Abstract
The emergence of lithic technology by ∼2.6 million years ago (Ma) is often interpreted as a correlate of increasingly recurrent hominin acquisition and consumption of animal remains. Associated faunal evidence, however, is poorly preserved prior to ∼1.8 Ma, limiting our understanding of early archaeological (Oldowan) hominin carnivory. Here, we detail three large well-preserved zooarchaeological assemblages from Kanjera South, Kenya. The assemblages date to ∼2.0 Ma, pre-dating all previously published archaeofaunas of appreciable size. At Kanjera, there is clear evidence that Oldowan hominins acquired and processed numerous, relatively complete, small ungulate carcasses. Moreover, they had at least occasional access to the fleshed remains of larger, wildebeest-sized animals. The overall record of hominin activities is consistent through the stratified sequence – spanning hundreds to thousands of years – and provides the earliest archaeological evidence of sustained hominin involvement with fleshed animal remains (i.e., persistent carnivory), a foraging adaptation central to many models of hominin evolution.
Introduction
Unique among extant primates, modern humans are anatomically adapted to regularly consume substantial amounts of vertebrate animal tissues (meat, organs, etc.). Over the last several million years, the hominin gastrointestinal tract has evolved from a chimpanzee-like large-intestine-dominated configuration well adapted for digesting fruits and other plant parts (as well as the occasional small mammal) to a more carnivore-like small-intestine-dominated form well suited for extracting complex nutrients from animal remains [1], [2]. Increased consumption of animal tissues likely fueled brain expansion in the genus Homo [1], [3]–[5], and may have helped to facilitate initial hominin dispersals out of Africa (ca. 1.8 Ma) [3], [6]–[7].
Despite its central role in many models of hominin evolution [1]–[8], however, relatively little is known about the timing and nature of the emergence of persistent hominin carnivory. From an archaeological perspective, the appearance of flaked lithic technology around ∼2.6 Ma is often though to reflect increased levels of hominin interest in, and involvement with, animal remains [8]–[12]. Associated faunal evidence, though, is largely unknown prior to 1.8 Ma, limiting opportunities to test these and related hypotheses using the zooarchaeological record.
In addition, past efforts to integrate archaeologically-derived inferences of Oldowan hominin diet with broader issues of theory have often been hindered by a range of analytical and interpretive challenges. Setting aside the overall rarity of assemblages for a moment, three of these constraints are particularly noteworthy. First, the earliest archaeofaunal assemblages are generally associated with small analytical datasets; with most collections having relatively few specimens, poor bone surface preservation, or both [10]–[15]. The earliest sites (ca. 3.4–2.3 Ma), those from Ethiopia (Dikika, Gona, Bouri, and Hadar), and Kenya (Lokalalei), for instance, each preserve evidence of one or more hominin butchery acts within analytically-small faunal collections [10–15, but see 16]. These records demonstrate that Oldowan hominins acquired and consumed animal tissues, at least on occasion. Yet given the limited amount of behavioral information potentially recorded in any small assemblage, it is uncertain if these records reflect initial hominin forays into carnivory – i.e., the occasional meal – or something substantively more typical and adaptively important [17], [18].
Second, the earliest assemblages are, by-and-large, isolated points in time and space. This is potentially problematic on two fronts. When considering the record on a site-by-site basis, each of these assemblages formed in less than 101–3 years [10]–[15], [19]. Foraging activities documented at this time-scale, though, while certainly reflecting past hominin behaviors, need not reflect evolved patterns of behavior (i.e. adaptations). As a result, we are often left with open questions of ‘forays’ versus ‘fundamental shifts in foraging activities’ when trying to interpret the record. Moreover, these assemblages are rarely recovered in clear stratigraphic succession [10]–[15]. As a result, demonstrating any continuity in hominin behavior through time – especially relative to a single ecological context (the appropriate frame of reference) – is often fraught with considerable analytical and interpretive difficulties.
Lastly, behavioral inferences derived from the archaeofaunal record can often be equivocal, even in cases where there is an abundance of well-preserved remains. Numerous studies of FLK 22 Zinj (1.84 Ma; Olduvai Gorge, Tanzania), for instance, have returned diverse often mutually-exclusive interpretations of Oldowan hominin diet and behavior [20]–[42]. This disjunction is potentially attributable to conceptual and methodological differences among analysts [43], but may also reflect the difficult and often subjective task of teasing apart the behavioral roles and material contributions (if any) of both hominins and carnivores in site formation activities [39], [43], [44].
Given these limitations, how might researchers differentiate between ‘initial forays into the carnivorous realm’ [2], [3], [11], [13] and ‘persistent carnivory’ when studying the earliest archaeofaunal record? To do so requires a stratified series of relatively large assemblages, each with clear evidence of sustained and abundant hominin involvement with fleshed animal remains, with each assemblage sampling a relatively discrete period of time (i.e., sampling behavior at an ecological-scale), and with the sum of assemblages providing an unambiguous record of sustained carnivorous behavior persisting at geological/evolutionary time scales (i.e., at 103+ years). At present, the earliest archaeological evidence of this adaptation is found either in the stratified assemblages of Bed I, Olduvai Gorge, Tanzania (1.86–1.75 Ma) [21,25,33,35; though see 20], or, somewhat more conservatively, in the Okote Member, Koobi Fora, Kenya (∼1.5 Ma) [44].The relatively recent dates of these assemblages, though, pose a challenge for models of hominin paleobiology that posit an earlier appearance for this adaptation – particularly so for models that associate increased carnivory with the emergence and early evolutionary history of the genus Homo up to 1 million years earlier [e.g., 1–3,5–7; though see 46–48]. Recent zooarchaeological discoveries at Kanjera South, Kenya provide new data relevant to this debate. Here we report on three large, well-preserved, stratified archaeofaunal assemblages that date to ∼2.0 Ma and collectively provide the earliest material evidence of persistent hominin carnivory.
Materials and Methods
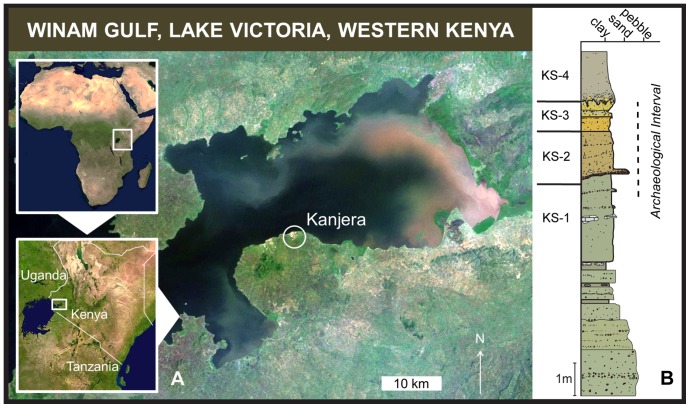
Kanjera South (KJS) is located on the southern shores of the Winam Gulf of Lake Victoria, southwestern Kenya (0°20′24″S, 34°32′16″E) (Figure 1). A relatively small (∼0.5 km2) amphitheater of stratified fluvial-lacustrine sediments, this locality has yielded in situ archaeological materials from each of its three lowermost beds (KS-1 though KS-3) [49]–[54]. The presence of the proboscidean Deinotherium sp. and the suid Metridiochoerus andrewsi provides a minimum age of 1.7 Ma for the sediments; and the equid Equus provides a maximum age of 2.3 Ma. The presence of the Olduvai subchron (1.77–1.95 Ma) in the uppermost bed, KS-5, further constrains the archaeological levels to ∼2.0 Ma [50].
Figure 1. Location of Kanjera along the modern shoreline of Lake Victoria, East Africa.
