Abstract
There has been a significant increase in studies of how global change parameters affect interacting species or entire communities, yet the combined or interactive effects of increased atmospheric CO2 and associated increases in global mean temperatures on chemically mediated trophic interactions are mostly unknown. Thus, predictions of climate-induced changes on plant-insect interactions are still based primarily on studies of individual species, individual global change parameters, pairwise interactions, or parameters that summarize communities. A clear understanding of community response to global change will only emerge from studies that examine effects of multiple variables on biotic interactions. We examined the effects of increased CO2 and temperature on simple laboratory communities of interacting alfalfa, chemical defense, armyworm caterpillars, and parasitoid wasps. Higher temperatures and CO2 caused decreased plant quality, decreased caterpillar development times, developmental asynchrony between caterpillars and wasps, and complete wasp mortality. The effects measured here, along with other effects of global change on natural enemies suggest that biological control and other top-down effects of insect predators will decline over the coming decades.
Introduction
Global change is currently one of the most important issues for ecology and for ecological applications, such as conservation and agriculture [1], [2]. Basic and applied ecologists have made considerable progress towards understanding the effects of rapid changes in biogeochemical cycles, climate, and species distributions [3]–[5]. While most theoretical and empirical predictions of the consequences of such changes are still focused on individual species distributions, an increasing number of studies have tackled the more difficult goal of predicting the effects of these large scale changes on interacting species [4], [6]–[22]. Similarly, the majority of ecological climate change studies examine effects of single climate change variables on biological systems, but some studies have examined interacting effects of different global change parameters, such as increased CO2 and increased temperature, on species interactions or on entire biotic communities [23]–[30]. Nevertheless, the theoretical framework for studying climate change and biotic interactions is still underdeveloped [4], [31]–[33] and there are insufficient numbers of empirical studies to effectively guide theory or synthesis [20], [28]–[30]. Existing studies have revealed that the effects of interacting global change parameters on trophic interactions are variable, especially for upper trophic levels, and more studies are necessary to determine factors that alter responses of biotic interactions to climate change [29]. To this end, we examined the effects of changes in temperature and CO2 on direct and indirect tritrophic interactions between plants (alfalfa, Medicago sativa L., Fabaceae), plant chemistry (sapogenins and saponins), herbivores (caterpillars, Spodoptera exigua Hübner, Noctuidae), and natural enemies (parasitic wasps, Cotesia marginiventris Cresson, Braconidae).
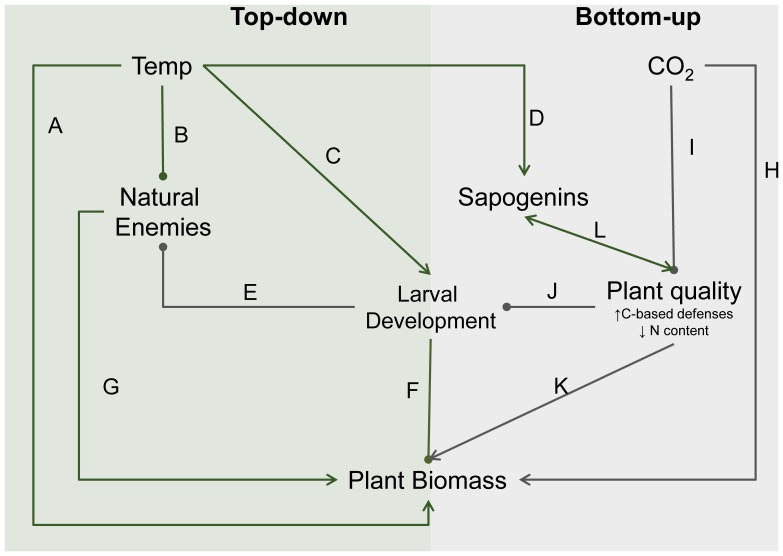
Increases in temperature and atmospheric CO2 interrupt the relationships between plants, herbivores, and their associated enemies through complex mechanisms [10], [12], [19], [25], [29], [34], but the existing body of literature allows for formulating hypotheses about the combined effects of temperature and CO2 on tritrophic interactions (Fig. 1, Table 1). For elevated temperature, tritrophic studies have demonstrated predominately top-down effects on plant biomass by directly altering herbivore and natural enemy development and survival. High temperatures (and extreme weather events) can have direct negative effects on survival of natural enemies, such as parasitic wasps [7], [10], [35]–[37], which are particularly sensitive to any temperature changes [21], [22]. One such negative effect of increased temperatures and heat waves is an increase in parasitoid development times [10], [28]. Elevated temperatures can also decrease larval development time [14], [30], [38], [39] and increase performance [30]. These combined temperature driven changes could negatively affect natural enemies by causing asynchrony between herbivore hosts and parasitoid development, resulting in a greater potential for herbivore outbreaks and lower plant biomass [40].
Figure 1. Path diagram summarizing direct and indirect effects of increased temperature and CO2 on tritrophic interactions.
The letters included as path coefficients correspond to direct effects that have been quantified in previous studies (cited in Table 1). Positive effects between variables are indicated by an arrow and negative effects are indicated by a filled circle. Temperature effects on top-down dominated interactions are in green and CO2 effects on chemically mediated interactions are in grey.
Table 1. Summary of selected studies that have demonstrated direct and indirect effects of increased temperature and CO2 on chemically mediated tritrophic interactions, as illustrated in Figure 1. “Path” refers to the letters included as path coefficients in Figure 1.
