Abstract
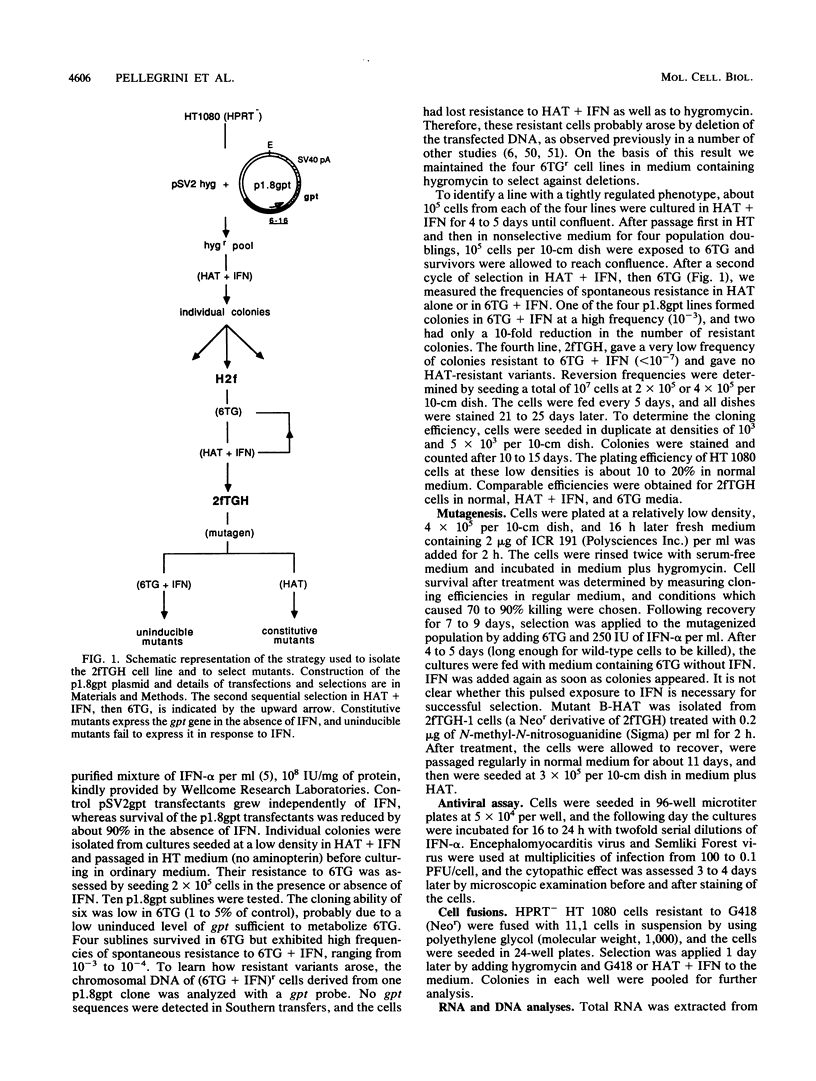
We have selected mutations in genes encoding components of the signaling pathway for alpha interferon (IFN-alpha) by using a specially constructed cell line. The upstream region of the IFN-regulated human gene 6-16 was fused to the Escherichia coli guanine phosphoribosyltransferase (gpt) gene and transfected into hypoxanthine-guanine phosphoribosyltransferase-negative human cells. These cells express gpt only in the presence of IFN-alpha. They grow in medium containing hypoxanthine, aminopterin, and thymidine plus IFN and are killed by 6-thioguanine plus IFN. Two different types of mutants were obtained after treating the cells with mutagens. A recessive mutant, selected in 6-thioguanine plus IFN, was completely resistant to IFN-alpha but responded normally to IFN-gamma and, unexpectedly, partially to IFN-beta. A constitutive mutant, selected in hypoxanthine-aminopterin-thymidine alone, was abnormal in expressing endogenous genes in the absence of IFN. Both types revert infrequently, allowing selection for complementation of the defects by transfection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affabris E., Jemma C., Rossi G. B. Isolation of interferon-resistant variants of Friend erythroleukemia cells: effects of interferon and ouabain. Virology. 1982 Jul 30;120(2):441–452. doi: 10.1016/0042-6822(82)90044-7. [DOI] [PubMed] [Google Scholar]
- Aguet M., Gresser I., Hovanessian A. G., Bandu M. T., Blanchard B., Blangy D. Specific high-affinity binding of 125I-labeled mouse interferon to interfevon resistant embryonal carcinoma cells in vitro. Virology. 1981 Oct 30;114(2):585–588. doi: 10.1016/0042-6822(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Aguet M., Mogensen K. E. Interferon receptors. Interferon. 1983;5:1–22. [PubMed] [Google Scholar]
- Allen G., Fantes K. H., Burke D. C., Morser J. Analysis and purification of human lymphoblastoid (Namalwa) interferon using a monoclonal antibody. J Gen Virol. 1982 Nov;63(Pt 1):207–212. doi: 10.1099/0022-1317-63-1-207. [DOI] [PubMed] [Google Scholar]
- Ashman C. R., Davidson R. L. Sequence analysis of spontaneous mutations in a shuttle vector gene integrated into mammalian chromosomal DNA. Proc Natl Acad Sci U S A. 1987 May;84(10):3354–3358. doi: 10.1073/pnas.84.10.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R., Thacker J. The nature of mutants induced by ionising radiation in cultured hamster cells. I. Isolation and initial characterisation of spontaneous, ionising radiation-induced, and ethyl methanesulphonate-induced mutants resistant to 6-thioguanine. Mutat Res. 1984 Nov;129(2):269–281. doi: 10.1016/0027-5107(84)90160-x. [DOI] [PubMed] [Google Scholar]
