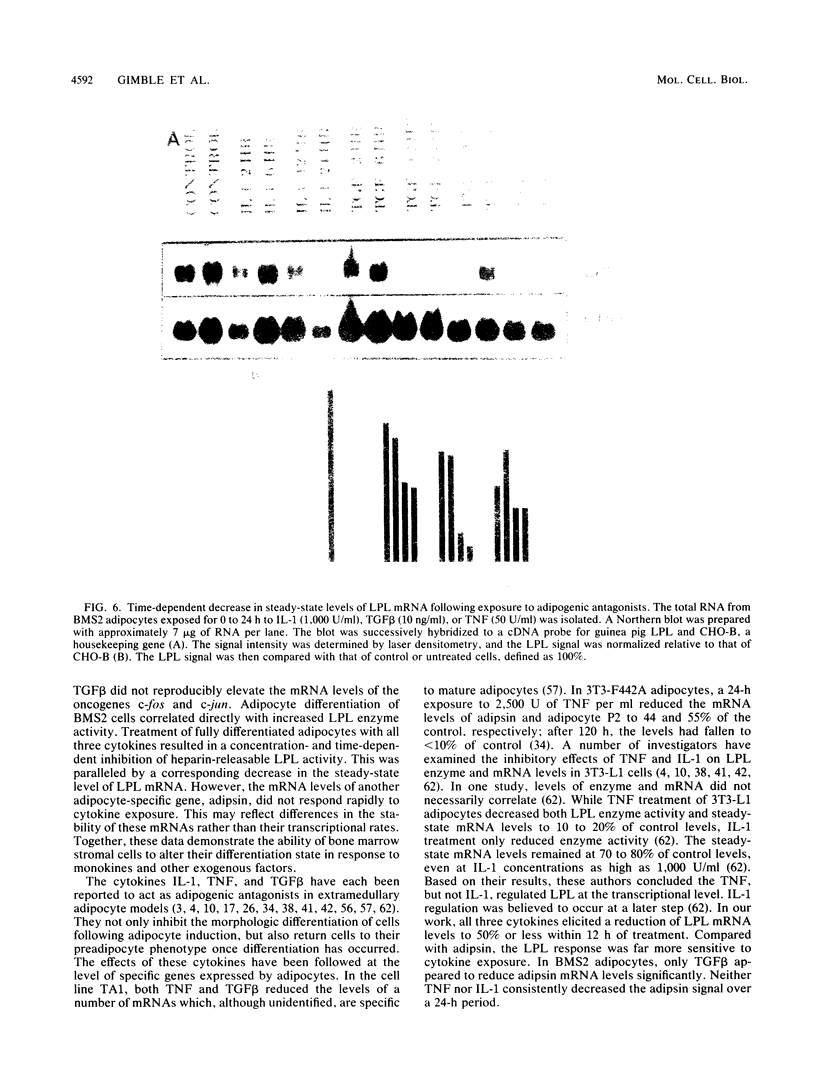



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathija A., Davis S., Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6(3):191–198. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- Bathija A., Davis S., Trubowitz S. Marrow adipose tissue: response to erythropoiesis. Am J Hematol. 1978;5(4):315–321. doi: 10.1002/ajh.2830050406. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Cornelius P., Enerback S., Bjursell G., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase mRNA content in 3T3-L1 cells by tumour necrosis factor. Biochem J. 1988 Feb 1;249(3):765–769. doi: 10.1042/bj2490765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. doi: 10.1146/annurev.cb.03.110187.002231. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Ellingsworth L. R., Brennan J. E., Fok K., Rosen D. M., Bentz H., Piez K. A., Seyedin S. M. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor beta. Immunohistochemical localization and association with differentiating cells. J Biol Chem. 1986 Sep 15;261(26):12362–12367. [PubMed] [Google Scholar]
- Enerbäck S., Semb H., Bengtsson-Olivecrona G., Carlsson P., Hermansson M. L., Olivecrona T., Bjursell G. Molecular cloning and sequence analysis of cDNA encoding lipoprotein lipase of guinea pig. Gene. 1987;58(1):1–12. doi: 10.1016/0378-1119(87)90023-0. [DOI] [PubMed] [Google Scholar]
- Enerbäck S., Semb H., Tavernier J., Bjursell G., Olivecrona T. Tissue-specific regulation of guinea pig lipoprotein lipase; effects of nutritional state and of tumor necrosis factor on mRNA levels in adipose tissue, heart and liver. Gene. 1988 Apr 15;64(1):97–106. doi: 10.1016/0378-1119(88)90484-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gimble J. M., Pietrangeli C., Henley A., Dorheim M. A., Silver J., Namen A., Takeichi M., Goridis C., Kincade P. W. Characterization of murine bone marrow and spleen-derived stromal cells: analysis of leukocyte marker and growth factor mRNA transcript levels. Blood. 1989 Jul;74(1):303–311. [PubMed] [Google Scholar]
- Greenberger J. S. Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro. 1979 Oct;15(10):823–828. doi: 10.1007/BF02618309. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S. Sensitivity of corticosteroid-dependent insulin-resistant lipogenesis in marrow preadipocytes of obese-diabetic (db/db) mice. Nature. 1978 Oct 26;275(5682):752–754. doi: 10.1038/275752a0. [DOI] [PubMed] [Google Scholar]
- HUDSON G. BONE-MARROW VOLUME IN THE HUMAN FOETUS AND NEWBORN. Br J Haematol. 1965 Jul;11:446–452. doi: 10.1111/j.1365-2141.1965.tb06607.x. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hirata J., Kaneko S., Nishimura J., Motomura S., Ibayashi H. Hydrocortisone modulates colony-stimulating activity produced by human bone marrow-derived adherent cells. Eur J Haematol. 1988 Mar;40(3):215–221. doi: 10.1111/j.1600-0609.1988.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Pietrangeli C. E., Hayashi S., Gimble J. M. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 1989;7:111–143. doi: 10.1146/annurev.iy.07.040189.000551. [DOI] [PubMed] [Google Scholar]
- Lanotte M., Metcalf D., Dexter T. M. Production of monocyte/macrophage colony-stimulating factor by preadipocyte cell lines derived from murine marrow stroma. J Cell Physiol. 1982 Jul;112(1):123–127. doi: 10.1002/jcp.1041120118. [DOI] [PubMed] [Google Scholar]
- Lanotte M., Scott D., Dexter T. M., Allen T. D. Clonal preadipocyte cell lines with different phenotypes derived from murine marrow stroma: factors influencing growth and adipogenesis in vitro. J Cell Physiol. 1982 May;111(2):177–186. doi: 10.1002/jcp.1041110209. [DOI] [PubMed] [Google Scholar]
- Liboi E., Di Francesco P., Gallinari P., Testa U., Rossi G. B., Peschle C. TGF beta induces a sustained c-fos expression associated with stimulation or inhibition of cell growth in EL2 or NIH 3T3 fibroblasts. Biochem Biophys Res Commun. 1988 Feb 29;151(1):298–305. doi: 10.1016/0006-291x(88)90593-1. [DOI] [PubMed] [Google Scholar]