| Path | Relevant direct and indirect trophic interactions |
| A | Direct effects of increased temperature on plant biomass are often positive [65]. |
| B | High temperatures can have direct negative effects on natural enemy survival [8]–[9]. |
| C | Elevated temperatures can decrease larval development time [12], [15]. |
| D | Increased temperature can cause increased production of sapogenins [66]. |
| E | Faster developing larvae reduce their window of vulnerability to predators and parasitoids [67].Developmental asynchrony between host and parasitoid can result in high parasitoid larval mortality [68], [69].Changes in larval performance can affect natural enemies; poor host quality negatively affects parasitoids [70]. |
| F | Herbivore larvae consume plant tissues, reducing biomass [71]. This reduction in biomass is increased by other factors: elevated CO2 can increase consumption rate and total plant biomass removed, and herbivores can increase consumption rates to compensate for poor leaf quality [19], [20], [24]. |
| G | The trophic cascade. Enemies have an indirect positive effect on plant biomass via controlling heribivory [72]. |
| H | Elevated CO2 directly increases plant growth by increasing photosynthesis [15]–[18], [24]. |
| I | Elevated CO2 reduces plant quality by increasing some carbon based defenses or by decreasing plant N content [19]–[24]. |
| J | Poor leaf quality due to elevated CO2 can negatively affect herbivore performance [20], [26]. |
| K | There is a trade-off between investing resources in plant defense versus growth [34]. |
| L | Saponins are derived from sapogenins and concentrations are positively correlated. |
In contrast to the consumer-driven effects of higher temperatures on plant biomass, elevated atmospheric CO2 is hypothesized to directly affect plant quality and biomass (Fig. 1). Elevated CO2 increases plant growth by increasing photosynthesis [41]–[45] and indirectly by reducing plant quality for herbivores by increasing concentrations of plant secondary metabolites [46]–[48] and decreasing leaf nitrogen content [45], [46], [48]–[50]. While these changes in plant quality can have negative bottom-up effects on herbivore performance [46], [51], [52], they can cause compensatory increases in consumption rates and total feeding by herbivore larvae [45], [46], [50]. Changes in herbivore performance mediated by plant quality can also negatively affect parasitoid performance [53]–[55]. Both the decrease in parasitoid performance and compensatory herbivore feeding cause a decrease in plant biomass, despite the direct increases in plant growth due to elevated CO2 (Fig. 1).
The studies that have examined effects of climate change on tritrophic interactions [10], [12], [19], [25], [28], [29], [34], [56] suggest that effects on these interactions will favor outbreaks in natural and managed ecosystems, including most agriculture. Most notably, increases in climatic variability and extreme weather events associated with climate change disrupt regulation of herbivores by predators and parasitoids [7], [10], [57], [58]. Based on this literature and any summary of pairwise interactions or direct effects (Fig. 1), it is not possible to make adequate predictions about effects of one climatic variable on trophic interactions because of confounding effects caused by changes in other variables [29], [30]. Including manipulations of multiple global change parameters, in this case, both CO2 and temperature, is important in plant-herbivore-parasitoid systems because parasitoid communities are particularly sensitive to temperature changes [21], [22] and this sensitivity could alter responses to increased CO2.
It is clear that natural enemies and plant chemistry both can negatively affect herbivore populations in terrestrial systems. But how will these interactions respond to changes of multiple climatic variables [29], [30]? Will the abundance of pest herbivores simply increase due to higher temperatures [14], [30], [38], [39]? Will increases in extreme weather events lead to disruption of normal pest control by natural enemies [7], [10], [57], [58]? Will carbon-based plant defenses increase in response to warming and enhanced CO2 [30]? Or will unpredictable patterns emerge due to interactions, synergies, or complex indirect pathways [6], [29], [30], [32]? We addressed these general questions using a model study organism, alfalfa, Medicago sativa, which is a high quality forage crop grown worldwide. It is particularly important in the western United States, where it is ranked third in forage crop acreage and accounts for nearly forty percent of the domestic alfalfa production [59]. The saponins and sapogenins found in alfalfa are antiherbivore compounds and can have significant effects on associated arthropods; concentrations of these compounds are affected by a variety of perturbations [60]. The arthropod communities associated with alfalfa are complex, with hundreds of species, diverse trophic assemblages and the potential for dampening or enhancing interactions [60]–[62]. For this study, we examined a simple but biologically significant slice of this food web and addressed specific hypotheses about how changes in temperature and CO2 interact to affect relationships between plant chemistry and one species of plant, caterpillar, and parasitoid.
Methods
Experimental Overview
The research described here was conducted from January 2006 to December 2009 at Tulane University (New Orleans, LA, USA) and the University of Nevada (Reno, NV, USA). We examined the effects of increased CO2 and temperature on alfalfa chemical defenses and biomass, growth and survivorship of the generalist herbivore Spodoptera exigua, and growth and survivorship of the parasitoid Cotesia marginiventris in chamber experiments. We utilized a full factorial design manipulating temperature (ambient and elevated), CO2 (ambient and elevated) and food chain length (plants only, plants+herbivores, plants+herbivores+parasitoids). Thus, alfalfa plants, grown from seeds, were subjected to 12 different treatment combinations. Temperature and CO2 were manipulated both independently and simultaneously in sequential experiments with these combinations of treatments: ambient temperature and CO2, elevated temperature and ambient CO2, elevated CO2 and ambient temperature, and elevated CO2 and temperature. Ambient CO2 was 380 ppm and elevated CO2 was 650 ppm, which is within the range of the projected increase in ambient levels by 2080 [63], [64]. Ambient temperatures were the mean monthly high (30°C day) and low (18°C night) temperatures during July (mid growing season) in northern Colorado. The high temperature was 5°C above ambient temperature (35°C day–23°C night), the maximum projected increase due to global warming [63], [64]. The experiments took place inside 3 VWR CO2 Incubators, which control CO2 and temperature, with the light regime of 14 hour days and 10 hour nights. Levels of CO2 and temperature were randomly assigned to each incubator for each trial. Within the incubators, food chain treatments were randomly assigned to separate cages (plastic microcosms, 24×24×32 cm), resulting in interspersion across space and time. There were 30 replicate cages utilized for each treatment combination, for a total of 360 cages. Cages that did not meet conditions of the experiment (i.e. larvae or plants were not ready for introduction of parasitoids or caterpillars) were discarded, and when possible these replicates were repeated at later dates.
Plants
Each cage contained nine pots with four alfalfa plants each (36 plants total) and when plants were harvested, all plants and associated insects within a cage were combined for biomass and other measures. Pots contained watered soils at the start of experiments, and incubator relative humidity was greater than 80%, so plants were not watered during the course of the experiment. At the end of each experiment, plants were harvested, weighed, and air-dried for analysis of total carbon, total nitrogen, and quantification of secondary metabolites; for these chemical analyses, plants were combined from 10 replicate cages (with the same treatment combinations) to allow for sufficient plant material.