- Cohen B., Peretz D., Vaiman D., Benech P., Chebath J. Enhancer-like interferon responsive sequences of the human and murine (2'-5') oligoadenylate synthetase gene promoters. EMBO J. 1988 May;7(5):1411–1419. doi: 10.1002/j.1460-2075.1988.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T. C., Rosen J. M., Guille M. J., Lewin A. R., Porter A. G., Kerr I. M., Stark G. R. Overlapping sites for constitutive and induced DNA binding factors involved in interferon-stimulated transcription. EMBO J. 1989 Mar;8(3):831–839. doi: 10.1002/j.1460-2075.1989.tb03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Deluca J. G., Kaden D. A., Krolewski J., Skopek T. R., Thilly W. G. Comparative mutagenicity of ICR-191 to S. typhimurium and diploid human lymphoblasts. Mutat Res. 1977 Feb 1;46(1):11–18. doi: 10.1016/0165-1161(77)90106-6. [DOI] [PubMed] [Google Scholar]
- Dron M., Tovey M. G. Isolation of Daudi cells with reduced sensitivity to interferon. I. Characterization. J Gen Virol. 1983 Dec;64(Pt 12):2641–2647. doi: 10.1099/0022-1317-64-12-2641. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-Boyé D. Interferon and cell division. IX. Interferon-resistant L1210 cells: characteristics and origin. J Natl Cancer Inst. 1974 Feb;52(2):553–559. doi: 10.1093/jnci/52.2.553. [DOI] [PubMed] [Google Scholar]
- Hannigan G. E., Gewert D. R., Fish E. N., Read S. E., Williams B. R. Differential binding of human interferon-alpha subtypes to receptors on lymphoblastoid cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):537–544. doi: 10.1016/0006-291x(83)91183-x. [DOI] [PubMed] [Google Scholar]
- Hannigan G., Williams B. R. Transcriptional regulation of interferon-responsive genes is closely linked to interferon receptor occupancy. EMBO J. 1986 Jul;5(7):1607–1613. doi: 10.1002/j.1460-2075.1986.tb04403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter P., Kikinis Z., Altus M. S., Pearson D., Nagamine Y. A new genetic approach for studying hormonal regulation of urokinase-type plasminogen activator gene expression in LLC-PK1 cells. Mol Cell Biol. 1987 Dec;7(12):4535–4541. doi: 10.1128/mcb.7.12.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Gilbert C. S., Stark G. R., Kerr I. M. Differential regulation of interferon-induced mRNAs and c-myc mRNA by alpha- and gamma-interferons. Eur J Biochem. 1985 Dec 2;153(2):367–371. doi: 10.1111/j.1432-1033.1985.tb09312.x. [DOI] [PubMed] [Google Scholar]
- Kelly J. M., Porter A. C., Chernajovsky Y., Gilbert C. S., Stark G. R., Kerr I. M. Characterization of a human gene inducible by alpha- and beta-interferons and its expression in mouse cells. EMBO J. 1986 Jul;5(7):1601–1606. doi: 10.1002/j.1460-2075.1986.tb04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. S., Pine R., Pfeffer L. M., Levy D. E., Darnell J. E., Jr Cells resistant to interferon are defective in activation of a promoter-binding factor. EMBO J. 1988 Dec 1;7(12):3779–3783. doi: 10.1002/j.1460-2075.1988.tb03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Levy D., Larner A., Chaudhuri A., Babiss L. E., Darnell J. E., Jr Interferon-stimulated transcription: isolation of an inducible gene and identification of its regulatory region. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8929–8933. doi: 10.1073/pnas.83.23.8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Baker R. M. Chromosome-mediated gene transfer of HPRT and APRT in an intraspecific human cell system. Somatic Cell Genet. 