(A) Kanjera lies to the immediate northeast of Homa Mountain, a volcanic complex active from the middle Miocene to the Pleistocene. The Winam Gulf fills the western end of the Nyanza Rift, an E-W graben with origins in the early Miocene. (B) Beds KS-1 through KS-3 of the Kanjera Formation (Southern Member) sample floodplain and low-aspect channel contexts originally deposited between the mountain and the nearby shores of a shallow lake [49]. Satellite imagery from USGS and NASA.
Three excavations set along a ∼50 m transect of outcrop have recovered several thousand well-preserved, spatially-associated, faunal and lithic artifact specimens (Table 1) [49]–[55]. When limiting ourselves to the archaeological interval (total depth ∼3.1 m), Excavation 1 (169 m2) samples beds KS-1 through KS-3; Excavation 2 (15 m2) and Excavation 5 (4 m2) sample KS-3. Clear lithostratigraphic correlations among excavations, short distances between excavations, and an absence of purposeful spatial organization of materials within beds or excavations allows faunal materials from KS-3 to be collectively considered a single assemblage [17].
Table 1. Faunal and lithic inventory.
| Bed | Total NISP | Macro-mammal NISP | Macro-mam. MNI | Principal fauna (% NISP, % MNI) | Lithics |
| KS-1 | 982 | 975 (525) | 25 | Bovid (92.4, 72.0), Equid (4.4, 8.0), Suid (1.5, 8.0), Hippo (0.2, 4.0) | 179 |
| KS-2 | 2190 | 2153 (886) | 40 | Bovid (82.6, 67.5), Equid (11.6, 10.0), Suid (0.9, 5.0), Hippo(1.0, 2.5) | 2533 |
| KS-3 | 491 | 470 (172) | 16 | Bovid (77.9, 68.8), Equid (4.7, 6.3), Suid (0.6, 6.3), Hippo (14.0,12.5) | 171 |
NISP (number of identified specimens) and MNI (minimum number of individuals) are defined and quantified following the literature [43]. ‘Total NISP’ reflects the sum of specimens recovered with coordinate data and included in this study. Tens of thousands of non-identifiable bone and tooth fragments <2 cm are omitted from this study. Fossils from conglomeratic facies (CP levels) are poorly preserved [49], and are likewise excluded from this study: KS-2CP (n = 259), KS-3CP (n = 102). Macro-mammals are defined here as weighing >5 kg. Macro-mammal NISP values are total sums and, in parentheses, the sum of specimens identified beyond Linnean class. %NISP and %MNI include macro-mammals only. Faunal and lithic counts are from the literature [17], [55].
Fossils and artifacts were recovered in situ by experienced excavators using lightweight hammers and awls [49]–[51]. Sediments were excavated in 1 m×1 m squares and 5 cm levels, with levels following natural stratigraphic units whenever possible. Recovered materials were individually numbered, piece-plotted using a Topcon total station, lifted, and individually bagged. Excavated sediments were further sieved through 1 mm mesh, resulting in the recovery of additional specimens.
Paleoenvironmental analyses indicate that the assemblages formed on a grassy plain set between a freshwater lake and the wooded slopes of nearby hills and mountains. The recovered faunas consist primarily of grassland-adapted bovids (Parmularius, Antidorcas), equids (Equus), and suids (Metridiochoerus), with water-dependent taxa (e.g., Hippopotamus, Crocodylus, and reduncine bovids) also present in limited numbers. Isotopic analyses of dental enamel and pedogenic carbonates concordantly indicate a grassland setting at KJS [49]–[52].
Site formation studies indicate that the assemblages were neither formed nor significantly winnowed by water flow [17], [49]. Fossils and artifacts are outsized clasts relative to the surrounding clays, silts, and fine-to-medium sands; and the overall abundance and taxonomic diversity of faunal remains far surpass natural landscape accumulation norms [49], [50]. When coupled with the results of previous taphonomic analyses [17], [49], biological agents of site formation are indicated for each of the assemblages. The vertical distribution of materials, deposit depths, and estimated rates of sedimentation and pedogenesis suggest that faunal and artifactual materials accumulated relatively rapidly over a period of decades to hundreds of years per bed. As the stratified assemblages formed both rapidly and recurrently [50], the record from KJS provides a rare opportunity to explore ancient foraging behaviors with regard to both shorter-term ecological and longer-term evolutionary dynamics.
We report here on the zooarchaeological record of bovid remains. These dominate the assemblages in terms of overall abundances (representing a minimum of 56 individuals), and are amenable to analysis using published protocols and experimental datasets [21]–[30], [56]–[63]. Analytically, we group remains by bed (e.g., ‘KS-1’, ‘KS-3’) rather than by excavation [49]. We further sort specimens by body size class [21], grouping animals into ‘small’ (e.g., Grant’s gazelle, Gazella granti) and ‘medium’ (e.g., Topi, Damaliscus lunatus) sizes. Extinct bovids of intermediate size and weight (e.g., Parmularius sp.) are treated as medium-size animals. Larger bovids (e.g., buffalo, Syncerus caffer) are poorly represented in the assemblages and are not treated in detail here. Following convention, we incorporate taxonomically-unidentifiable long bone fragments in all appropriate analyses.
In our study of bone surface modifications, three investigators (JVF, BLP, and JSO) jointly analyzed specimens, shared observations, and discussed interpretations before providing individual assessments of bone damage [17]. Analysts employed low–power magnification (10×-40×) and strong light sources to identify modifications. They attributed agency (e.g., hominin, carnivore) to modifications only after excluding all possible alternatives (including potential confounds detailed in [32], [64]–[69]).
Values for minimum numbers of skeletal elements (MNE) reflect considerations of animal size and developmental age, extensive refitting efforts, and, for long bones, element identification of shaft portions [21]. High-survival elements (HSE) include the cranium, mandible, humerus, radius, metacarpal, femur, tibia, and metatarsal [61]. Point estimates of Shannon evenness follow published methods [30], [70], whereas interval estimates are constructed using Bayesian models [71].
Results and Discussion
Bone surface modification frequencies are known to accurately reflect the timing and context of both hominin and carnivore involvement with animal remains [22–25,56–58; though also see discussions in 16,26]. We use them here to assess the identity and sequence of actors and behaviors responsible for forming and modifying the assemblages.
Hominin-modified specimens (i.e., fossil bones bearing cut marks and/or hammerstone percussion damage) are present through the entire KS-1 through KS-3 sequence (Table 2 and Table S1). These specimens provide unambiguous evidence of hominin processing of bovid remains (Figure 2), and indicate a functional relationship between artifactual and faunal materials. When considering the anatomical placement of cut marks, we report bone damage consistent with both defleshing and disarticulation activities [17]. Frequencies of cut-marked limb specimens range from 1.9% to 6.3% in summed (i.e., total bed) assemblages, with similar frequencies observed irrespective of analyst, bed, or animal body size. The overall uniformity of these results suggests a relatively consistent pattern of carcass exploitation through time (within-analyst test for the homogeneity of cut mark frequencies across beds: homogeneity not falsified, all p-values >0.1).
