Abstract
Background
Cerebrospinal fluid (CSF) sodium concentration increases during migraine attacks, and both CSF and vitreous humor sodium increase in the rat migraine model. The Na,K-ATPase is a probable source of these sodium fluxes. Since Na,K-ATPase isoforms have different locations and physiological roles, our objective was to establish which alpha isoforms are present at sites where sodium homeostasis is disrupted.
Methods
Specific Na,K-ATPase alpha isoforms were identified in rat tissues by immunohistochemistry at the blood-CSF barrier at the choroid plexus, at the blood-CSF-trigeminal barrier at the meninges, at the blood-retina barrier, and at the blood-aqueous barrier at the ciliary body. Calcitonin gene-related peptide (CGRP), occludin, or von Willibrand factor (vWF) were co-localized with Na,K-ATPase to identify trigeminal nociceptor fibers, tight junctions, and capillary endothelial cells respectively.
Results
The Na,K-ATPase alpha-2 isoform is located on capillaries and intensely at nociceptive trigeminal nerve fibers at the meningeal blood-CSF-trigeminal barrier. Alpha-1 and −3 are lightly expressed on the trigeminal nerve fibers but not at capillaries. Alpha-2 is expressed at the blood-retina barriers and, with alpha-1, at the ciliary body blood aqueous barrier. Intense apical membrane alpha-1 was associated with moderate cytoplasmic alpha-2 expression at the choroid plexus blood-CSF barrier.
Conclusion
Na,K-ATPase alpha isoforms are present at the meningeal, choroid plexus, and retinal barriers. Alpha-2 predominates at the capillary endothelial cells in the meninges and retinal ganglion cell layer.
Keywords: Cerebrospinal fluid (CSF), Choroid plexus (CP), Meninges, Retina
Background
Sodium is crucial for neuronal excitability: when the membrane potential of a neuron depolarizes, sodium channels open. When the potential reaches a threshold, the sodium current becomes regenerative and initiates the action potential. Hodgkin and Katz showed that altered extracellular sodium concentration changes the action potential amplitude in squid axon [1,2]. Recent experiments revealed that sodium increases in human lumbar CSF by 3 – 4 mM during migraine attacks [3], and in the intracranial CSF and ocular vitreous humor by over 20 mM in a rat model of central sensitization [4]. Since retinal ganglion cells participate in the photophobia of migraine [5], this intraocular increase of sodium may implicate a local retinal stimulation from elevated sodium concentrations. The degree of these sodium changes are sufficient to increase neuronal excitability by more than 1.75 fold in simulated [4] and primary cultured neurons [6]. Stimulation of trigeminal meningeal nociceptors and retinal neurons might contribute to the cranial pain and light sensitivity of migraine [7]. The enzyme Na,K-ATPase is a potential source to change [Na+], and the expression of Na,K-ATPase alpha isoforms in the related barriers will be reported in this study.
Cerebrospinal fluid (CSF) cations are tightly regulated at distinctly different concentrations than in blood, with higher sodium and lower potassium in CSF, made possible because the blood-CSF barrier [8] limits diffusional correction of the gradient. Choroid plexuses (CP) in the lateral, third, and fourth ventricles generate most of the CSF, including these cation gradients. These CP vascular structures have fenestrated capillaries from which the blood exudes to face the basal wall of a single layer of epithelial cells. Tight junctions between these epithelial cells [9] prohibit diffusion from this exudate directly into CSF, and vice versa. The Na,K-ATPase alpha-1 isoform at the apical membrane of these cells [10,11], together with other transporters [12-14] including the Na+-K+-2Cl- cotransporter and the Na+-H+ exchanger, is critical for the Na+ secretion and K+ absorption from CSF that result in this characteristic CSF-blood cation gradient. We, therefore, studied the Na,K-ATPase isoforms at the CP.
Once released from the CP, CSF is in direct contact with capillaries extensively throughout the meninges. Capillaries in the dura, arachnoid, and pia mater have tight junctions [9] in place of their normal fenestrations, although some meningeal vessels have been reported to be fenestrated [15]. Meningeal capillaries, however, have not been as extensively studied as those at the blood–brain capillary interface, where it is clear that surrounding pericytes, astrocyte endfeet, and neuronal processes bolster the endothelial cell tight junctions at the blood–brain barrier [9]. In addition, peripheral sensory nerves have tight junctions between intraneural capillary endothelial cells and the perineurium, forming a blood-nerve-barrier [16], though these have not yet been studied in the meningeal nerves. We define the barrier in meningeal blood vessels between blood and CSF and the barrier in meningeal nerve fibers between nerve and blood/CSF together as the blood-CSF-trigeminal barrier.
The blood supply for the retina includes the intraretinal vessels for the inner 2/3 and choroidal vessels for the outer 1/3. There are two blood-retina barriers: tight junctions between the inner intraretinal capillary endothelial cells at the retinal ganglion cell layer protect the inner 2/3 of the retina, and tight junctions between the retinal pigment epithelial (RPE) cells form the barrier to the outer, fenestrated choroidal blood supply [17-19]. A third barrier in the eye, the blood-aqueous barrier, is formed by the tight junctions in the non-pigmented epithelium (NPE) of the ciliary body [20], where the Na,K-ATPase contributes to sodium and aqueous humor secretion [21].
How sodium dysregulation arises in the migraine model is not defined, and prompted the anatomical studies reported in this paper. Altered Na,K-ATPase activity is considered primarily responsible for the sodium changes found in CSF [7]. The 3 alpha isoforms of Na,K-ATPase in brain have different distributions and activities related to their varied functions, and yet they have not been completely characterized at these blood-fluid barriers. To gain further definition of the Na,K-ATPase isoforms at these barriers, we localize the Na,K-ATPase alpha −1, -2, and −3 isoform expression at the principal source of CSF (in the CP), at the sites of intracranial trigeminal nociception (in the meninges), and at the sites of ocular sodium regulation (in the ciliary body and retina).
Methods
Fourty-three Sprague–Dawley male rats (Harlan) between the weights of 180 and 240 g were used in these studies. The HMRI Animal Care and Use Committee approved animal procedures.