Insect Colonies
Spodoptera exigua were purchased from a supplier (Agripest) and immatures of the parasitoid Cotesia marginiventris were collected from northern Colorado fields; both were maintained as colonies in the laboratory. Two newly eclosed (within 12 hours) first instar caterpillars were placed on each alfalfa plant after the appearance of the second trifoliate leaf (approximately 4 weeks after planting). This resulted in 72 caterpillars per cage. In parasitoid addition treatments, the caterpillars were allowed to feed undisturbed for 7 days, at which point a mated female parasitoid was added to each cage and remained in the cages for the duration of the experiment. We ensured that all caterpillars were in the third instar at this point, regardless of experimental treatment. Each trial ended when the last caterpillar pupated or died. Herbivore days to eclose, pupal mass, survival, and parasitism rate were recorded; cage means were calculated for days to eclose and pupal mass, while total survivorship and total percent parasitism for the 72 caterpillars was calculated as a single value for the cage.
Chemical Analysis
To analyze saponin content, we utilized a modified isolation and quantification procedure [65]. In preparation for chemical extraction, leaf samples were dried overnight in an oven at 40°C and ground to a coarse powder, and subsamples of the dry leaf powder were analyzed by the Nevada Stable Isotopes Lab for total carbon and nitrogen content. One hundred milligrams of dry leaf powder were placed into a centrifuge tube and compounds were extracted from the leaf material in 30 ml of 80% ethanol with stirring. The samples were then centrifuged and the sample plus solvent was separated from the leaf material and dried under a vacuum. The process was repeated to completely extract compounds from the leaves. The dried samples were then dissolved in 15 ml methanol and defatted by shaking the solution with 15 ml of hexanes (98.5% hexane plus a mixture of isomers) in a centrifuge tube. The hexane layer was pipetted off and the process was repeated. The hexanes plus lipids were dried under N2 with heat. The defatted methanol layer was dried under a vacuum, and the samples were dissolved in 20 ml water. This solution was centrifuged to separate any remaining leaf material from the dissolved sample.
C-18 SepPak cartridges (Waters Corp.) were then preconditioned with 15 ml acetone followed by 15 ml water. The water with dissolved sample was passed through the cartridge, and the elution was dried under a vacuum. The cartridge was then sequentially eluted with 20 ml each of 35%, 60%, 80% and 100% methanol. The elutions were transferred directly to a pre-weighed scintillation vials and dried under N2 with heat. Samples were stored in the freezer. According to previous work with this method, the 35% fraction contains flavans, the 60% fraction is comprised of flavones, the 80% fraction contains saponins and the 100% fraction contains sapogenins. The water fraction has sugars and organic acids, and the hexanes have lipids. Samples were completely dried overnight in an oven at low temperature. Vials with samples were then weighed to determine the mass of each class of compounds contained in the leaf material. The weights of samples from the elutions were used in analyses.
Content of elutions was confirmed by HPLC with a matrix-assisted laser desorption ionization source. The main components for the 80% elution fraction were the alfalfa saponins soyasapogenol B-3-O-Rhamnose-Galactose-Glucose carboxylic acid (mass is 965.5 for the sodiated ion +H) and Hederagenin-3-O- [Beta D glucose acid methyl ester] -28-O- [Beta D glucose] (mass is 847.5 for the sodiated ion +H). The main components for the sapogenin (100%) fractions were hederagenin (mass is 685.5 for the ion and 494.6 for the sodiated ion +H), and zahnic acid (mass is 508.6 for the sodiated ion +H), medicagenic acid (mass is 522.6 for the sodiated ion −2 H+).
Statistical Analysis
The focal statistical analyses were path analyses based on our causal hypotheses presented in Figure 1. In order to identify which variables were best to test in our focal path analyses, the main and interaction effects of temperature, CO2 and parasitoid treatment on all response variables were estimated using analysis of variance (ANOVA). For ANOVAs, replication for plant chemistry was lower than for other response variables because plants from different cages (replicates with the same levels of all treatments) were combined to provide enough material for chemical analysis. Cage means of caterpillar and plant response variables, including survival and percent parasitism (for 72 caterpillars), were used as response variables, and residuals from these variables met assumptions of normality. In addition, we used a Mann-Whitney z statistic to test the specific hypothesis that percentage parasitism was associated with the 4 combinations of temperature and CO2.
We examined direct and indirect effects of CO2 and temperature on alfalfa biomass with path analysis (Proc CALIS, SAS Institute Inc., NC). We proposed 2 general models based on previous literature (Fig. 1) as well as an alternative, simpler model, all of which elucidated direct versus indirect effects of CO2 and temperature on alfalfa biomass, quality, larval development, and parasitoid performance. While other important interaction pathways, including numerous indirect effects, can be proposed from the literature, several reviews suggest that the direction and magnitude of those effects are still too variable to predict [20], [29], [30]. Path models yielding a goodness of fit chi-square with a P-value greater than 0.5 were considered a good fit to the data.
Results
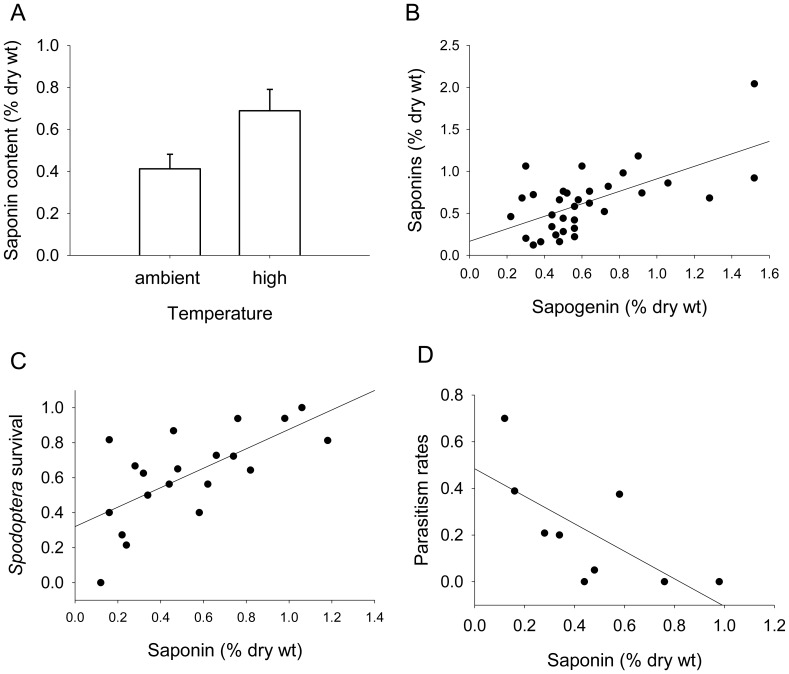
The effects of temperature on plant quality were consistent with predictions of overall decreases in plant quality with projected climate change. Warmer temperatures caused an increase in saponin content (Fig. 2), which was positively correlated with the concentration of metabolically unrelated (i.e. these compounds were not simply the same saponins without the sugars) sapogenins (Fig. 2). In contrast, based on ANOVA, there were no significant effects of CO2 on saponins or sapogenins and there were no complex interactions. Also based on ANOVAs, there were no significant effects of temperature and CO2 on carbon and nitrogen content, C: N, lipids, flavones, sugars, and flavans. Saponin content was correlated with both herbivore and parasitoid success: Spodoptera survival was positively associated with saponin content, while parasitism rates were negatively associated with saponin content (Fig. 2).