Table 2. Bone surface modification frequencies.
| Epiphyseal Fragments (EPI) | Mid-Shaft Fragments (MSH) | |||||||
| Bed | TM % | CM % | PM % | N | TM % | CM % | PM % | N |
| KS-1 | 18, 24, 18 | 3, 3, 3 | 0, 0, 0 | 34 | 8, 10, 8 | 2, 4, 4 | 9, 9, 10 | 96 |
| KS-2 | 13, 17, 11 | 6, 8, 11 | 3, 8, 8 | 64 | 9, 16, 12 | 1, 3, 3 | 4, 5, 8 | 207 |
| KS-3 | 9, 9, 0 | 0, 0, 0 | 0, 0, 0 | 11 | 5, 11, 2 | 2, 0, 0 | 5, 2, 14 | 44 |
| Sum | 14, 18, 12 | 5, 6, 7 | 2, 5, 5 | 109 | 8, 14, 10 | 1, 3, 3 | 6, 6, 9 | 347 |
Modifications detailed by long bone portion [22]–[24], bed, and analyst. Epiphyseal fragments (EPI) bear at least some of the proximal or distal articular surface. Mid-shaft fragments (MSH) are diaphyseal specimens that lack cancellous bone on medullary surfaces. Bone modifications follow the literature [17 and references therein], and include tooth marks (TM: pits, scores, furrows), cut marks (CM), and percussion marks (PM: pits, striae). Bone modification frequencies are listed by analyst: Ferraro, Pobiner, and Oliver (in order from left to right). Samples are bovid and taxonomically-indeterminate long bone specimens (i.e., humerus, radius, metacarpal, femur, tibia, metatarsal, or ‘long bone shaft fragment’), ≥2 cm in length, from body sizes 1–3 (i.e., small and medium-sized) [21], with ‘very good’ to ‘excellent’ bone surface preservation (i.e., surface conditions 4–5 [17]) and without recent or geological fractures. Data for summed body sizes, including ‘size indet’.
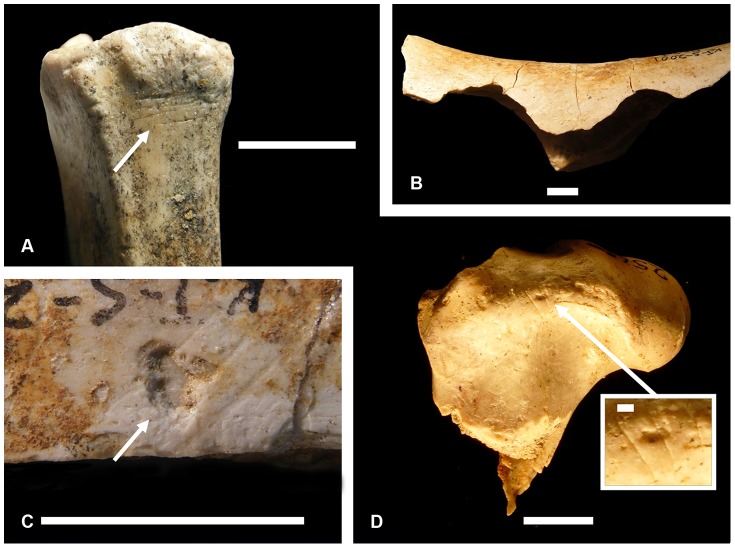
Figure 2. Bone surface modifications.
(A) KJS 7472, a small bovid metatarsal from KS-2 bearing cut marks; (B) KJS 7379, a medium-sized bovid humerus from KS3 bearing pair of hammerstone notches, the specimen is also cut-marked (not figured); (C) KJS 5447, a mammal limb bone shaft fragment from KS-2 with percussion pit and striae, the specimen is also cut-marked (not figured); (D) KJS 2565, a small bovid femur from KS-2 with numerous cut marks. Scale is 1 cm in panels (A-D); 1 mm in the panel (D) close-up. Specimen numbers are field designations, not KNM accession numbers.
In addition, numerous cut-marked rib specimens reflect recurrent hominin defleshing of axial skeletons of both small and medium-sized animals. In KS-2, 9.7% to 12.9% of smaller-bodied rib specimens (N = 31) and 5.0% to 7.5% of medium-bodied rib specimens (N = 40) bear cut marks (with ranges reflecting multiple analysts’ interpretations). This evidence clearly indicates the repeated tool-mediated removal of soft tissues. Numerous hammerstone-percussed specimens in each assemblage also indicate repeated hominin exploitation of within-bone food resources (Table 2 and Figure 2). From a comparative perspective, frequencies of hominin-induced bone surface modifications are consistent with values recorded from a number of similarly analyzed Early Pleistocene anthropogenic faunal assemblages from East Africa (e.g., BK, Olduvai Gorge; Table S2) [72]–[74].
Carnivore-damaged specimens (e.g., tooth-marked remains) reflect the actions of additional agents of assemblage modification. Looking at summed (i.e., total bed) assemblages of long bones, <25% of epiphyseal specimens and <17% of midshaft fragments bear tooth-marks (Table 2). Similar frequencies are observed irrespective of bed or animal body size, reflecting some regularity in carnivore feeding behaviors through time (within-analyst homogeneity not falsified for most pairs, all p-values >0.04). Relative to data derived from reference assemblages, tooth-mark frequencies at KJS are indistinguishable from scenarios in which carnivores had secondary access to previously defleshed and demarrowed bone refuse [23]–[26], [56]–[58] (Figure 3, Figure 4).
Figure 3. Tooth-marked mid-shaft fragments: results from experimental assemblages and excavations at KJS.
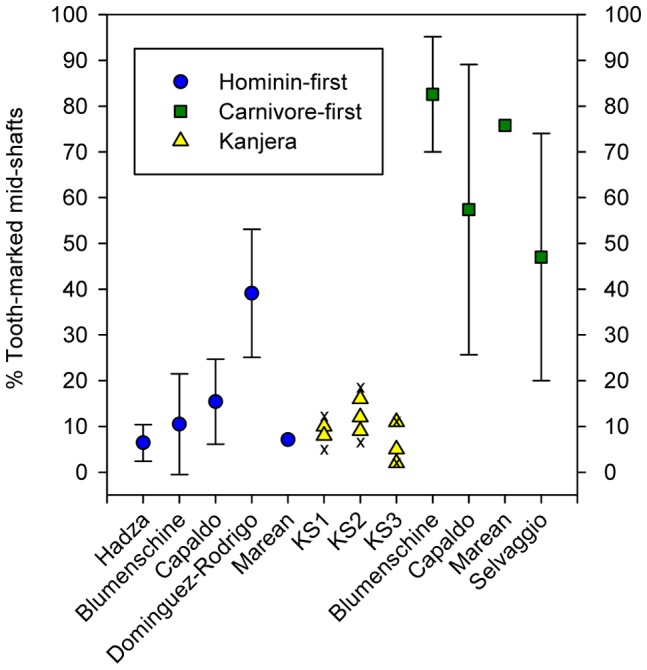
Figure follows a published model [26]. Hominin-first assemblages refer to remains initially defleshed and demarrowed by hominins, then subsequently exposed to large-bodied carnivores (primarily hyenas). Carnivore-first assemblages refer to remains initially defleshed and/or demarrowed by large-bodied carnivores (primarily hyenas and/or lions). Data for body sizes 1–4 [21]. Modern data (with single standard deviations where available) derived from the literature [23]–[26], [56]–[58]. KJS frequencies are from Table 2 and Table S1. Multiple symbols for KJS indicate the results of multiple analysts. X’s indicate minimum and maximum estimates of damage (see Table S1).
Figure 4. Tooth-mark frequencies and long bone portion representation: results from modern experiments and excavations at KJS.