- Liboi E., Pelosi E., Di Francesco P., Gallinari P., Petrini M., Sposi N. M., Testa U., Rossi G. B., Peschle C. The EL2 rat fibroblasts line: differential effects of growth factors (EGF, PDGF, FGF, TPA and TGF beta) on cell proliferation and c-fos expression. Ann N Y Acad Sci. 1987;511:318–328. doi: 10.1111/j.1749-6632.1987.tb36260.x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Min H. Y., Spiegelman B. M. Adipsin, the adipocyte serine protease: gene structure and control of expression by tumor necrosis factor. Nucleic Acids Res. 1986 Nov 25;14(22):8879–8892. doi: 10.1093/nar/14.22.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J., Andrews D. F., Crittenden C., Kaushansky K., Singer J. W. Response of simian virus 40 (SV40)-transformed, cultured human marrow stromal cells to hematopoietic growth factors. J Clin Invest. 1989 Feb;83(2):593–601. doi: 10.1172/JCI113922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara L., Sistonen L., Bos T. J., Vogt P. K., Keski-Oja J., Alitalo K. Enhanced jun gene expression is an early genomic response to transforming growth factor beta stimulation. Mol Cell Biol. 1989 Mar;9(3):1255–1262. doi: 10.1128/mcb.9.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangeli C. E., Hayashi S., Kincade P. W. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur J Immunol. 1988 Jun;18(6):863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- Price S. R., Mizel S. B., Pekala P. H. Regulation of lipoprotein lipase synthesis and 3T3-L1 adipocyte metabolism by recombinant interleukin 1. Biochim Biophys Acta. 1986 Dec 19;889(3):374–381. doi: 10.1016/0167-4889(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor--the primary regulatory role of the hormone in 3T3-L1 adipocytes. Arch Biochem Biophys. 1986 Dec;251(2):738–746. doi: 10.1016/0003-9861(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Quantin B., Breathnach R. Epidermal growth factor stimulates transcription of the c-jun proto-oncogene in rat fibroblasts. Nature. 1988 Aug 11;334(6182):538–539. doi: 10.1038/334538a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M., Keating A., Bowen-Pope D. F., Singer J. W., Ross R. Responsiveness of the in vitro hematopoietic microenvironment to platelet-derived growth factor. Leuk Res. 1985;9(4):427–434. doi: 10.1016/0145-2126(85)90001-3. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Bjorndahl J. M., Wang C. Y., Kao H. T., Fu S. M. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. J Exp Med. 1988 Mar 1;167(3):937–953. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Jung L. K., Walters J. A., Chen W., Wang C. Y., Fu S. M. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988 Nov 1;168(5):1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Marrow adipose cells and hemopoiesis: an interpretative review. Exp Hematol. 1984 Feb;12(2):139–146. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Torti S. V., Larrick J. W., Ringold G. M. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. J Cell Biol. 1989 Mar;108(3):1105–1113. doi: 10.1083/jcb.108.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Sakai H. The hematopoietic stroma. Am J Anat. 1984 Jul;170(3):447–463. doi: 10.1002/aja.1001700317. [DOI] [PubMed] [Google Scholar]
- Whitlock C., Denis K., Robertson D., Witte O. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Tsai S., Wong G. G., Clark S. C. Interleukin-1 regulation of hematopoietic growth factor production by human stromal fibroblasts. J Cell Physiol. 1988 Feb;134(2):292–296. doi: 10.1002/jcp.1041340217. [DOI] [PubMed] [Google Scholar]
- Zechner R., Newman T. C., Sherry B., Cerami A., Breslow J. L. Recombinant human cachectin/tumor necrosis factor but not interleukin-1 alpha downregulates lipoprotein lipase gene expression at the transcriptional level in mouse 3T3-L1 adipocytes. Mol Cell Biol. 1988 Jun;8(6):2394–2401. doi: 10.1128/mcb.8.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]