Tissue preparation
For the retina, ciliary body, and CP studies, rats were deeply anesthetized with pentobarbital (50–100 mg/Kg). After euthanasia by decapitation, eyes were enucleated and brains were removed. Brain regions were identified with reference to the Paxinos and Watson brain atlas [22]. Coronal sections were cut from eyes and brains, frozen in O.C.T. compound (Tissue Tek, Torrance, CA) in tissue blocks (Pathology Innovations, Wyckoff, NJ), and sliced into 12 μm sections using a cryostat (Mikron, Vista, CA). Each section was mounted on Fisherbrand Superfrost® Plus precleaned slides (Fisher Scientific, Pittsburgh, PA) and stored at −30°C until stained. These tissues were fresh-frozen without cryoprotection, and ethanol fixed on their slide at the start of immunostaining. For meningeal studies, rats were deeply anesthetized with pentobarbital, then transcardially perfused with PBS followed by fresh 2% paraformaldehyde. The dura with meninges were carefully stripped from the inner calvarium, cut around the base of the brain and removed in one piece from its surface. The meninges over the cerebellum were removed; the remainder cut into half, stained as floating sections, and then mounted on slides. This preparation was predominantly dura, but we refer to it as meninges as it also includes significant arachnoid and possibly some pial fragments.
Antibodies
The following antibodies were used in this study: mouse monoclonal anti-Na,K-ATPase alpha-1 (Cat# 05–369, Millipore); rabbit anti-Na,K-ATPase alpha-2 (Cat# 07–674, Millipore); rabbit anti-Na,K-ATPase alpha-3 (Cat#: 06–172, Millipore); mouse monoclonal anti-vWF (Cat# LS-B4034, Lifespan Biosciences); rabbit anti-vWF (Cat#: ECM 590, Millipore); guinea pig anti-CGRP (Cat#: T-5027, Peninsula Laboratories, LLC); rabbit anti-occludin (Cat#: sc-5562, Santa Cruz Biotechnology); goat anti-guinea pig IgG (Cat#: BA-7000, Vector Laboratories); goat anti-rabbit IgG (Cat# BA-1000; Vector Laboratories); horse anti-mouse rat adsorbed IgG (Cat# BA-2001, Vector Laboratories). The following combinations were used in fluorescence experiments: For Figure 1, guinea pig anti-CGRP primary antibody, goat anti-guinea pig IgG secondary antibody, with fluorescein; followed by rabbit anti-Na,K-ATPase alpha-2 primary antibody (1:500), goat anti-rabbit IgG secondary antibody, with Texas red. For Figure 2, guinea pig anti-CGRP primary antibody, goat anti-guinea pig IgG secondary antibody, with Texas red; followed by mouse anti-Na,K-ATPase alpha-1 primary antibody, horse anti-mouse rat adsorbed IgG secondary antibody, with fluorescein. For Figure 3, rabbit anti-Na,K-ATPase alpha-2 primary antibody, goat anti-rabbit IgG secondary antibody, with fluorescein; followed by mouse anti-Na,K-ATPase alpha-1 primary antibody, horse anti-mouse rat adsorbed IgG secondary antibody, with Texas red. For Figures 4 and 5, mouse anti-Na,K-ATPase alpha-1, or rabbit anti-Na,K-ATPase alpha-2, or rabbit anti-Na,K-ATPase alpha-3 primary antibody, horse anti-mouse rat adsorbed IgG (for alpha-1) or goat anti-rabbit IgG (for alpha-2 and alpha-3) secondary antibody, with fluorescein; followed by rabbit anti-vWF (for alpha-1) or mouse anti-vWF (for alpha-2 and alpha-3) primary antibody, goat anti-rabbit IgG (for alpha-1) or horse anti-mouse (for alpha-2 and alpha-3) IgG secondary antibody, with Texas red. To reduce background, all wash solutions contained 2% normal serum from the host of the secondary antibody. Since the dura is around 50 μm thick and tends to have high background, all dura rinses between treatments were 4 times15 min.
Figure 1.
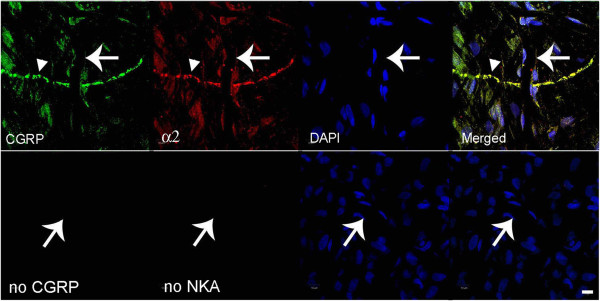
Fluorescence images of CGRP and Na,K-ATPase alpha-2 in meninges. In the top row, CGRP is labeled with fluorescein on the left, Na,K-ATPase alpha-2 is visualized with Texas red, nuclei are DAPI-stained, and the merged image is on the right. The large arrow indicates a capillary and the smaller arrowhead indicates a trigeminal nerve. The lower row images are negative controls when no primary antibody was used. The notable finding is that Na,K-ATPase alpha-2 is expressed at the capillary and nerve fiber. Scale bar = 10 μm.
Figure 2.
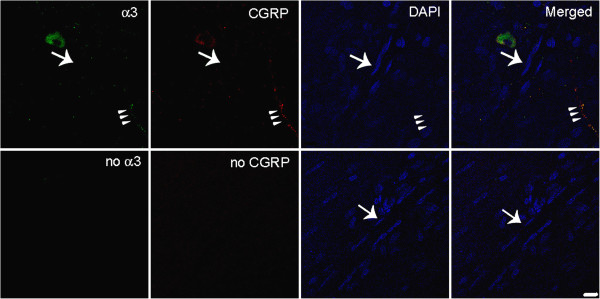
Fluorescence images of Na,K-ATPase alpha-3 and CGRP in meninges. In the top row, Na,K-ATPase alpha-3 is labeled with fluorescein in the left, CGRP is visualized with Texas red, nuclei are DAPI-stained, and the merged image is on the right. The large arrow indicates a capillary and the arrowhead triplet indicate a trigeminal nerve. The lower horizontal images are negative controls when no primary antibody was used. The notable finding is that Na,K-ATPase alpha-3 is expressed at the nerve fiber, not on the capillary. Scale bar = 10 μm.