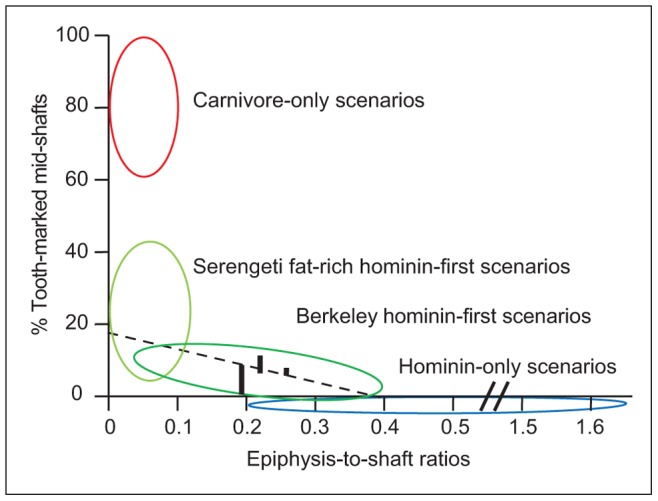
Portions defined in Table 2 and Table S1. ‘Shafts’ include both near-epiphyseal and mid-shaft specimens. Complete bones are not included in epiphysis-to-shaft calculations (number of complete bones = 2, 7, and 1; beds KS-1 through KS-3, respectively). Ellipses outline the range of results in experimental feeding scenarios involving: carnivores-only, hominins-only, or a sequence of hominins-then-carnivores (i.e., ‘hominin-first’). The dashed line is a published least-squares regression for hominin-first scenarios [22]. Hominin-only scenarios have no tooth marks, hence the placement of the ellipse beneath the x-axis. KJS data from Table 2 are for summed body sizes. KJS epiphysis-to-shaft ratios: 0.26, 0.22, and 0.19 for beds KS-1 though KS-3 respectively. KJS tooth-mark data displayed as solid vertical bars, with bars representing the range of analysts’ results. Results from Kanjera are consistent with hominin-first contexts.
Skeletal element analyses further detail the nature of hominin involvement with animal remains (Table S3). Smaller-sized bovids are relatively abundant, both with respect to counts of bony elements and numbers of individuals represented. Specimens from all skeletal regions are present in each assemblage, with high-density cranial and appendicular elements predominating (Figure 5A). Lower-density axial elements (e.g., vertebrae, ribs) are also present, though at proportionately lower frequencies. In each assemblage, skeletal element abundances are positively correlated with bone density values (rs range: 0.368 to 0.655; p-values: 0.110 to 0.002) [59], a pattern consistent with scenarios in which scavenging carnivores removed many of the lower-density remains originally present on-site [22], [25], [27], [60], [61] (Table S4). For cranial and long bone specimens (i.e., ‘high-survival elements’ [HSE]) [61], skeletal element abundances are not significantly correlated with either standardized food utility values (rs range: −0.457 to 0.145; all p-values >0.20) [62] or within-bone nutrient values (rs range: −0.618 to 0.505; all p-values >0.10) [28], [29], suggesting relatively little selectivity in hominin transport with respect to these variables (Tables S5–S7). This latter observation is consistent with Shannon evenness values of HSE’s (range: 0.924 to 0.955), which suggest that small bovid carcasses were transported and deposited as relatively complete units (Table S8) [30]. When considered in sum, the pattern of small bovid skeletal part representation is similar across assemblages, and is consistent with scenarios in which numerous relatively complete carcasses were deposited on-site by hominins [22], [27]–[30], [59]–[62]. Coupled with the results of bone surface modification studies, these data reflect an ecological context in which Oldowan hominins had sustained primary access to the meat and organ tissues of a substantial number of small bovids throughout the deposition of all three geological beds: a period spanning hundreds to thousands of years [50].
Figure 5. Skeletal element representation for (A) small and (B) medium-sized bovids, Bed KS-1.
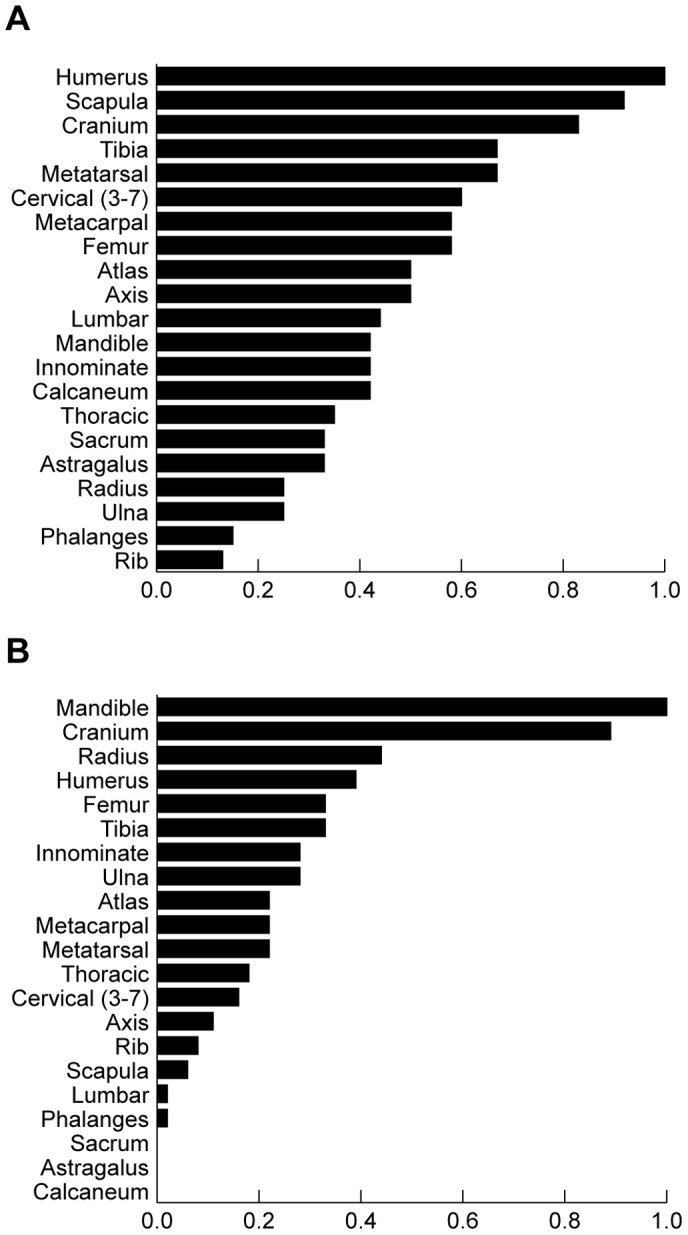
Abundance data presented as percent minimum animal units (%MAU), calculated following the literature [43]. KJS data derived from Table S3. 100% MAU = 6 for small bovids, 9 for medium-sized bovids. Similar patterns of skeletal element representation are present in Beds KS-2 and KS-3.
With respect to the timing of hominin access to these smaller-sized individuals, actualistic studies in a modern East African grassland (the Serengeti) show that small bovid carcasses are, almost without exception, completely consumed by lions and/or hyenas within the first few minutes to hours following death [63]. Given the relative abundance of small bovid carcasses at KJS (Table S3), the relative dearth of carnivore tooth marks on their remains (Table S1), and the inferred rarity of such scavenging opportunities in grassland settings, our results strongly suggest that hominins acquired many of these animals very early in their resource lives (i.e., fairly close to the moment of death). At present, the summed evidence that Oldowan foragers acquired, defleshed, and demarrowed numerous, complete, small bovids throughout the formation of all three assemblages plausibly represents the earliest archaeological record of hominin hunting activities.
The skeletal remains of medium-sized bovids reflect a slightly different taphonomic history. Although specimens from all skeletal regions are represented, cranial remains predominate (Figure 5B). Within each assemblage, skeletal element abundances are positively correlated with bone densities (rs range: 0.401 to 0.666; all p-values <0.10) [59], and HSE abundances are not significantly correlated with either standardized food utility values (rs range: −0457 to −0.241; all p-values >0.20) [62] or within-bone nutrient values (rs range: 0.107 to 0.657; all p-values >0.10) [28], [29]. When considering the sum of surface modification data, Shannon evenness values (range: 0.808 to 0.944), and theoretical considerations of transport behaviors [61], [62], the record from KJS most parsimoniously indicates that Oldowan hominins introduced the partial remains of medium-sized carcasses to the site, with specific foraging behaviors varying with respect to body region (e.g., head versus postcrania) and timing of access to carcasses [63].