Figure 2. Saponin content in alfalfa (A, B) and the effects on Spodoptera survival (C)(R2 = 0.379 F1,19 = 10.964 P<0.01) and parasitism rates (D)(R2 = 0.477 F1,8 = 6.383 P<0.05).
Values in panel A are mean saponin content (±1 SE) under ambient and high temperatures; ANOVA, F1,22 = 4.762, P<0.05. The sapogenins in panel B are biosynthetically unrelated to the corresponding saponins; R2 = 0.40, F1,33 = 21.351, P<0.001.
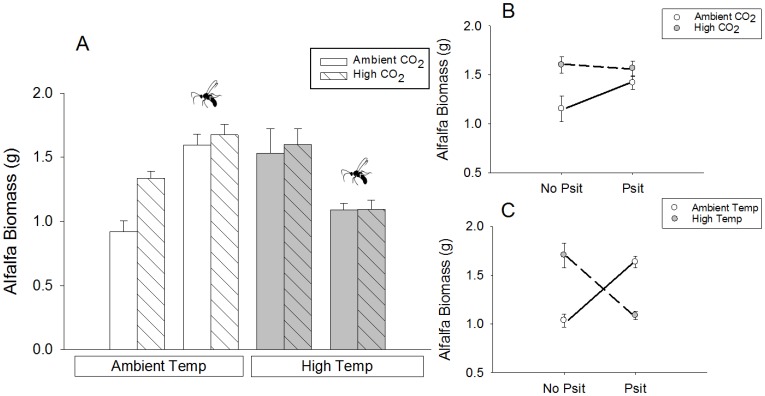
Complex interactions between abiotic and biotic factors were most evident when examining alfalfa biomass as the ANOVA response variable. The indirect positive effect of parasitoids on alfalfa biomass (i.e. the trophic cascade) had the strongest effect on alfalfa for all combinations of temperature, CO2, and parasitoid presence (Fig. 3); the lowest mean biomass per microcosm was found under conditions of no parasitoids+ambient temperature+ambient CO2. However, the positive effects of parasitoids on alfalfa biomass were attenuated with increasing CO2 (Fig. 3B) and disappeared with increasing temperature (Fig. 3C). These significant interactions were corroborated and refined by our path analyses (Fig. 4), which generally supported predictions from previous literature (Fig. 1).
Figure 3. Alfalfa biomass with herbivores under ambient and elevated temperatures and CO2.
(A) Mean alfalfa biomass (±1 SE) under different treatments; the wasp icon indicates the parasitoid addition treatments. There was a significant effect of CO2 on alfalfa biomass; F1,195 = 10.30, P<0.01. (B) The interaction between CO2 and parasitoid treatment (Psit); F1,195 = 8.02, P<0.01. (C) The interaction between temperature and parasitoid treatment; F1,195 = 51.57, P<0.001.
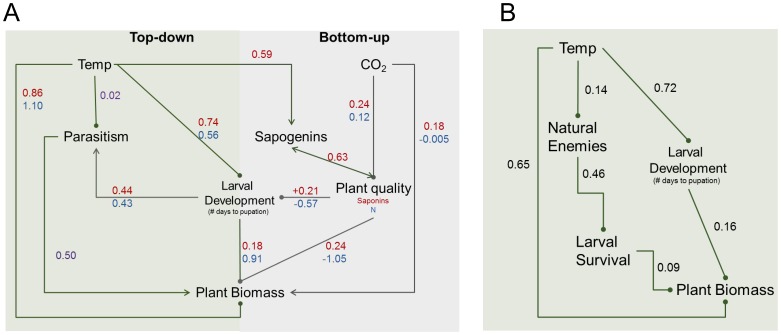
Figure 4. Path diagrams summarizing 2 models of direct and indirect effects of temperature and CO2 on alfalfa biomass.
Positive effects between variables are indicated by an arrow and negative effects are indicated by a filled circle. Standardized path coefficients are from the two general path models – blue coefficients are from a model that examined total leaf nitrogen (N) as a plant quality variable and red coefficients are from a model that examined saponins and sapogenins as plant quality variables. (A) Temperature and CO2 effects on plant chemistry and the effects on alfalfa biomass. (B) A nested path model that demonstrates that the positive effects of natural enemies on plant biomass are mediated by decreased larval survivorship.
The path analyses, which were focused on effects on biomass and saponin/sapogenins, helped clarify potential mechanisms for the strong negative effect of temperature on alfalfa biomass. Both general models included a pathway that incorporates the known tradeoff between plant quality (saponin production or total N content) and growth (Pearson et al. 2008)(Saponin, goodness of fit, χ2 = 4.66, df = 8, P = 0.79; N content, goodness of fit, χ2 = 2.52, df = 4, P = 0.64, Fig. 4A). The strong indirect negative effects of temperature on alfalfa biomass via increased saponin content (path coefficients in Fig. 4A) only partly explained the negative effect of temperature on biomass, since there was still a strong direct effect of temperature. The direct and indirect negative effects of temperature on plant biomass were also attenuated by the increase in larval development at higher temperatures and the indirect effects of parasitism (via larval development). The apparent trophic cascade (“direct” effect of parasitism in 4A) was confirmed by the path analysis focused on effects of parasitism on larval development (goodness of fit, χ2 = 0.01, df = 1, P = 0.80, Fig. 4B).
