Abstract
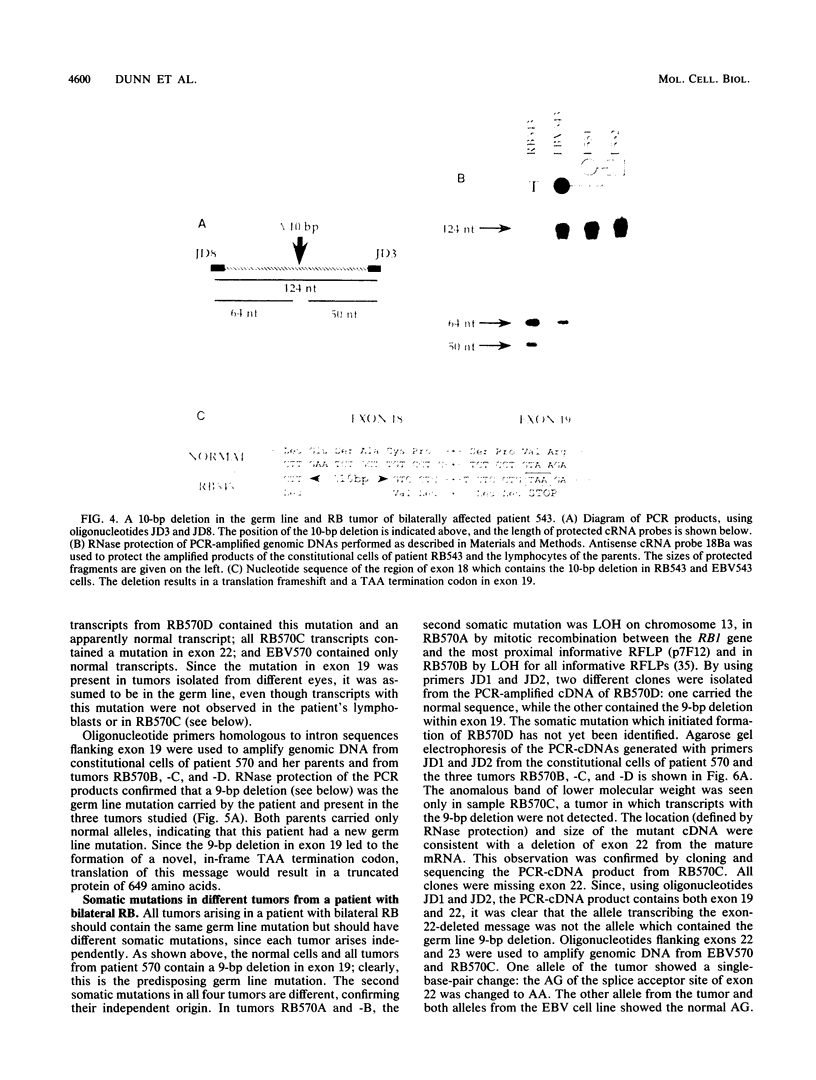
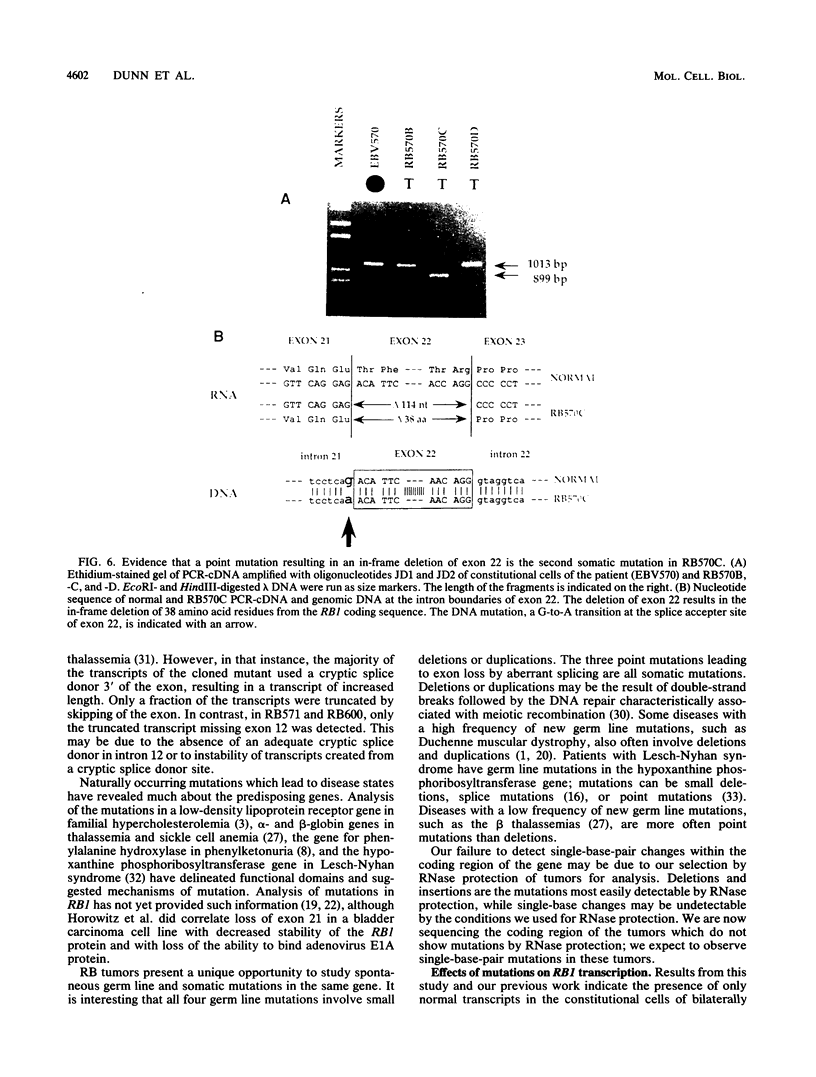
Inactivation of both alleles of the RB1 gene during normal retinal development initiates the formation of a retinoblastoma (RB) tumor. To identify the mutations which inactivate RB1, 21 RB tumors isolated from 19 patients were analyzed with the polymerase chain reaction or an RNase protection assay or both. Mutations were identified in 13 of 21 RB tumors; in 8 tumors, the precise errors in nucleotide sequence were characterized. Each of four germ line mutations involved a small deletion or duplication, while three somatic mutations were point mutations leading to splice alterations and loss of an exon from the mature RB1 mRNA. We were unable to detect expression of the mutant allele in lymphoblasts of three bilaterally affected patients, although the mutation was present in the genomic DNA and transcripts containing the mutations were obvious in the RB tumors in the absence of a normal RB1 allele. The variations in the level of expression of mutant transcripts suggest deregulation of RB1 transcription in the absence of a functional RB1 gene product.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodrug S. E., Ray P. N., Gonzalez I. L., Schmickel R. D., Sylvester J. E., Worton R. G. Molecular analysis of a constitutional X-autosome translocation in a female with muscular dystrophy. Science. 1987 Sep 25;237(4822):1620–1624. doi: 10.1126/science.3629260. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Lee E. Y., To H., Young L. J., Sery T. W., Hayes R. C., Friedmann T., Lee W. H. Human retinoblastoma susceptibility gene: genomic organization and analysis of heterozygous intragenic deletion mutants. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2210–2214. doi: 10.1073/pnas.85.7.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Hansen M. F., Nordenskjold M., Kock E., Maumenee I., Squire J. A., Phillips R. A., Gallie B. L. Genetic origin of mutations predisposing to retinoblastoma. Science. 1985 Apr 26;228(4698):501–503. doi: 10.1126/science.3983638. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Cavenee W., White R., Rapaport J. M., Petersen R., Albert D. M., Bruns G. A. Homozygosity of chromosome 13 in retinoblastoma. N Engl J Med. 1984 Mar 1;310(9):550–553. doi: 10.1056/NEJM198403013100902. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Becker A. J., Gallie B. L. Identification of germline and somatic mutations affecting the retinoblastoma gene. Science. 1988 Sep 30;241(4874):1797–1800. doi: 10.1126/science.3175621. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gallie B. L., Albert D. M., Wong J. J., Buyukmihci N., Pullafito C. A. Heterotransplantation of retinoblastoma into the athymic "nude" mouse. Invest Ophthalmol Vis Sci. 1977 Mar;16(3):256–259. [PubMed] [Google Scholar]