The overall taphonomic history of medium-sized postcrania is thus fairly equivalent to that of the smaller-sized carcasses. In both cases, remains are present at abundances that far exceed natural landscape accumulation norms (Table 1), and bone surface modification frequencies and skeletal part analyses indicate that hominins had primary access to soft tissues (Table 2, Figure 3, Figure 4). The evidence is consistent with scenarios in which hominins introduced a relative abundance of fleshed medium-sized postcrania to the site. In contrast to the record of smaller-sized bovids, however, skeletal element representation and element evenness scores suggest an increased measure of hominin selectivity in skeletal part choice and transport decisions when dealing with medium-sized remains (Table S3, Table S8). Long bone elements are fairly numerous relative to axial remains (as measured by %MAU) (Figure 5B, Table S3); and the more proximal limb elements (i.e., humerus, radio-ulna, femur, and tibia) are relatively more abundant than metapodials (Figure 5B, Table S3). This patterning differs substantially from that of the smaller-sized bovids. The latter’s remains are more evenly-distributed across the entire postcranial skeleton (HSE’s+low survival elements [LSE’s]), as well as across the six major long bones (Figure 5A, Table S3), and presumably reflects the introduction of numerous, fairly complete small bovids to the site. At issue here: what strategies did hominins follow when selecting and transporting medium-sized remains?
The record is potentially consistent with two main scenarios. In the first, hominins introduce an abundance of compete (or relatively complete) medium-sized carcasses to the site. This follows a ‘food maximizing’ strategy in which hominins face negligible-to-minor transport constraints and transfer most or all of the edible remains from death sites to KJS [75]. As a result, they treat both small and medium-sized bovids in a relatively similar manner when making carcass transport decisions. Observed differences in skeletal element records on-site (smalls vs. mediums) would then presumably reflect systematic differences in post-depositional carnivore scavenging behaviors. In the second scenario, hominins preferentially transport limb remains from medium-sized carcasses, plus some axial elements whenever possible. This follows a ‘weight minimizing’ strategy in which transport constraints (e.g., the number of available carriers, distance to destination, predation risk, etc.) limit hominins to carrying away only a subset of all edible tissues [75]. In this case, carnivore treatment of skeletal remains on-site would be relatively consistent across size groups [25], and observed differences in the skeletal element record (small vs. medium) would instead predominantly reflect systematic size-based differences in hominin transport practices.
Here, comparisons between size groups are particularly informative. For small bovids, LSE values are not grossly disproportionate to those of HSE’s (Figure 5A, Table S3). In fact, their overall skeletal record corresponds fairly well to expectations for dual-patterned hominin-first assemblages, [22], [25], [27], [29]. Note too that skeletal remains of smaller-sized individuals are usually at far greater risk of destruction than those of medium-sized animals, especially in grassland contexts [43], [63].This makes the latter’s record at KJS all the more interesting. In each of the assemblages, medium-sized bovids are fairly depauperate in postcranial axial remains relative to both head and limb elements (Figure 5B, Table S3). As the smaller-sized bovids are more evenly represented across the skeleton (both with and without considerations of cranial remains), we discount the possibility that hominins introduced a substantial amount of medium-sized postcranial axial elements to the assemblages (or, alternatively, that those remains were somehow introduced ‘naturally’; e.g., via mass death). In short, if an abundance of medium-sized axial remains were originally present on-site in substantial numbers, and they were largely deleted by scavenging carnivores, then the overall skeletal record of smaller-sized bovids should reflect a substantially more biased record (both in terms of head remains relative to postcrania, and HSE’s relative to LSE’s). The alternative, a null hypothesis in which all bovids were originally present on-site as similarly-apportioned carcasses, would require that medium-sized postcrania (LSE’s+HSE’s) were preferentially deleted by carnivores relative to all smaller-sized remains. We argue that this is unlikely (especially for the record of HSE’s), and note that tooth-mark frequencies are relatively similar across the remains of both size groups (Table S1). In turn, we argue that the KJS record provides robust evidence that hominins largely – but certainly not exclusively – followed a ‘weight-minimizing’ strategy at KJS when selecting and transporting remains from fleshed medium-sized carcasses.
The record of medium-sized cranial elements requires a bit more explanation. Specifically, these remains are disproportionately abundant within each of the assemblages (Figure 5B, Table S3). If hominins largely followed a ‘weight-minimizing’ strategy, and solely had access to complete medium-sized carcasses, they would not have preferentially transported crania and mandibles to KJS. The reason is clear: head remains are quite heavy given their tissue yields, and will often be ignored at death sites in favor of a slew of higher-ranked remains [75]. These same arguments hold when discussing medium-sized limb HSE’s. The preponderance of head remains on-site (as well as the paucity of long bone remains) is thus unlikely to reflect either simple utility or density-related phenomena. Instead, the record strongly suggests the purposeful introduction of a fair number of isolated heads to the site by Oldowan foragers.
But why acquire, transport, and process an abundance of medium-sized heads? In living animals, these remains contain a wealth of fatty, calorie-packed, nutrient-rich tissues: a rare and valuable food resource in a grassland setting where alternate high-value foodstuffs (fruits, nuts, etc.) are often unavailable [2], [3], [29], [49], [52], [63], [76]–[78]. Medium-sized heads are also relatively dense and durable elements, and their internal contents are generally inaccessible to all but hyenas and tool-wielding hominins [63], [79], [80]. As a result, they are often seasonally-available as scavengable resources in East African grasslands [63], [76], [79]–[83]. Additionally, bone surface modification studies at KJS clearly demonstrate that hominins accessed internal head contents: several cranial vault and mandibular fragments bear evidence of percussion striae. Considered in sum, the presumed availability of these isolated remains across the landscape, the relative abundance of these remains in the KJS assemblages, and unambiguous material evidence that hominins exploited their contents on-site is most parsimoniously interpreted as reflecting very early archaeological evidence of a distinct hominin scavenging strategy – one that included a strong focus on acquiring and exploiting fatty, nutrient-rich, energy-dense within-head food resources (e.g., brain matter, mandibular nerve and marrow, etc.) [e.g., 24,63,76,82,84–86].
The total abundance of remains on site, (Table 1), the number of animals represented (Table 1), the high taxonomic diversity present [17], [50], [52], the relatively low frequency of tooth-marked specimens (Figure 3, Figure 4, Table S1), and a sedimentological record wholly inconsistent with a fluvial accumulation of remains [49], [52] also combine to suggest that the KJS assemblages are unlikely to represent in situ death or ‘background scatter’ accumulations formed by non-hominin agencies. Similarly, the skeletal element record of medium-sized bovids suggests that they were unlikely to have been present on-site as complete carcasses, an expectation of most ‘kill-site’ and/or landscape accumulation models. When limiting discussion to medium-sized postcrania, the high abundance of limb remains (including many isolated epiphyses) relative to axial elements is also the inverse expectation for landscape assemblages (Figure 5B) [63].