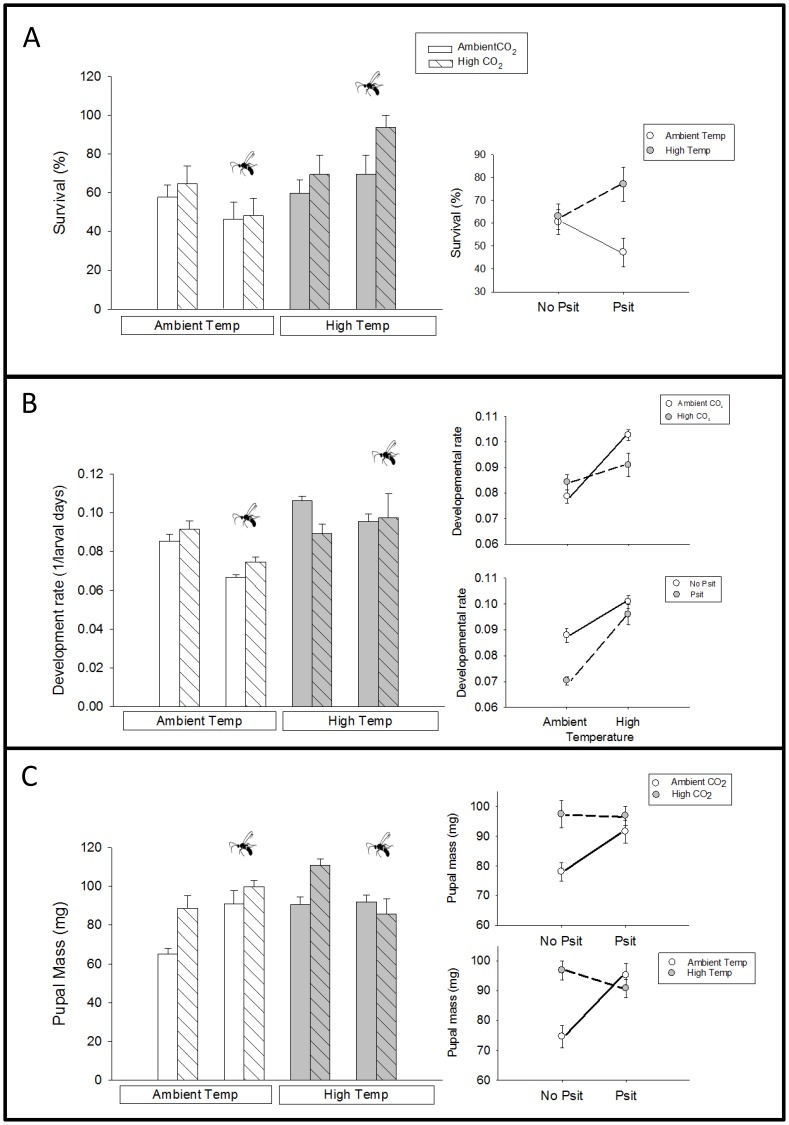
Spodoptera survival, development, pupal mass and parasitism rates were significantly affected by temperature. In the presence of parasitoids, larval survival was the highest under high temperatures and the lowest under ambient temperatures (Fig. 5). All larvae developed faster with increased temperatures, however this increase was dampened by elevated CO2 (Fig. 5). Under ambient temperatures, larvae developed significantly slower in the parasitoid treatments – these development results only included Spodoptera larvae that pupated, so development rates of individuals that died from parasitoids could not be included in these calculations. The negative effect of parasitoids on herbivore developmental rates disappeared at elevated temperature (Fig. 5).
Figure 5. Differences in Spodoptera performance between treatments; significant interactions are graphically summarized in the smaller figures to the right.
(A) Significant temperature (F1,145 = 32.63, P<0.001) and parasitoid addition (F1,145 = 9.56, P<0.01) effects on larval developmental rates (mean number of days until pupation−1± SE) and interactions between temperature and parasitoid addition (F1,145 = 7.41, P<0.01) and temperature and CO2 (F1,145 = 5.58, P<0.05). (B) Significant temperature (F1,196 = 4.48, P<0.05) and CO2 effects (F1,196 = 7.85, P<0.01) on pupal mass (mean ± SE) and interactions between temperature and parasitoid presence (F1,196 = 13.51, P<0.001) and CO2 and parasitoid presence (F1,196 = 6.22, P<0.05). (C) Significant temperature effect (F1,195 = 7.83, P<0.01) on survival (mean ±1 SE) and interaction between temperature and parasitoid presence (F1,195 = 5.187, P<0.05).
Both temperature and CO2 significantly increased Spodoptera pupal mass (Fig. 5) and there were significant interactions with parasitoid presence. Under both ambient temperature and CO2, larvae from the parasitoid treatments that survived to pupation had significantly greater pupal masses than those without parasitoids. However, elevated temperature and CO2 diminished this effect of parasitoid presence on the pupal mass of surviving individuals.
High temperature and CO2 caused dramatic reductions in parasitism (Mann-Whitney, Z = −2.949, P = 0.003), with levels declining from 29.6±8.1% at ambient temperature and CO2 to zero at elevated levels of both variables. Even at low CO2, increased temperature had strong negative effects on parasitism, causing decreases from 26.9±8.3% to 2.8±2.8% parasitism at high versus low temperature.
Discussion
The most notable result of our experiments was the indirect effect of increased temperature, which caused decreased development times for caterpillars, resulting in a dramatic negative effect on parasitism. Caterpillars developed rapidly at the higher temperatures and pupated before parasitoids were able to eclose from the late larval stages, resulting in death of the developing parasitoid. This developmental asynchrony could have been exacerbated by the timing of parasitoid introduction to the chambers – had they been introduced earlier, perhaps parasitoid success would have been higher in all treatments. However, the time of introduction was chosen to maximize parasitism based on the phenology of the lab colony – introduction of adults at third instar was optimal for successful parasitism for this particular colony. The 29.6% decline in parasitism recorded at the higher temperature is biologically significant [58] and such developmental mismatches can contribute to overall phenological asynchrony, since the parasitoid must pupate before its host, and if it does manage to eclose, this species has a very short adult stage for mating and finding an early instar host.