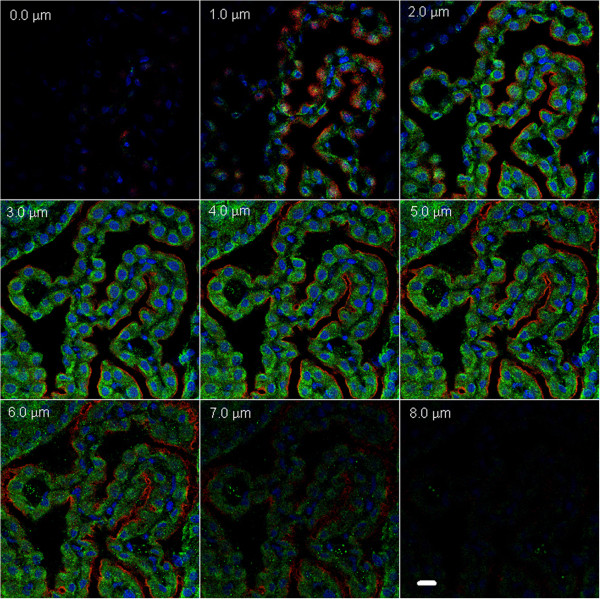
Figure 3.
Representative immunofluorescence images of a choroid plexus from the 3rd ventricle, sequentially acquired at 1 μm steps in a z-stack to reveal the cellular location of Na,K-ATPase alpha-1 and −2 isoforms. The same Na,K-ATPase expression patterns were found in the choroid plexuses in the lateral ventricles (data not shown). Texas red is used to visualize alpha-1 and fluorescein for alpha-2, and the nuclei are DAPI stained. Negative controls (without primary antibody; data not shown) had no membrane or cytoplasmic staining. Scale bar = 10 μm.
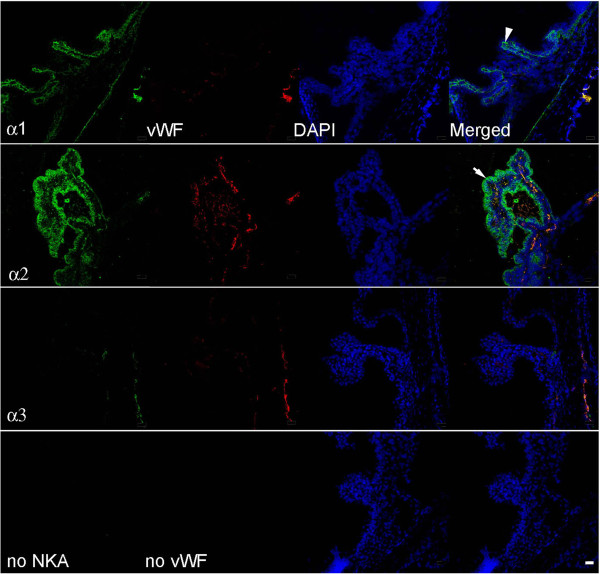
Figure 4.
Representative immunofluorescence images of the blood-aqueous barrier at the rat ciliary body with Na,K-ATPase alpha-1, -2, and −3 isoform expression. Na,K-ATPase isoforms labeled with fluorescein in left hand column images, vWF visualized with Texas red in second column, nuclei are DAPI stained, and the merged image for each horizontal panel is in the right hand column. Lower horizontal images are negative controls with no primary antibody. Merged images show alpha-1 expressed on the PE, alpha-2 on the NPE, and alpha-2 expressed on ciliary body capillaries. The arrowhead points to the PE on the alpha-1 merged image, and the arrow points to the NPE on the alpha-2 merged image. Scale bar = 20 μm.
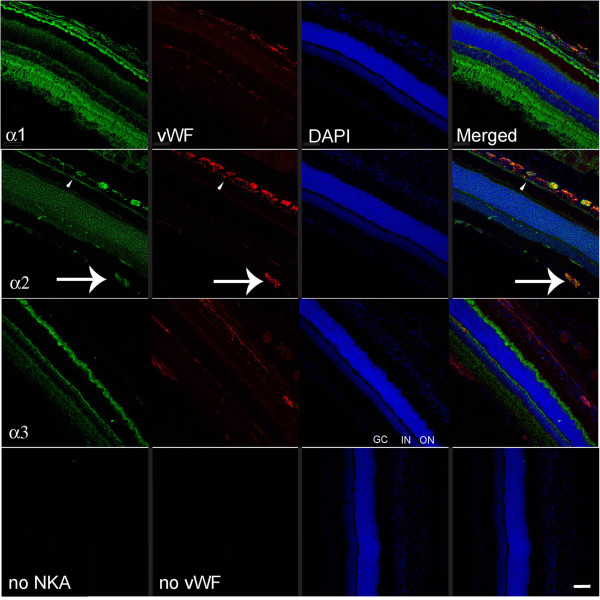
Figure 5.
Representative immunofluorescence images of Na,K-ATPase alpha-1, -2, and −3 expression at the rat retina. Na,K-ATPase isoforms labeled with fluorescein in the left hand column images, vWF visualized with Texas red in the second column, nuclei are DAPI-labeled, and the merged image for each horizontal panel is in the right hand column. Lower horizontal images are negative controls when no primary antibody was used. Unique Na,K-ATPase alpha-1 & -3 specific expression in the retinal cell layers is seen, similar to previous reports [23,44]. The arrow indicates alpha-2 expression in the capillaries at the retinal ganglion cell layer. GC: ganglion cell layer; IN: inner nuclear layer; ON: outer nuclear layer are labeled for orientation on the alpha-3 DAPI image. Scale bar = 50 μm.
The aforementioned Na,K-ATPase alpha-2 antibody we used was imperfect for retinal layer staining in comparison to the published work [23], but we demonstrate its specificity on retinal capillaries in the retinal ganglion cell layer and CP.
Immunohistochemistry
Meninges, CPs from the lateral and 3rd ventricles, and eyes were labeled with antibodies specific for the Na,K-ATPase alpha-1, alpha-2, and alpha-3 isoforms (1:1,000 unless otherwise stated, Millipore, Billerica, MA), von Willebrand factor (vWF) (1:500 dilution from Millipore or 1:100 dilution from Lifespan Biosciences, Seattle, WA), calcitonin gene-related peptide (CGRP) (1:5000 in Figure 2, 1:1,000 dilution in all other occasions, Peninsula Laboratories, LLC, San Carlos, CA), and occludin (1:200 dilution from Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Species-specific, biotinylated secondary antibodies were optimized at 1:200 or 1:500. Negative control procedures included identical steps, but without primary antibody. Pre-absorption of the alpha-2-antiserum with peptide in molar excess abrogated specific immunoreactive alpha-2 at the CP epithelium, the retinal ganglion cell layer, and the retinal pigment epithelium.