Finally, as with many zooarchaeological assemblages, the KJS skeletal inventories are dominated by numerous unidentifiable long bone shaft fragments. At issue: who or what created these fragments from whole bones? The relative rarity of ‘dry bone’ fractures, coupled with abundant evidence of ‘green bone’ breakage, strongly suggests the involvement of behavioral agents of modification, especially given the inferred low-energy depositional setting at KJS [17], [49]–[52]. Bone surface modifications (e.g., percussion marks and notches; tooth marks and notches) indicative of access to within-bone resources, however, are found at relatively low frequencies in each of the assemblages (Figure 3; Figure 4; Table 2; Table S1; Table S2) [17]. This result is surprising as it is inconsistent with known outcomes of both hominin and carnivore bone breakage practices, where surface modification frequencies are, on average, substantially higher [e.g., 22,23,25,57,58]. A similar pattern of an abundance of shattered but largely unmodified long bone specimens is observed in many other Paleolithic assemblages [31,45,72,73; Table S2], suggesting to us that current bone breakage models may not fully account for all relevant variables. Notably, at KJS there is no evidence that post-depositional sediment compaction and/or bone weathering influenced the bone breakage record [17]. Further experimental research may be required to fully explain these observations.
Conclusions
The zooarchaeological record from Kanjera South, Kenya provides a rare opportunity to explore early hominin diet and foraging behaviors at ∼2.0 Ma. In each of three large well-preserved faunal assemblages, there is definitive evidence that Oldowan hominins acquired, transported, and consumed the remains of numerous small bovid individuals. Surface modification studies and skeletal element analyses indicate that hominins acquired most or all of these remains relatively early in their resource lives (i.e., in a complete or relatively complete state), providing foragers with access to meat, organ, and within-bone food resources. Given their overall abundance and skeletal representation, unambiguous evidence of their butchery, and their presumed limited availability as potentially-scavengable resources in a grassland setting [17], [27], [63], [87], the small bovid remains at KJS may reflect the earliest archaeological evidence of hominin hunting activities.
The record of medium-sized bovids is slightly more complex. Within each assemblage, there is clear evidence that hominins acquired the postcranial remains of at least some medium-sized individuals relatively early in their resource lives (i.e., with at least some adhering flesh), perhaps mirroring, to some extent, the record of their involvement with smaller-sized bovids [Table S1]. The disproportionate abundance of medium-sized heads, however, likely reflects a separate but complementary foraging activity, one specifically focused on scavenging these remains for their internal food resources (e.g., brain tissues) [17], [63], [84]. This latter portion of the record may represent the earliest archaeological evidence of a distinct hominin scavenging strategy.
With regard to evolutionary ecology, the relative uniformity of hominin activities documented through the KJS sequence indicates an evolved foraging adaptation well-tuned to local ecological contexts. This point implies that hominin involvement with, and their presumed consumption of, animal remains had substantial fitness implications. In turn, sufficiently strong selective pressures are implicated as having favored the evolution of persistent hominin carnivory no later than 2.0 million years ago. This date is approximately 200,000–500,000 years earlier than previously documented [11], [20], [33], [45], and increases the known time depth of this adaptation within the hominin lineage (range of dates reflects varied interpretations of faunal materials from Olduvai [20]–[42]).
Lastly, our findings are directly relevant to a number of interrelated debates within Oldowan hominin paleobiology. These include many of the formative issues of the field, including those that explore the possible relationship(s) between the emergence of persistent hominin carnivory and the evolution of novel social and foraging ecologies [2]–[6], [8], [76], [78], [84], [88]–[90], brain expansion [1]–[5], [19], [78], [88]–[91], range extension [3], [6], [7], [78], [89], life history adaptations [3], [4], [78], [88], [89], [91]–[93], and, potentially, the interplay of some or all of these topics as they relate to the emergence and early evolutionary history of the genus Homo [1]–[3], [5]–[7], [78], [88], [89], [91], [92], [94].
Supporting Information
Bone surface modifications. Modifications detailed by long bone portion, bed, animal size group, and analyst.
(DOC)
East African Earlier Stone Age zooarchaeological assemblages. Surface modification data for bovid and taxonomically-indeterminate long bone specimens.
(DOC)
Minimum number of elements (MNE) for small and medium-sized bovids.
(DOC)
Skeletal element abundances and bone mineral densities.
(DOC)
Skeletal element abundances and standardized food utility indices.
(DOC)
Skeletal element abundances and within-bone nutrients.
(DOC)
Skeletal element abundances and within-bone resource extraction rates.
(DOC)
Skeletal element evenness. Evenness calculated using the Shannon evenness index. Bone frequencies modeled using a Bayesian multinomial model.
(DOC)
Acknowledgments
We thank the Office of the President of the Republic of Kenya and the National Museums of Kenya for permission to conduct this study. Research at Kanjera was undertaken through a cooperative understanding between the National Museums of Kenya and the Smithsonian Institution. All necessary permits were obtained for the described study, which complied with all relevant regulations. We thank I.O. Farah, E. Mbua, M. Muungu and the staff of the Palaeontology Section, NMK Department of Earth Sciences, for logistical support; C.M. Kilonzi for curatorial care of the collections; and J.M. Muteti and the excavation crew for their expertise in the field.
Funding Statement
This research was supported by funding from the National Science Foundation, Leakey Foundation, Wenner-Gren Foundation, National Geographic Society, The Leverhulme Trust, University of California, Baylor University, and the City University of New York. Additional logistical support was provided by the Smithsonian Institution’s Human Origins Program and the Peter Buck Fund for Human Origins Research, the British Institute in Eastern Africa, and the National Museums of Kenya. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aiello LC, Wheeler P (1995) The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36: 199–221 Available: http://www.jstor.org/stable/2744104. [Google Scholar]
- 2. Milton K (1999) A hypothesis to explain the role of meat-eating in human evolution. Evol Anthropol 8: 11–21 doi:10.1002/(SICI)1520–6505(1999). [Google Scholar]
- 3.Foley R (2001) The evolutionary consequences of increased carnivory in hominids. In: Stanford CB, Bunn HT, editors. Meat-Eating and Human Evolution. Oxford: Oxford University Press. 305–331.
- 4. Kaplan H, Hill K, Lancaster J, Hurtado M (2000) A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol 9: 156–185 doi: 10.1002/1520–6505 (2000). [Google Scholar]
- 5. Leonard WR, Snodgrass JJ, Robertson ML (2007) Effects of brain evolution on human nutrition and metabolism. Annu Rev Nutr 27: 311–327 doi: 10.1146/annurev.nutr.27.061406.093659. [DOI] [PubMed] [Google Scholar]
- 6. Walker A, Shipman P (1989) The costs of becoming a predator. J Hum Evol 18: 373–392 doi:10.1016/0047–2484(89)90037–7. [Google Scholar]
- 7. Anton SC, Leonard WR, Robertson ML (2002) An ecomorphological model of the initial hominid dispersal from Africa. J Hum Evol 43: 773–785 doi:10.1006/jhev.2002.0602. [DOI] [PubMed] [Google Scholar]
- 8. Isaac GL (1978) Food sharing in human evolution: archaeological evidence from the Plio-Pleistocene of East Africa. J Anthropol Res 34: 311–325 (doi: 10.2307/3629782).. [Google Scholar]
- 9. Semaw S (2000) The World’s Oldest Stone Artefacts from Gona, Ethiopia: Their Implications for Understanding Stone Technology and Patterns of Human Evolution Between 2.6–1.5 Million Years Ago. J Archaeol Sci 27: 1197–1214 doi:10.1006/jasc.1999.0592. [Google Scholar]
- 10. de Heinzelin J, Clark JD, White T, Hart W, Renne P, et al. (1999) Environment and behavior of 2.5 million-year-old Bouri hominids. Science 284: 625–629 doi:10.1126/science.284.5414.625. [DOI] [PubMed] [Google Scholar]
- 11. Plummer T (2004) Flaked stones and old bones: biological and cultural evolution at the dawn of technology. Yearb Phys Anthropol 47: 118–164 doi:10.1002/ajpa.20157. [DOI] [PubMed] [Google Scholar]
- 12. Dominguez-Rodrigo M, Pickering TR, Semaw S, Rogers MJ (2005) Cutmarked bones from Pliocene archaeological sites at Gona, Afar, Ethiopia: implications for the function for the world’s oldest stone tools. J Hum Evol 48: 109–121 (doi:10.1016/j.jhevol.2004.09.004).. [DOI] [PubMed] [Google Scholar]
- 13. McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, et al. (2010) Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466: 857–860 doi:10.1038/nature09248. [DOI] [PubMed] [Google Scholar]
- 14. Kibunjia M (1994) Pliocene archaeological occurrences in the Lake Turkana basin. J Hum Evol 27: 159–171 doi:10.1006/jhev.1994.1040. [Google Scholar]
- 15. Kimbel WH, Walter RC, Johanson DC, Reed KE, Aronson JL, et al. (1996) Late Pliocene Homo and Oldowan Tools from the Hadar Formation (Kada Hadar Member), Ethiopia. J Hum Evol 31: 549–561 (doi:10.1006/jhev.1996.0079).. [Google Scholar]
- 16. Dominguez-Rodrigo M, Pickering TR, Bunn HT (2010) Configurational approach to identifying the earliest hominin butchers. Proc Nat Acad Sci USA 107: 20929–20934 doi: 10.1073/pnas.1013711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraro JV (2007) Broken bones and shattered stones: on the foraging ecology of Oldowan hominins [PhD dissertation]. Los Angeles: University of California, Los Angeles. 340 p.