In contrast to temperature, increases in CO2 indirectly increased larval development times by decreasing plant quality, both of which were associated with lower levels of parasitism. For both temperature and CO2 effects on trophic interactions, the host-parasitoid developmental mismatch could contribute to the phenological asynchrony predicted by other climate change scenarios [40] and is likely to synergize with similar global change parameters that delink parasitoids from their hosts. For example, extreme weather events, such as floods and droughts, are increasing with global warming, and these climatic events are likely to cause decreases in caterpillar parasitism rates due to delinking the phenologies of host-parasitoid populations [58]. The fact that increased temperature and CO2 each cause temporal developmental shifts between parasitoids and hosts provides a clear mechanism by which alfalfa biomass is not enhanced by parasitoids under a changing climate: parasitoids simply cannot track the variable development and quality of their hosts.
Interestingly, rather than increasing alfalfa biomass indirectly via killing their caterpillar hosts, parasitoids at high temperature caused a decrease in biomass via increased consumption by their caterpillar hosts with no associated mortality. In any biotic community, this effect on biomass could be maintained by immigration of mated adults from adjacent patches or by host shifts by other parasitoids, but in the absence of genetic variation in development rates, the parasitoids would go locally extinct and the direct effects of herbivory on plant biomass would be more important. This parasitoid-biomass result is more relevant to classical biological control, since the continual release of parasitoids could sustain this indirect negative effect on biomass in warmer and CO2 enriched conditions. Literature syntheses on climate change and biological control [5], [27] indicate that parasitoid-host developmental mismatches could be common.
Herbivore populations are affected by a combination of abiotic factors, natural enemies, and plant quality and availability. Changes in climate have direct effects on the autecology of herbivores via changes in growth rate, metabolic activity, survivorship, and related factors, but as shown in our chamber experiments, indirect effects can modify significantly the outcomes of consumer-resource interactions [66]. At first glance, the predictions from previous studies on associations between climate variables and consumer-resource relationships are adequate for predicting more complex interactions (i.e. comparing Figs. 1 and 4). However, quantifications of only the direct effects do not uncover important indirect mechanisms, such as developmental asynchrony and trade-offs between growth and plant quality. Experiments on simple tritrophic systems that include controlled manipulations of multiple variables that are changing globally provide data that allow for considerable insight into basic questions about the regulation of herbivore populations. More experiments, coupled with observational data and models, will help clarify the conditions under which factors like connectance, parasitism, herbivore, outbreaks, and ecosystem services will increase or decrease in response to interacting climate change variables [8]–[28]. Furthermore, understanding relationships between global climatic changes and tritrophic interactions is particularly important in agricultural systems, where herbivore outbreaks are predicted to increase.
There are multiple consequences to the fact that the responses of biotic interactions to climate change are complex. This includes the possibility that effects acting via different direct or indirect pathways could cancel each other out or lead to changes not predicted by single factor models or experiments. For example, increases in plant biomass due to temperature can be counteracted by changes in parasitism, larval development, and increases in production of secondary metabolites, such that the overall effects of temperature (direct plus indirect) are negative (e.g., Fig. 4). It is clear that such interactions must be examined in order to produce realistic predictions for future impacts of climate change on biotic communities.
Our results are limited to an unnatural experimental setting; most notably, the community is an unrealistically simple chain, the three trophic levels did not evolve together, and levels of temperature and CO2 will gradually increase to our experimental levels over a number of decades. However, models and experiments are necessarily artificial, and in this case our experiments provided relevant insight into mechanisms by which trophic asynchrony can occur. Based on our experimental results here and accompanying models [4] and observational field studies [5], [30], [58], we conclude that any efforts to conserve natural enemies or to enhance natural biological control will be negatively affected by complex interactions between multiple climate change metrics and biotic communities. These effects are likely to be exacerbated by increases in extreme weather events [7], [10], [11], [58], contributing to increased insect outbreaks through a number of direct and indirect pathways.
Acknowledgments
Thanks to M. Forister, T. Massad, C. Jeffrey, the chemical ecology group at UNR, N. Mills, and two anonymous reviewers for comments and edits. J. Ruberson graciously supplied insects for our lab colonies. T. Massad developed the methods for quantifying alfalfa saponins. The authors thank the staff at Earthwatch, M. Tobler, G. Rodriguez-Castaneda, M. Olson, and many Earthwatch volunteers for their hard work and enthusiasm for the project.