Diaminobenzidine tetrahydrochloride (DAB) staining: after washing out the fixative with phosphate-buffered saline containing 0.5% Triton X-100 (PBST), meninges were incubated in avidin for 15 min, rinsed with PBST, biotin blocking solution (Vector Laboratories, Burlingame, CA) for 15 min, followed by 1–2 h in a solution of 5% normal serum (from same species as the host of the secondary antibody) and 0.3% H2O2 in PBST. Slices were incubated in primary antibody (alpha-1, alpha-2, alpha-3, CGRP, occludin, and vWF) or peptide pre-absorbed alpha-2 antibody with 2% normal serum in PBST at 4°C overnight, washed in PBS, incubated for 2 h at room temperature with biotinylated secondary antibody with 2% normal goat serum in PBS, washed in PBS, incubated in freshly prepared ABC complex (Vector laboratories) for 30 min, washed in PBS, incubated in DAB (Invitrogen Life Technologies, Grand Island, NY) solution for 5–10 min, and rinsed in PBS to terminate the reaction. The edges of meninges were scored to flatten their curve and mounted on glass slides. After drying overnight, meninges were dehydrated with ethanol (70, 95, 100, 100%; 10 min each step), xylene for 10 min, and covered in mounting medium (Thermo Scientific) with cover slides.
Fluorescence double labeling: Eyes and brain slides were fixed in 100% ethanol for 2 h. Fixed tissues on slides or freshly prepared, floating meninges were stained using the aforementioned procedure but replacing the ABC complex with 30 min to 1 h incubation in Fluorescein Avidin DCS, or Texas Red Avidin D (Vector Laboratories). This and all subsequent procedures were carried out in the dark. After PBST washes, the same process was repeated for the second antibody and the opposite fluorophore from that used for the first antibody. The slices were rinsed in PBS, mounted with ProLong Gold antifade reagent with 4’, 6-diamidino-2-phenylindole (DAPI) (Invitrogen, Sunnyvale, CA), a cover slide was attached and sealed with clear fingernail polish. Preparations were cured in darkness, usually for > 24 h.
DAB-stained sections were imaged on a Nikon eclipse TS100 microscope. Large tiled images (up to 8x8 tiles, See Figures 1 &6) were acquired on a Zeiss Observer Z1 microscope (Carl Zeiss, Jena, Germany), 5x/0.5NA objective, Zeiss AxioCamMrC5 color camera, and AxioVision software v.4.8. Fluorescence images were captured on a Zeiss LSM 510 NLO microscope (Carl Zeiss, Jena, Germany) using a 20x/0.8 or 63x/1.4 lens. Samples were illuminated with Argon/ion (488nm), HeNe (543nm), and Chameleon Ultra 2-photon (790nm) lasers. Subtraction of paired images (Figures 7 &8) was achieved using MIPAV (http://mipav.cit.nih.gov, Version 12.5).
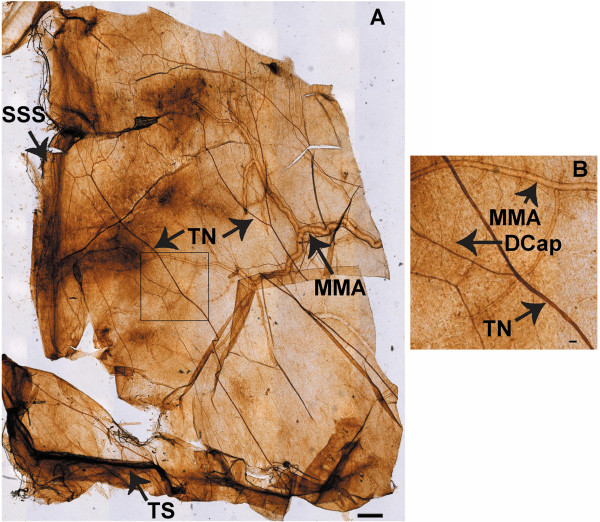
Figure 6.
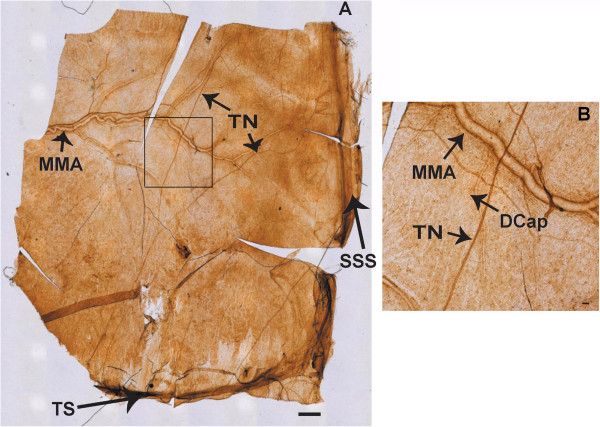
A is a tiled DAB image of Na,K-ATPase alpha-2 immunostaining at the rat meninges. The field of view is half of the meninges, extending from the transverse sinus (TS) to the superior sagittal sinus (SSS) centrally and the middle meningeal artery (MMA) laterally, with the trigeminal nerve fibers (TN) crossing the dura. Scale bar 500 μm. B: Enlarged region of meninges, magnified from the rectangle area in Figure 9A, illustrating branches of the MMA, a TN, and a capillary (DCap). Scale bar = 50 μm. For control, see Figure 10.
Figure 7.
A is a tiled DAB image, locating occludin at the rat meninges. The field of view is half of the meninges, extending from the TS to the SSS centrally, the MMA laterally, with the TN crossing the dura. Scale bar 500 μm. B: Enlarged region of meninges, magnified from the rectangle area in Figure 6A, illustrating branches of the MMA, a TN, and a capillary (DCap). Scale bar = 50 μm. For control, see Figure 10.
Figure 8.