- 18. Reeve HK, Sherman PW (1993) Adaptation and the goals of evolutionary research. Q Rev Biol 68: 1–32 doi: 10.2307/2832133. [Google Scholar]
- 19. Braun DR, Harris JWK, Levin NE, McCoy JT, Herries AIR, et al. (2010) Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proc Nat Acad Sci USA 107: 10002–10007 doi:10.1073/pnas.1002181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez-Rodrigo M, Barba R, Egeland CP (2007) Deconstructing Olduvai: a Taphonomic Study of the Bed I Sites. Dordrecht, Netherlands: Springer. 337 p.
- 21.Bunn HT (1982) Meat-eating and human evolution: studies on the diet and subsistence patterns of Plio-Pleistocene hominids in East Africa [PhD dissertation]. Berkeley: University of California, Berkeley. 514 p.
- 22.Blumenschine RJ, Marean CW (1993) A carnivore’s view of archaeological bone assemblages. In: Hudson J, editor. From Bones to Behavior: Ethnoarchaeological and Experimental Contributions to the Interpretation of Faunal Remains. Carbondale: University of Southern Illinois Press. Pp. 273–300.
- 23. Blumenschine RJ (1995) Percussion marks, tooth marks, and experimental determinations of the timing of hominid and carnivore access to long bones at FLK Zinjanthropus, Olduvai Gorge, Tanzania. J Hum Evol 29: 21–51 doi:10.1006/jhev.1995.1046. [Google Scholar]
- 24. Capaldo SD (1997) Experimental determinations of carcass processing by Plio-Pleistocene hominids and carnivores at FLK 22 (Zinjanthropus), Olduvai Gorge, Tanzania. J Hum Evol 33: 555–597 doi:10.1006/jhev.1997.0150. [DOI] [PubMed] [Google Scholar]
- 25.Capaldo SD (1995) Inferring hominid and carnivore behavior from dual-patterned archaeofaunal assemblages [PhD dissertation]. New Brunswick, New Jersey: Rutgers University. 428 p.
- 26. Lupo KD, O’Connell JF (2002) Cut and tooth mark distributions on large animal bones: ethnoarchaeological data from the Hadza and their implications for current ideas about early human carnivory. J Archaeol Sci 29: 85–109 doi:10.1006/jasc.2001.0690. [Google Scholar]
- 27. Marean CW, Spencer LM, Blumenschine RJ, Capaldo SD (1992) Captive hyaena bone choice and destruction, the Schlepp Effect and Olduvai archaeofaunas. J Archaeol Sci 19: 101–121 doi:10.1016/0305–4403(92)90009-R. [Google Scholar]
- 28. Blumenschine RJ, Madrigal TC (1993) Variability in long bone marrow yields of East African ungulates and its zooarchaeological implications. J Archaeol Sci 20: 555–587 doi:10.1006/jasc.1993.1034. [Google Scholar]
- 29. Lupo KD (1998) Experimentally derived extraction rates for marrow: implications for body part exploitation strategies of Plio-Pleistocene hominid scavengers. J Archaeol Sci 25: 657–675 doi:10.1006/jasc.1997.0261. [Google Scholar]
- 30. Faith JT, Gordon AD (2007) Skeletal element abundances in archaeofaunal assemblages: economic utility, sample size, and assessment of carcass transport strategies. J Archaeol Sci 34: 872–882 doi:10.1016/j.jas.2006.08.007. [Google Scholar]
- 31. Dominguez-Rodrigo M (2002) Hunting and scavenging by early humans: the state of the debate. Journal of World Prehistory 16: 1–54 doi:10.1023/A:1014507129795. [Google Scholar]
- 32. Dominguez-Rodrigo M, Barba R (2006) New estimates of tooth mark and percussion mark frequencies at the FLK Zinj site: the carnivore-hominid-carnivore hypothesis falsified. J Hum Evol 50: 170–194 doi:10.1016/j.jhevol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 33. Bunn HT, Kroll EM (1986) Systematic butchery by Plio/Pleistocene hominids at Olduvai Gorge, Tanzania. Curr Anthropol 27: 431–452. [Google Scholar]
- 34. Oliver JS (1994) Estimates of hominid and carnivore involvement in the FLK Zinjanthropus fossil assemblage: some socioecological implications. J Hum Evol 27: 267–294 doi:10.1006/jhev.1994.1046. [Google Scholar]
- 35.Potts R (1988) Early Hominin Activities at Olduvai. New York: Aldine de Gruyter. 396 p.
- 36. Madrigal TC, Blumenschine RJ (2000) Preferential processing of high return rate marrow bones by Oldowan hominids: a comment on Lupo. J Archaeol Sci 27: 739–741 doi:10.1006/jasc.1999.0497. [Google Scholar]
- 37. Faith JT, Dominguez-Rodrigo M, Gordon AD (2009) Long-distance carcass transport at Olduvai Gorge? A quantitative examination of Bed I skeletal element abundances. J Hum Evol 56: 247–256 doi:10.1016/j.jhevol.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Binford LR (1981) Bones: Ancient Men and Modern Myths. Orlando: Academic Press. 320 p.
- 39. Bunn HT (1991) A taphonomic perspective on the archaeology of human origins. Annu Rev Anthropol 20: 433–467 doi:10.1146/annurev.an.20.100191.002245. [Google Scholar]
- 40. Dominguez-Rodrigo M, Bunn HT, Mabulla AZP, Ashley GM, Diez-Martin F, et al. (2010) New excavations at the FLK Zinjanthropus site and its surrounding landscape and their behavioral implications. Quat Res 74: 315–332 doi: 10.1016/j.yqres.2010.07.003. [Google Scholar]
- 41. Bunn HT, Pickering TR (2010) Bovid mortality profiles in paleoecological context falsify hypotheses of endurance running–hunting and passive scavenging by early Pleistocene hominins. Quat Res 74: 395–404 doi: 10.1016/j.qres.2010.07.012. [Google Scholar]
- 42. Pante MC, Blumenschine RJ, Capaldo SD, Scott RS (2012) Validation of bone surface modification models for inferring fossil hominin and carnivore feeding interactions, with reapplication to FLK 22, Olduvai Gorge, Tanzania. J Hum Evol 63: 395–407 doi: 10.1016/jhevol2011.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Lyman RL (1994) Vertebrate Taphonomy. Cambridge: Cambridge University Press. 524 p.