Funding Statement
This work was supported by: Earthwatch Institute (www.Earthwatch.org), University of Nevada Reno (www.unr.edu), Department of Energy (National Institute for Global Environmental Change and National Institute for Climatic Change Research, http://niccr.nau.edu/), and National Science Foundation (nsf.gov) grants - DEB1020509 and DEB0849361. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beddington J (2010) Food security: contributions from science to a new and greener revolution. Philos T R Soc B 365: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hillebrand H, Matthiessen B (2009) Biodiversity in a complex world: consolidation and progress in functional biodiversity research. Ecol Lett 12: 1405–1419. [DOI] [PubMed] [Google Scholar]
- 3. Berggren A, Bjorkman C, Bylund H, Ayres MP (2009) The distribution and abundance of animal populations in a climate of uncertainty. Oikos 118: 1121–1126. [Google Scholar]
- 4. Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trend Ecol Evol 25: 325–331. [DOI] [PubMed] [Google Scholar]
- 5. Thomson LJ, Macfadyen S, Hoffmann AA (2010) Predicting the effects of climate change on natural enemies of agricultural pests. Biol Control 52: 296–306. [Google Scholar]
- 6. Araujo MB, Luoto M (2007) The importance of biotic interactions for modelling species distributions under climate change. Global Ecol Biogeogr 16: 743–753. [Google Scholar]
- 7. Bannerman JA, Gillespie DR, Roitberg BD (2011) The impacts of extreme and fluctuating temperatures on trait-mediated indirect aphid-parasitoid interactions. Ecol Entomol 36: 490–498. [Google Scholar]
- 8. Brose U, Dunne JA, Montoya JM, Petchey OL, Schneider FD, et al. (2012) Climate change in size-structured ecosystems. Philos T R Soc B 367: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Sassi C, Tylianakis JM (2012) Climate change disproportionately increases herbivore over plant or parasitoid biomass. PLoS ONE 7: e40557 doi: 10.1371/journal.pone.0040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gillespie DR, Nasreen A, Moffat CE, Clarke P, Roitberg BD (2012) Effects of simulated heat waves on an experimental community of pepper plants, green peach aphids and two parasitoid species. Oikos 121: 149–159. [Google Scholar]
- 11. Harmon JP, Moran NA, Ives AR (2009) Species response to environmental change: impacts of food web interactions and evolution. Science 323: 1347–1350. [DOI] [PubMed] [Google Scholar]
- 12. Harrington R, Woiwod I, Sparks T (1999) Climate change and trophic interactions. Trend Ecol Evol 14: 146–150. [DOI] [PubMed] [Google Scholar]
- 13. Hoekman D (2010) Turning up the heat: Temperature influences the relative importance of top-down and bottom-up effects. Ecology 91: 2819–2825. [DOI] [PubMed] [Google Scholar]
- 14. Klapwijk MJ, Grobler BC, Ward K, Wheeler D, Lewis OT (2010) Influence of experimental warming and shading on host-parasitoid synchrony. Glob Change Biol 16: 102–112. [Google Scholar]
- 15. Kratina P, Greig HS, Thompson PL, Carvalho-Pereira TSA, Shurin JB (2012) Warming modifies trophic cascades and eutrophication in experimental freshwater communities. Ecology 93: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 16. Lurgi M, Lopez BC, Montoya JM (2012) Climate change impacts on body size and food web structure on mountain ecosystems. Philos T R Soc B 367: 3050–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lurgi M, Lopez BC, Montoya JM (2012) Novel communities from climate change. Philos T R Soc B 367: 2913–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merrill RM, Gutierrez D, Lewis OT, Gutierrez J, Diez SB, et al. (2008) Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J Anim Ecol 77: 145–155. [DOI] [PubMed] [Google Scholar]
- 19. Petchey OL, McPhearson PT, Casey TM, Morin PJ (1999) Environmental warming alters food-web structure and ecosystem function. Nature 402: 69–72. [Google Scholar]
- 20. Traill LW, Lim MLM, Sodhi NS, Bradshaw CJA (2010) Mechanisms driving change: altered species interactions and ecosystem function through global warming. J Anim Ecol 79: 937–947. [DOI] [PubMed] [Google Scholar]
- 21. Voigt W, Perner J, Davis AJ, Eggers T, Schumacher J, et al. (2003) Trophic levels are differentially sensitive to climate. Ecology 84: 2444–2453. [Google Scholar]
- 22. Voigt W, Perner J, Jones TH (2007) Using functional groups to investigate community response to environmental changes: two grassland case studies. Glob Change Biol 13: 1710–1721. [Google Scholar]
- 23. Binzer A, Guill C, Brose U, Rall BC (2012) The dynamics of food chains under climate change and nutrient enrichment. Philos T R Soc B 367: 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Sassi C, Lewis OT, Tylianakis JM (2012) Plant-mediated and nonadditive effects of two global change drivers on an insect herbivore community. Ecology 93: 1892–1901. [DOI] [PubMed] [Google Scholar]
- 25. Hoover JK, Newman JA (2004) Tritrophic interactions in the context of climate change: a model of grasses, cereal Aphids and their parasitoids. Glob Change Biol 10: 1197–1208. [Google Scholar]
- 26. Newman JA (2004) Climate change and cereal aphids: the relative effects of increasing CO2 and temperature on aphid population dynamics. Glob Change Biol 10: 5–15. [Google Scholar]
- 27. Shurin JB, Clasen JL, Greig HS, Kratina P, Thompson PL (2012) Warming shifts top-down and bottom-up control of pond food web structure and function. Philos T R Soc B 367: 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stacey DA (2003) Climate and biological control in organic crops. Int J Pest Manage 49: 205–214. [Google Scholar]
- 29. Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11: 1351–1363. [DOI] [PubMed] [Google Scholar]
- 30. Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob Change Biol 12: 27–41. [Google Scholar]
- 31. de Sassi C, Staniczenko PPA, Tylianakis JM (2012) Warming and nitrogen affect size structuring and density dependence in a host-parasitoid food web. Philos T R Soc B 367: 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Connor MI, Gilbert B, Brown CJ (2011) Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am Nat 178: 626–638. [DOI] [PubMed] [Google Scholar]
- 33. Petchey OL, Brose U, Rall BC (2010) Predicting the effects of temperature on food web connectance. Philos T R Soc B 365: 2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pounds JA, Puschendorf R (2004) Ecology - Clouded futures. Nature 427: 107–109. [DOI] [PubMed] [Google Scholar]
- 35. Butler CD, Trumble JT (2010) Predicting population dynamics of the parasitoid Cotesia marginiventris (Hymenoptera: Braconidae) resulting from novel interactions of temperature and selenium. Biocontrol Sci Techn 20: 391–406. [Google Scholar]
- 36. Mironidis GK, Savopoulou-Soultani M (2009) Development, survival and growth rate of the Hyposoter didymator-Helicoverpa armigera parasitoid-host system: Effect of host instar at parasitism. Biol Control 49: 58–67. [Google Scholar]