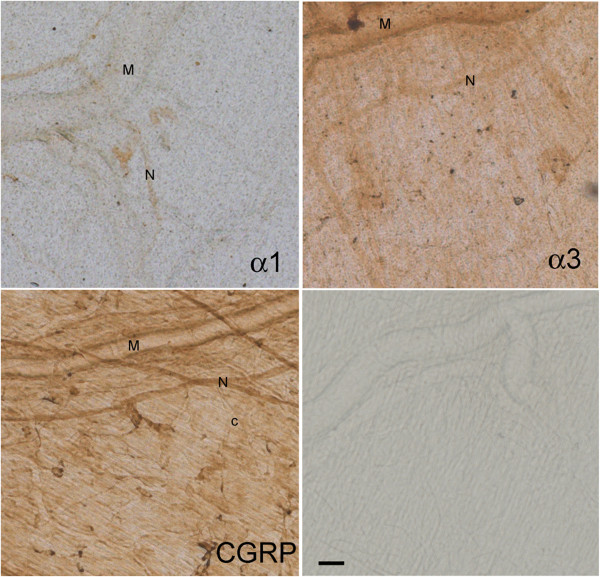
Representative images of meningeal areas showing DAB-stained Na,K-ATPase alpha-1, alpha-3, CGRP, and control, as indicated in the sections. M: branch of middle meningeal artery; N: trigeminal nerve fiber; c: capillary. Scale bar = 50 μm.
Results
Meninges
We used CGRP with immunoreactivity for trigeminal meningeal nerve fibers, some of which are on or near meningeal vessels [24], and because they are altered in migraine [25]. We observed that Na,K-ATPase alpha-2 was expressed in trigeminal nerve fibers, the transverse and sagittal sinuses, large middle meningeal artery branches (Figure 9), and capillaries (Figure 9B, Figure 1). The tight junction protein, occludin, was also expressed on trigeminal meningeal nerve fibers, venous sinuses, MMA branches, and capillaries (Figure 6), with the same distribution as Na,K-ATPase alpha-2 (Figure 9) and CGRP (Figure 10). The anti-CGRP stained the nerve fiber, but not the capillaries in Figure 2, probably because the concentration is much lower (1:5,000), and Texas red, a weaker signal than fluorescein, was used for CGRP in this experiment. Na,K-ATPase alpha-1 and alpha-3 were lightly expressed in MMA branches and nerve fibers, but not at capillaries (Figure 10). Alpha-3 expression was confirmed in fluorescence labeling at trigeminal nerve fibers, but not at capillaries (Figure 2).
Figure 9.
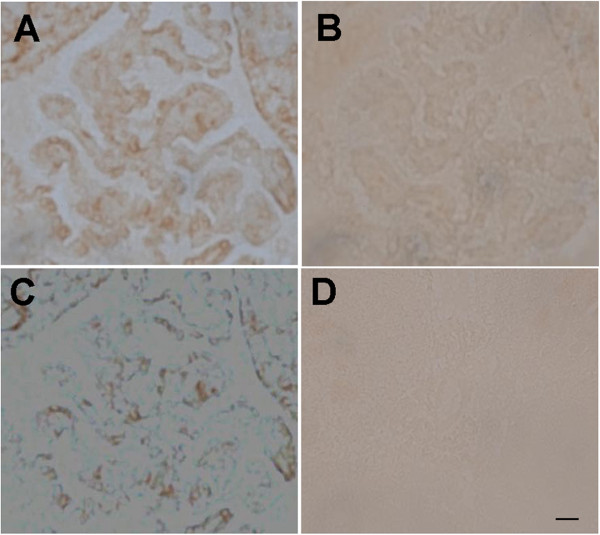
Specificity of Na,K-ATPase alpha-2 immunoreactivity at the choroid plexus. The CP was labeled with anti-alpha-2 (A) or with peptide pre-absorbed anti-alpha-2 (B). The subtraction image is in C. D: no primary control. Scale bar = 20 μm.
Figure 10.
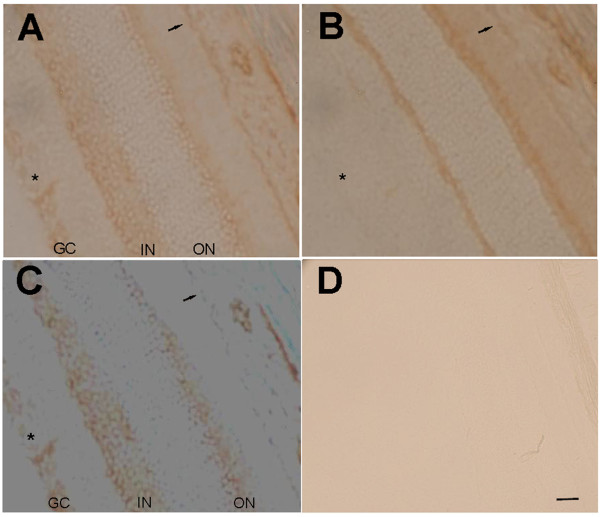
Specificity of Na,K-ATPase alpha-2 immunoreactivity at the retina. The retina was stained with anti-alpha-2 (A) or with pre-absorbed anti-alpha-2 (B). Subtraction image is shown in C. D: no primary negative control. GC: ganglion cell layer; IN: inner nuclear layer; ON: outer nuclear layer; asterisk: ganglion cell layer; small arrow: RPE. Scale bar = 20 μm.
Choroid plexus
Na,K-ATPase alpha-1 was intensely expressed along the apical membrane of the choroidal epithelial cells, while the alpha-2 was moderately expressed in epithelial cell cytoplasm. The cellular locations of these isoforms are distinct on the z-stacked images (Figure 3). Na,K-ATPase alpha-2 labeling in the CP was abrogated with peptide pre-absorption (Figure 7), confirming the specificity of the cytoplasmic alpha-2 immunoreactivity. Na,K-ATPase alpha-2 was also expressed in ventricular ependymal cells and in the capillaries of the CP (Figure 7 and data not shown). Na,K-ATPase alpha-3 was not expressed in the choroid epithelium or in its blood supply (data not shown).
Ciliary body
The Na,K-ATPase alpha-1 isoform is expressed on the basolateral surface of the pigmented epithelium (PE), while alpha-2 is located densely in the basolateral membrane of the NPE on the side facing the aqueous humor. Furthermore, the alpha-2 co-localized with vWF, the endothelial cell marker, in the ciliary body capillaries. Alpha-3 was not expressed in the ciliary body epithelium or in the capillaries (Figure 4).
Retina
Na,K-ATPase alpha-1 and alpha-2 were expressed in the RPE, but only the alpha-2 isoform co-localized with vWF on the blood vessel endothelial cells around the retinal ganglion cells (Figure 5). The alpha-3 isoform did not co-localize with any blood vessels in the retina. After peptide pre-absorption, alpha-2 expression in the ganglion cell layer and RPE disappeared, indicating specificity of alpha-2 immunoreactivity at these locations (Figure 8).