1983 Mar;9(2):175–188. doi: 10.1007/BF01543176. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Merlin G., Falcoff E., Aguet M. 125I-labelled human interferons alpha, beta and gamma: comparative receptor-binding data. J Gen Virol. 1985 May;66(Pt 5):1149–1152. doi: 10.1099/0022-1317-66-5-1149. [DOI] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Aguet M., Gressner I. Binding of 125I-labelled human alpha interferon to human lymphoid cells. Int J Cancer. 1981 Nov 15;28(5):575–582. doi: 10.1002/ijc.2910280508. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Vogel S., Silverstein S. Phenotypic switching in cells transformed with the herpes simplex virus thymidine kinase gene. Mol Cell Biol. 1982 Jun;2(6):708–714. doi: 10.1128/mcb.2.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rutherford M. N., Hannigan G. E., Williams B. R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988 Mar;7(3):751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W., Bradley W. E., Thompson L. H. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol Cell Biol. 1982 Sep;2(9):1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell. 1987 May 8;49(3):295–297. doi: 10.1016/0092-8674(87)90277-7. [DOI] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Griffin D. Epitopes of human interferon-alpha defined by the reaction of monoclonal antibodies with alpha interferons and interferon analogues. J Immunol. 1987 Nov 15;139(10):3375–3381. [PubMed] [Google Scholar]
- Thacker J., Debenham P. G., Stretch A., Webb M. B. The use of a cloned bacterial gene to study mutation in mammalian cells. Mutat Res. 1983 Sep;111(1):9–23. doi: 10.1016/0027-5107(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Thacker J. The molecular nature of mutations in cultured mammalian cells: a review. Mutat Res. 1985 Jun-Jul;150(1-2):431–442. doi: 10.1016/0027-5107(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Analyses of mutation in pSV2gpt-transformed CHO cells. Mutat Res. 1986 Apr;160(2):121–131. doi: 10.1016/0027-5107(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Detection of deletion mutations in pSV2gpt-transformed cells. Mol Cell Biol. 1984 Jul;4(7):1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey M. G., Dron M., Mogensen K. E., Lebleu B., Mechti N., Begonlours-Guymarho J. Isolation of Daudi cells with reduced sensitivity to interferon. II. On the mechanisms of resistance. J Gen Virol. 1983 Dec;64(Pt 12):2649–2653. doi: 10.1099/0022-1317-64-12-2649. [DOI] [PubMed] [Google Scholar]
- Weck P. K., Apperson S., May L., Stebbing N. Comparison of the antiviral activities of various cloned human interferon-alpha subtypes in mammalian cell cultures. J Gen Virol. 1981 Nov;57(Pt 1):233–237. doi: 10.1099/0022-1317-57-1-233. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Yonehara-Takahashi M., Ishii A. Binding of human interferon alpha to cells of different sensitivities: studies with internally radiolabeled interferon retaining full biological activity. J Virol. 1983 Mar;45(3):1168–1171. doi: 10.1128/jvi.45.3.1168-1171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]