- 37. Hance T, van Baaren J, Vernon P, Boivin G (2007) Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol 52: 107–126. [DOI] [PubMed] [Google Scholar]
- 38. Ali A, Luttrell RG, Schneider JC (1990) Effects of temperature and larval diet on development of the fall armyworm (Lepidoptera, Noctuidae). Ann Entomol Soc Am 83: 725–733. [Google Scholar]
- 39. Williams RS, Lincoln DE, Norby RJ (2003) Development of gypsy moth larvae feeding on red maple saplings at elevated CO2 and temperature. Oecologia 137: 114–122. [DOI] [PubMed] [Google Scholar]
- 40. Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol S 37: 637–669. [Google Scholar]
- 41. Agrell J, Anderson P, Oleszek W, Stochmal A, Agrell C (2004) Combined effects of elevated CO2 and herbivore damage on alfalfa and cotton. J Chem Ecol 30: 2309–2324. [DOI] [PubMed] [Google Scholar]
- 42. Curtis PS, Wang XZ (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113: 299–313. [DOI] [PubMed] [Google Scholar]
- 43. Ding Li ZZ-P, Li S-Y, Zhang C-H, Bai K-Z, Kuang T-Y (1996) Assimilation and allocation of carbon and nitrogen in alfalfa under doubled CO2 environment. Acta Bot Sin 38: 83–86. [Google Scholar]
- 44. Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: Plants face the future. Annu Rev Plant Biol 55: 591–628. [DOI] [PubMed] [Google Scholar]
- 45. Schadler M, Roeder M, Brandl R, Matthies D (2007) Interacting effects of elevated CO2, nutrient availability and plant species on a generalist invertebrate herbivore. Glob Change Biol 13: 1005–1015. [Google Scholar]
- 46. Bezemer TM, Jones TH (1998) Plant-insect herbivore interactions in elevated atmospheric CO2: quantitative analyses and guild effects. Oikos 82: 212–222. [Google Scholar]
- 47. Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global Change Effects on Plant Chemical Defenses against Insect Herbivores. J Integr Plant Biol 50: 1339–1354. [DOI] [PubMed] [Google Scholar]
- 48. Korner C (2000) Biosphere responses to CO2 enrichment. Ecol Appl 10: 1590–1619. [Google Scholar]
- 49. Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob Change Biol 4: 43–54. [Google Scholar]
- 50. Lindroth RL (2010) Impacts of Elevated Atmospheric CO2 and O3 on Forests: Phytochemistry, Trophic Interactions, and Ecosystem Dynamics. J Chem Ecol 36: 2–21. [DOI] [PubMed] [Google Scholar]
- 51. Dyer LA, Letourneau DK, Dodson CD, Tobler MA, Stireman JO, et al. (2004) Ecological causes and consequences of variation in defensive chemistry of a Neotropical shrub. Ecology 85: 2795–2803. [Google Scholar]
- 52. Roth S, Lindroth RL, Volin JC, Kruger EL (1998) Enriched atmospheric CO2 and defoliation: effects on tree chemistry and insect performance. Glob Change Biol 4: 419–430. [Google Scholar]
- 53. Harvey JA, Van Nouhuys S, Biere A (2005) Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J Chem Ecol 31: 287–302. [DOI] [PubMed] [Google Scholar]
- 54. Lampert EC, Dyer LA, Bowers MD (2011) Chemical Defense Across Three Trophic Levels: Catalpa bignonioides, the Caterpillar Ceratomia catalpae, and its Endoparasitoid Cotesia congregata. J Chem Ecol 37: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 55. Smilanich AM, Dyer LA (2012) Effects of banana plantation pesticides on the immune response of lepidopteran larvae and their parasitoid natural enemies. Insects 3: 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bezemer TM, Jones TH, Knight KJ (1998) Long-term effects of elevated CO2 and temperature on populations of the peach potato aphid Myzus persicae and its parasitoid Aphidius matricariae. Oecologia 116: 128–135. [DOI] [PubMed] [Google Scholar]
- 57. Logan JD, Wolesensky W, Joern A (2006) Temperature-dependent phenology and predation in arthropod systems. Ecol Model 196: 471–482. [Google Scholar]
- 58. Stireman JO, Dyer LA, Janzen DH, Singer MS, Lill JT, et al. (2005) Climatic unpredictability and parasitism of caterpillars: Implications of global warming. Proc Nat Acad Sci 102: 17384–17387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putnam DH, Brummer J, Cash D, Gray A, Griggs T, et al.. (2000) The Importance of Western Alfalfa Production.: Proceedings, 2000 National Alfalfa Symposium, 10–12 December, 2000 University of California Alfalfa Workgroup, Alfalfa Council. Las Vegas, NV. 9 pages.
- 60. Pearson CV, Massad TJ, Dyer LA (2008) Diversity cascades in alfalfa fields: From plant quality to agroecosystem diversity. Environ Entomol 37: 947–955. [DOI] [PubMed] [Google Scholar]
- 61.Barbosa HA (2004) Vegetation dynamics over the Northeast region of Brazil and their connections with climate variability during the last two decades of the twentieth century.: University of Arizona. Tucson, Arizona.
- 62. Dyer LA, Stireman JO (2003) Community-wide trophic cascades and other indirect interactions in an agricultural community. Basic Appl Ecol 4: 423–432. [Google Scholar]
- 63.Houghton JT, Ding Y, Griggs DJ, Noquer M, van der Linden PJ, et al.. (2001) Climate Change 2001: the Scientific Basis. Cambridge: Cambridge University Press. 944 p.
- 64. Hulme M, Viner D (1998) A climate change scenario for the tropics. Climatic Change 39: 145–176. [Google Scholar]
- 65. Kursar TA, Dexter KG, Lokvam J, Pennington RT, Richardson JE, et al. (2009) The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Nat Acad Sci 106: 18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Suttle KB, Thomsen MA, Power ME (2007) Species interactions reverse grassland responses to changing climate. Science 315: 640–642. [DOI] [PubMed] [Google Scholar]
- 67. Sallas L, Luomala EM, Utriainen J, Kainulainen P, Holopainen JK (2003) Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol 23: 97–108. [DOI] [PubMed] [Google Scholar]
- 68. Pecetti L, Tava A, Romani A, De Benedetto MG, Corsi P (2006) Variety and environment effects on the dynamics of saponins in lucerne (Medicago sativa L.). Eur J Agron 25: 187–192. [Google Scholar]
- 69. Haggstrom H, Larsson S (1995) Slow larval growth on a suboptimal willow results in high predation mortality in the leaf beetle Galerucella lineola . Oecologia 104: 308–315. [DOI] [PubMed] [Google Scholar]
- 70. Harvey JA, Jervis MA, Gols R, Jiang NQ, Vet LEM (1999) Development of the parasitoid, Cotesia rubecula (Hymenoptera: Braconidae) in Pieris rapae and Pieris brassicae (Lepidoptera: Pieridae): evidence for host regulation. J Insect Physiol 45: 173–182. [DOI] [PubMed] [Google Scholar]
- 71. Harvey JA, Sano T, Tanaka T (2010) Differential host growth regulation by the solitary endoparasitoid, Meteorus pulchricornis in two hosts of greatly differing mass. J Insect Physiol 56: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 72. Ode PJ (2006) Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annu Rev Entomol 51: 163–185. [DOI] [PubMed] [Google Scholar]