Discussion
We report that the ouabain-sensitive Na,K-ATPase alpha-2 isoform is located at CP, meningeal, retinal, and ciliary body barriers, either by itself or with alpha-1, and the alpha-2 is the consistent capillary endothelial cell isoform at these blood-barrier locations. The endothelial cell does not always choose the Na,K-ATPase alpha-2 in the nervous system; for instance, the alpha-1 is the endothelial isoform in the labyrinthine barrier [26]. It is plausible that the specific Na,K-ATPase isoforms at these locations are important in sodium flux, and thus contribute to the sodium disturbance of migraine. One distinction for Na,K-ATPase regulation conferred by alpha chain isoforms is that alpha-1 is relatively insensitive to ouabain (Kd around 30 μM), whereas alpha-2 and −3 are sensitive with Kd around 15 – 41 nM [27], close to the concentration of circulating endogenous ouabain-like compounds [28]. In humans, the Na,K-ATPase Kd for ouabain appears similar for the three alpha isoforms in studies thus far, while varying considerably between different cardiac glycoside compounds [29].
Meninges
The meningeal barrier is created at the tight junctions between the meningeal vascular endothelial cells, separating capillary blood from CSF, and at the trigeminal nerve barrier. The faint presence of Na,K-ATPase alpha-1 and −3 isoforms in meningeal sensory nerve fibers and the intense alpha-2 isoform expression in both meningeal nerve fibers and capillaries indicate an important neurovascular role for Na,K-ATPase alpha-2 in regulating sodium at the trigeminal nerves. These results provide the first evidence that both occludin and alpha-2 Na,K-ATPase are expressed in the rat meninges, both in blood vessels and around CGRP-positive, nociceptive nerve fibers. Occludin expression in the nerve fiber is most likely in the endoneurial capillary endothelial cells and perineurium as previously reported in peripheral nerves [16]. Precise localization of Na,K-ATPase alpha-2 and occludin within the meningeal nerve fiber compartments will require further study.
Our studies offer anatomical support for Na,K-ATPase alpha-2 as a modulator of intra-meningeal sodium at the blood-CSF-trigeminal barrier and, to a lesser extent, for alpha-1 and −3 at the trigeminal nerve fibers.
Choroid plexus
Consistent with previous studies in the CP [10,11], we found the alpha-1 subunit is intensely expressed in the apical membrane of the epithelial cells. The alpha-1 isoform has substantially lower affinity (Kd in the μM range) for the specific Na,K-ATPase inhibitor, ouabain, than is required to inhibit the alpha-2 and −3 Na,K-ATPase (Kd in the nM range), consistent with the demonstration that 1 mM ouabain is required to retain sodium in the CP [30]. Using both immunocytochemistry and in situ hybridization, CP apical membrane Na,K-ATPase alpha1 and AQP1 were reported to decrease with age, thus decreasing CSF secretion in aged rats [11]. Zlokovic and colleagues reported alpha-1, beta-1, and beta-2 in the rat CP, but not alpha-2 [31]. Multiple lines of evidence, therefore, indicate that the apical epithelium Na,K-ATPase alpha-1 is critical for CSF and sodium regulation at the choroidal blood-CSF barrier.
The CP barrier, however, may have more complex regulation of sodium homeostasis, as we found moderate cytoplasmic Na,K-ATPase alpha-2 expression at choroidal epithelial cells. Since our CP alpha-2 immunoreactivity is not consistent with the previous study [31], we pre-absorbed alpha-2 binding sites with excess peptide, and the results confirm cytoplasmic alpha-2 expression (Figure 7). Furthermore, we defined the cellular localization of the two alpha isoforms with 2-photon microscopy (Figure 3). The difference between our results and previous reports might arise from different methods: tissue preparation — previous authors used fixed tissues, while we stained fresh slices of retina; antibodies — previous authors used polyclonal antibodies derived from purified rat brainstem Na,K-ATPase preparations (no longer available), while we used synthetic peptide-derived commercial antibodies that are not as specific immunohistochemically for retinal neurons, but are specific for capillaries; sensitivity — some authors used Western blotting while we used fluorescence immunostaining.
CSF production and Na,K-ATPase in the CP are regulated at many levels. Studies have demonstrated that 5-HT or noradrenaline, known migraine triggers, can reduce CSF production, an activity that was synergistically inhibited by 5-HT and isoproterenol through PKC, or inhibited through PKA [32]. Cholinergic agents via the NO/cGMP pathway have been shown to inhibit CP Na,K-ATPase activity in bovine studies [33]. Thus, modulation of Na,K-ATPase activity is important for CP functions, and includes pathways known to be involved in migraine.
The overall regulation by alpha, beta, or gamma subunits of the CP Na,K-ATPase at the blood-CSF barrier remains to be elucidated, but we concur with previous studies that the Na,K-ATPase alpha-1 is the primary regulator of CSF sodium at the CP epithelium, while the role for alpha-2 in the CP epithelial cytoplasm merits further study.
Ciliary body
Na,K-ATPase in the ciliary body is modulated by various factors that affect intraocular pressure and aqueous fluid [34-36]. Our finding that Na,K-ATPase alpha-2 is expressed in the endothelial cells of the fenestrated capillaries, and very densely in the NPE, while alpha-2 is expressed in the PE, is consistent with previous work [21]. Earlier work suggested that the Na,K-ATPase alpha-1 in PE might control overall sodium secretion, and alpha-2 in the NPE may be more responsive to environmental factors [37].
Retina
Our vWF expression data is consistent with the previously described distribution of intraretinal blood vessels [38]. The Na,K-ATPase alpha-1, -3 expression we find in the retina is also consistent with previous reports [23]. The Na,K-ATPase alpha-2 expression (Figure 8), however, does not match the immunoreactivity shown in the retinal layers in these earlier studies (with a different antibody). Nevertheless, peptide pre-absorption demonstrates alpha-2 specific expression strongly in the ganglion cell layer and modestly at the RPE. The Na,K-ATPase expression in the rat RPE is also consistent with previous light and electron microscopy, and cell culture studies [39,40], however, we are cautious about interpreting our modest RPE specific immunoreactivity: previous immunoblotting demonstrated alpha-1, beta-1, and beta-2 Na,K-ATPase subunits from human RPE cells [41], but these authors found no alpha-2 or −3 RNA transcripts in the human.