- Gallie B. L., Holmes W., Phillips R. A. Reproducible growth in tissue culture of retinoblastoma tumor specimens. Cancer Res. 1982 Jan;42(1):301–305. [PubMed] [Google Scholar]
- Gibbs R. A., Caskey C. T. Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science. 1987 Apr 17;236(4799):303–305. doi: 10.1126/science.3563511. [DOI] [PubMed] [Google Scholar]
- Goddard A. D., Balakier H., Canton M., Dunn J., Squire J., Reyes E., Becker A., Phillips R. A., Gallie B. L. Infrequent genomic rearrangement and normal expression of the putative RB1 gene in retinoblastoma tumors. Mol Cell Biol. 1988 May;8(5):2082–2088. doi: 10.1128/mcb.8.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hu X. Y., Burghes A. H., Ray P. N., Thompson M. W., Murphy E. G., Worton R. G. Partial gene duplication in Duchenne and Becker muscular dystrophies. J Med Genet. 1988 Jun;25(6):369–376. doi: 10.1136/jmg.25.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Bookstein R., Young L. J., Lin C. J., Rosenfeld M. G., Lee W. H. Molecular mechanism of retinoblastoma gene inactivation in retinoblastoma cell line Y79. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6017–6021. doi: 10.1073/pnas.85.16.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- McGee T. L., Yandell D. W., Dryja T. P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene. 1989 Aug 1;80(1):119–128. doi: 10.1016/0378-1119(89)90256-4. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Squire J., Gallie B. L., Phillips R. A. A detailed analysis of chromosomal changes in heritable and non-heritable retinoblastoma. Hum Genet. 1985;70(4):291–301. doi: 10.1007/BF00295364. [DOI] [PubMed] [Google Scholar]
- Sun H., Treco D., Schultes N. P., Szostak J. W. Double-strand breaks at an initiation site for meiotic gene conversion. Nature. 1989 Mar 2;338(6210):87–90. doi: 10.1038/338087a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Stout J. T., Palella T. D., Davidson B. L., Kelley W. N., Caskey C. T. A molecular survey of hypoxanthine-guanine phosphoribosyltransferase deficiency in man. J Clin Invest. 1986 Jan;77(1):188–195. doi: 10.1172/JCI112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Tarr G. E., Kelley W. N. Human hypoxanthine (guanine) phosphoribosyltransferase: an amino acid substitution in a mutant form of the enzyme isolated from a patient with gout. Proc Natl Acad Sci U S A. 1983 Feb;80(3):870–873. doi: 10.1073/pnas.80.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger L., Winger C., Shastry P., Russell A., Longenecker M. Efficient generation in vitro, from human peripheral blood cells, of monoclonal Epstein-Barr virus transformants producing specific antibody to a variety of antigens without prior deliberate immunization. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4484–4488. doi: 10.1073/pnas.80.14.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]