Dysregulation of retinal Na,K-ATPase leads to many pathophysiologies [42,43]. Our findings suggest that the Na,K-ATPase alpha-2 isoform in the blood-retinal barrier at the retinal ganglion cell layer may play a critical role in maintaining sodium homeostasis in the retina.
Conclusions
1. Our study provides anatomical evidence of Na,K-ATPase, mainly the alpha-2 isoform, at the meningeal trigeminal nerve fibers and capillaries, and at the retinal ganglion cell layer.
2. At the CP blood-CSF and ciliary body blood-aqueous barriers, the alpha-1 is the dominant Na,K-ATPase, though alpha-2 is also present.
Abbreviations
CGRP: Calcitonin gene-related peptide; CSF: Cerebrospinal fluid; CP: Choroid plexus; DAB: Diaminobenzidine tetrahydrochloride; NPE: Non-pigmented epithelium; Oatp2: Organic anion transporting polypeptide 2; PE: Pigmented epithelium; RPE: Retinal pigment epithelium; vWF: Von willebrand factor
Competing interests
The authors declare that they have no competing interests.
Author contributions
XA was involved in study design, data acquisition, data analysis, and helped to draft and critically review the manuscript. PM was involved in data acquisition and data analysis. MT, JC, and LK participated in data acquisition. AF participated in study design and critically reviewed the manuscript. BA participated in data acquisition. DL helped in dura preparation and immunostaining, and critically reviewed the manuscript. MH designed the study, was involved in data acquisition, data analysis, and wrote the manuscript. All authors helped review the manuscript, and approved the final version of the manuscript.
Contributor Information
Xianghong Arakaki, Email: xianghong@hmri.org.
Paige McCleary, Email: pmccleary12@students.claremontmckenna.edu.
Matthew Techy, Email: mktechy@wustl.edu.
Jiarong Chiang, Email: abby.chiang@hmri.org.
Linus Kuo, Email: kuolinus@gmail.com.
Alfred N Fonteh, Email: afonteh@hmri.org.
Brian Armstrong, Email: BArmstrong@coh.org.
Dan Levy, Email: dlevy1@bidmc.harvard.edu.
Michael G Harrington, Email: mghworks@hmri.org.
Acknowledgments
Funding for this project was received from NIH NS072497, HMRI, and the Altadena Women’s Guild. We acknowledge help from manuscript reviewers.
References
- Hodgkin AL. The Ionic Basis of Nervous Conduction. Science. 1964;145:1148–1154. doi: 10.1126/science.145.3637.1148. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MG, Fonteh AN, Cowan RP, Perrine K, Pogoda JM, Biringer RG, Huhmer AF. Cerebrospinal fluid sodium increases in migraine. Headache. 2006;46(7):1128–1135. doi: 10.1111/j.1526-4610.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Harrington MG, Chekmenev EY, Schepkin V, Fonteh AN, Arakaki X. Sodium MRI in a rat migraine model and a NEURON simulation study support a role for sodium in migraine. Cephalalgia. 2011;31(12):1254–1265. doi: 10.1177/0333102411408360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki X, Foster H, Su L, Do H, Wain AJ, Fonteh AN, Zhou F, Harrington MG. Extracellular sodium modulates the excitability of cultured hippocampal pyramidal cells. Brain Res. 2011;1401:85–94. doi: 10.1016/j.brainres.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MG, Fonteh AN, Arakaki X, Cowan RP, Ecke LE, Foster H, Huhmer AF, Biringer RG. Capillary endothelial Na(+), K(+), ATPase transporter homeostasis and a new theory for migraine pathophysiology. Headache. 2010;50(3):459–478. doi: 10.1111/j.1526-4610.2009.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS, Feder N, Brightman MW. Electron microscopic study of the blood–brain and blood-cerebrospinal fluid barriers with microperoxidase. J Neuropathol Exp Neurol. 1971;30(1):137–138. [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschenko MS, Donnet C, Wetzel RK, Asinovski NK, Jones LR, Sweadner KJ. Phospholemman, a single-span membrane protein, is an accessory protein of Na, K-ATPase in cerebellum and choroid plexus. J Neurosci. 2003;23(6):2161–2169. doi: 10.1523/JNEUROSCI.23-06-02161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J. Aging affects choroidal proteins involved in CSF production in Sprague–Dawley rats. Neurobiol Aging. 2005;26(6):917–927. doi: 10.1016/j.neurobiolaging.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Keep RF, Xiang J, Ulanski LJ, Brosius FC, Betz AL. Choroid plexus ion transporter expression and cerebrospinal fluid secretion. Acta Neurochir Suppl. 1997;70:279–281. doi: 10.1007/978-3-7091-6837-0_86. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Park HJ, Lee S, Kim YH, Choi I. The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res. 2011;1377:13–20. doi: 10.1016/j.brainres.2010.12.062. [DOI] [PubMed] [Google Scholar]
- Praetorius J, Nielsen S. Distribution of sodium transporters and aquaporin-1 in the human choroid plexus. Am J Physiol Cell Physiol. 2006;291(1):C59–C67. doi: 10.1152/ajpcell.00433.2005. [DOI] [PubMed] [Google Scholar]
- Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975;164(2):127–169. doi: 10.1002/cne.901640202. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–173. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
- Dunn JS, Wyburn GM. The anatomy of the blood brain barrier: a review. Scott Med J. 1972;17(1):21–36. doi: 10.1177/003693307201700106. [DOI] [PubMed] [Google Scholar]
- Vinores SA, McGehee R, Lee A, Gadegbeku C, Campochiaro PA. Ultrastructural localization of blood-retinal barrier breakdown in diabetic and galactosemic rats. J Histochem Cytochem. 1990;38(9):1341–1352. doi: 10.1177/38.9.2117624. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T. In: Blood–brain Interfaces: From Ontogeny to Artificial Barriers. Dermietzel DCS R, Nedergaard M, editor. Weinham: 2006 WILEY-VCH Verlag GmbH & Co; 2006. The blood retinal interface: similarities and contrasts with the blood–brain interface; pp. 701–724. [Google Scholar]
- Noske W, Stamm CC, Hirsch M. Tight junctions of the human ciliary epithelium: regional morphology and implications on transepithelial resistance. Exp Eye Res. 1994;59(2):141–149. doi: 10.1006/exer.1994.1092. [DOI] [PubMed] [Google Scholar]
- Wetzel RK, Sweadner KJ. Immunocytochemical localization of NaK-ATPase isoforms in the rat and mouse ocular ciliary epithelium. Invest Ophthalmol Vis Sci. 2001;42(3):763–769. [PubMed] [Google Scholar]
- Paxinos G. WC: The rat brain in stereotaxic coordinates. 6. London, UK: Academic Press; 2007. [Google Scholar]
- McGrail KM, Sweadner KJ. Complex Expression Patterns for Na+, K + −ATPase Isoforms in Retina and Optic Nerve. Eur J Neurosci. 1990;2(2):170–176. doi: 10.1111/j.1460-9568.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Weissner W, Williams M, Ali S, Levy D. Axon diameters and intradural trajectories of the dural innervation in the rat. J Comp Neurol. 2004;473(3):364–376. doi: 10.1002/cne.20106. [DOI] [PubMed] [Google Scholar]
- Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the Trigeminal System: Mechanisms and Role in Headache Generation. Headache. 2012;52(9):1411–1427. doi: 10.1111/j.1526-4610.2012.02212.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K + −ATPase alpha1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS One. 2011;6(1):e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi PA, Sweadner KJ. Postnatal changes in Na, K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J Biol Chem. 1991;266(14):9327–9331. [PubMed] [Google Scholar]
- Jacobs BE, Liu Y, Pulina MV, Golovina VA, Hamlyn JM. Normal pregnancy: mechanisms underlying the paradox of a ouabain-resistant state with elevated endogenous ouabain, suppressed arterial sodium calcium exchange, and low blood pressure. Am J Physiol Heart Circ Physiol. 2012;302(6):H1317–H1329. doi: 10.1152/ajpheart.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck C, Potter T, Bartz M, Wittwer T, Wahlers T, Mehlhorn U, Scheiner-Bobis G, McDonough AA, Bloch W, Schwinger RH. Isoform specificity of cardiac glycosides binding to human Na+, K + −ATPase alpha1beta1, alpha2beta1 and alpha3beta1. Eur J Pharmacol. 2009;622(1–3):7–14. doi: 10.1016/j.ejphar.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MS, Reza E, Wang H, Leenen FH. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertension. 2009;54(4):860–867. doi: 10.1161/HYPERTENSIONAHA.108.125807. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Mackic JB, Wang L, McComb JG, McDonough A. Differential expression of Na, K-ATPase alpha and beta subunit isoforms at the blood–brain barrier and the choroid plexus. J Biol Chem. 1993;268(11):8019–8025. [PubMed] [Google Scholar]
- Fisone G, Snyder GL, Aperia A, Greengard P. Na+, K(+)-ATPase phosphorylation in the choroid plexus: synergistic regulation by serotonin/protein kinase C and isoproterenol/cAMP-PK/PP-1 pathways. Mol Med. 1998;4(4):258–265. [PMC free article] [PubMed] [Google Scholar]
- Ellis DZ, Nathanson JA, Sweadner KJ. Carbachol inhibits Na(+)-K(+)-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am J Physiol Cell Physiol. 2000;279(6):C1685–C1693. doi: 10.1152/ajpcell.2000.279.6.C1685. [DOI] [PubMed] [Google Scholar]
- Ellis DZ, Nathanson JA, Rabe J, Sweadner KJ. Carbachol and nitric oxide inhibition of Na, K-ATPase activity in bovine ciliary processes. Invest Ophthalmol Vis Sci. 2001;42(11):2625–2631. [PubMed] [Google Scholar]
- Hou Y, Delamere NA. Influence of ANG II on cytoplasmic sodium in cultured rabbit nonpigmented ciliary epithelium. Am J Physiol Cell Physiol. 2002;283(2):C552–C559. doi: 10.1152/ajpcell.00459.2001. [DOI] [PubMed] [Google Scholar]
- Yadav UC, Srivastava SK, Ramana KV. Inhibition of aldose reductase attenuates endotoxin signals in human non-pigmented ciliary epithelial cells. Exp Eye Res. 2010;90(5):555–563. doi: 10.1016/j.exer.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hernando N, Martin-Alonso JM, Martin-Vasallo P, Coca-Prados M. Expression of multiple Na+, K(+)-ATPase genes reveals a gradient of isoforms along the nonpigmented ciliary epithelium: functional implications in aqueous humor secretion. J Cell Physiol. 1991;149(2):184–194. doi: 10.1002/jcp.1041490203. [DOI] [PubMed] [Google Scholar]
- Archer DB. Intraretinal, preretinal, and subretinal new vessels. Trans Ophthalmol Soc U K. 1977;97(4):449–456. [PubMed] [Google Scholar]
- Marrs JA, Andersson-Fisone C, Jeong MC, Cohen-Gould L, Zurzolo C, Nabi IR, Rodriguez-Boulan E, Nelson WJ. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J Cell Biol. 1995;129(2):507–519. doi: 10.1083/jcb.129.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okami T, Yamamoto A, Omori K, Takada T, Uyama M, Tashiro Y. Immunocytochemical localization of Na+, K(+)-ATPase in rat retinal pigment epithelial cells. J Histochem Cytochem. 1990;38(9):1267–1275. doi: 10.1177/38.9.2167328. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Bhat SP, Bok D. Expression and synthesis of the Na, K-ATPase beta 2 subunit in human retinal pigment epithelium. Gene. 1996;176(1–2):237–242. doi: 10.1016/0378-1119(96)00258-2. [DOI] [PubMed] [Google Scholar]
- Catanzaro O, Labal E, Andornino A, Capponi JA, Di Martino I, Sirois P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides. 2012;34(2):349–352. doi: 10.1016/j.peptides.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Di Leo MA, Santini SA, Cercone S, Lepore D, Gentiloni Silveri N, Caputo S, Greco AV, Giardina B, Franconi F, Ghirlanda G. Chronic taurine supplementation ameliorates oxidative stress and Na + K + ATPase impairment in the retina of diabetic rats. Amino Acids. 2002;23(4):401–406. doi: 10.1007/s00726-002-0202-2. [DOI] [PubMed] [Google Scholar]
- McGrail KM, Sweadner KJ. Immunofluorescent localization of two different Na, K-ATPases in the rat retina and in identified dissociated retinal cells. J Neurosci. 1986;6(5):1272–1283. doi: 10.1523/JNEUROSCI.06-05-01272.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]