- 44. Pickering TR, Egeland CP, Dominguez-Rodrigo M, Brain CK, Schnell AG (2008) Testing the “shift in the balance of power” hypothesis at Swartkrans, South Africa: hominid cave use and subsistence behavior in the early Pleistocene. J Anthropol Archaeol 27: 30–45 doi:10.1016/j.jaa.2007.07.002. [Google Scholar]
- 45. Pobiner BL, Rogers MJ, Monahan CM, Harris JWK (2008) New evidence for hominin carcass processing strategies at 1.5 Ma, Koobi Fora, Kenya. J Hum Evol 55: 103–130 doi:10.1016/j.jhevol.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 46. O’Connell JF, Hawkes K, Lupo KD, Blurton Jones NG (2002) Male strategies and Plio-Pleistocene archaeology. J Hum Evol 43: 831–872. [DOI] [PubMed] [Google Scholar]
- 47. O’Connell JF, Hawkes K, Blurton Jones NG (1999) Grandmothering and the evolution of Homo erectus. J Hum Evol 36: 461–485 doi: 10.1006/jhev.1998.0285. [DOI] [PubMed] [Google Scholar]
- 48. Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain NL (1999) The raw and the stolen: cooking and the ecology of human origins. Curr Anthropol 40: 567–594. [PubMed] [Google Scholar]
- 49. Plummer T, Bishop LC, Ditchfield P, Hicks J (1999) Research on Late Pliocene Oldowan sites at Kanjera South, Kenya. J Hum Evol 36: 151–170 doi:10.1006/jhev.1998.0256. [DOI] [PubMed] [Google Scholar]
- 50.Plummer TW, Ditchfield PW, Bishop LC, Kingston JD, Ferraro JV, et al.. (2009) Oldest evidence of toolmaking hominins in a grassland-dominated ecosystem. PLoS ONE 4, e7199. doi:10.1371/journal.pone.0007199. [DOI] [PMC free article] [PubMed]
- 51. Bishop LC, Plummer TW, Ferraro JV, Braun D, Ditchfield PW, et al. (2006) Recent research into Oldowan hominin activities at Kanjera South, western Kenya. Afr Archaeol Rev 23: 31–40 doi:10.1007/s10437–006–9006–1. [Google Scholar]
- 52.Plummer TW, Bishop LC, Ditchfield PW, Ferraro JV, Kingston JD, et al.. (2009) The environmental context of Oldowan hominin activities at Kanjera South, Kenya. In: Hovers E, Braun DR, editors. Interdisciplinary Approaches to the Oldowan. Dordrecht, Netherlands: Springer. 149–160.
- 53. Braun DR, Plummer T, Ditchfield P, Ferraro JV, Maina D, et al. (2008) Oldowan behavior and raw material transport: perspectives from the Kanjera Formation. J Archaeol Sci 35: 2329–2345 doi:10.1016/j.jas.2008.03.004. [Google Scholar]
- 54. Braun DR, Plummer T, Ferraro JV, Ditchfield P, Bishop LC (2009) Raw material quality and Oldowan hominin toolstone preferences: evidence from Kanjera South, Kenya. J Archaeol Sci 36: 1605–1614 doi:10.1016/j.jas.2009.03.025. [Google Scholar]
- 55.Braun DR (2006) The ecology of Oldowan technology: perspectives from Koobi Fora and Kanjera South [PhD dissertation]. New Brunswick, New Jersey: Rutgers University. 346 p.
- 56. Selvaggio MM (1994) Carnivore tooth marks and stone tool butchery marks on scavenged bones: archaeological implications. J Hum Evol 27: 215–228 doi:10.1006/jhev.1994.1043. [Google Scholar]
- 57.Dominguez-Rodrigo M (1999) Meat-eating and carcass procurement by hominids at the FLK Zinj 22 site, Olduvai Gorge (Tanzania): a new experimental approach to the old hunting-versus-scavenging debate. In: Ulrich H, editor. Lifestyles and Survival Strategies in Pliocene and Pleistocene Hominids. Gelsenkirchen, Germany: Edition Archaea. 89–111.
- 58. Marean CW, Abe Y, Frey CJ, Randall RC (2000) Zooarchaeological and taphonomic analysis of the Die Kelders Cave 1 Layers 10 and 11 Middle Stone Age larger mammal fauna. J Hum Evol 38: 197–233 doi:10.1006/jhev.1999.0356. [DOI] [PubMed] [Google Scholar]
- 59. Lam YM, Chen X, Pearson OM (1999) Intertaxonomic variability in patterns of bone density and the differential representation of bovid, cervid, and equid elements in the archaeological record. Am Antiq 64: 343–362 doi: 10.2307/2694283. [Google Scholar]
- 60. Marean CW, Spencer LM (1991) Impact of carnivore ravaging on zooarchaeological measures of element abundance. Am Antiq 56: 645–658 doi: 10.2307/281542. [Google Scholar]
- 61. Marean CW, Cleghorn N (2003) Large mammal skeletal element transport: applying foraging theory in a complex taphonomic system. Journal of Taphonomy 1: 15–42. [Google Scholar]
- 62. Metcalfe D, Jones KT (1988) A reconsideration of animal body part utility indices. Am Antiq 53: 486–504 doi: 10.2307/281213. [Google Scholar]
- 63. Blumenschine RJ (1987) Characteristics of an early hominid scavenging niche. Curr Anthropol 29: 382–407 doi: 10.2307/2743480. [Google Scholar]
- 64. Behrensmeyer AK, Gordon KD, Yanagi GT (1986) Trampling as a cause of bone surface damage and psuedo-cutmarks. Nature 319: 768–771 doi:10.1038/319768a0. [Google Scholar]
- 65. Behrensmeyer AK (1978) Taphonomic and ecological information from bone weathering. Paleobiology 4: 150–162 doi: 10.2307/2400283. [Google Scholar]
- 66. Olsen SL, Shipman P (1988) Surface modification on bone: trampling versus butchery. J Archaeol Sci 15: 535–553 doi:10.1016/0305–4403(88)90081–7. [Google Scholar]
- 67. Dominguez-Rodrigo M, de Juana S, Galan AB, Rodriquez M (2009) A new protocol to differentiate trampling marks from butchery marks. J Archaeol Sci 36: 2643–2654 doi: 10.1016/j.jas.2009.07.017. [Google Scholar]
- 68. Njau JK, Blumenschine RJ (2006) A diagnosis of crocodile feeding traces on larger mammal bone, with fossil examples from the Plio-Pleistocene Olduvai Basin, Tanzania. J Hum Evol 50: 142–162 doi:10.1016/j.jhevol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 69. Dominguez-Solera SD, Dominguez-Rodrigo M (2009) A taphonomic study of bone modifications and of tooth-mark patterns on long limb bone portions by suids. Int. J Osteoarchaeol 19: 345–363 doi:10.1002/oa.987. [Google Scholar]
- 70.Magurran AE (1988) Ecological Diversity and its Measurement. Princeton: Princeton University Press. 179 p.
- 71.Seaman JW III (2010) Topics in Bayesian inference: induced priors, proof loading for combination drugs, and distribution of archaeological skeletal assemblages [PhD dissertation]. Waco, Texas: Baylor University. 112 p.
- 72. Monahan CM (1996) New zooarchaeological data from Bed II, Olduvai Gorge, Tanzania: implications for hominid behavior in the Early Pleistocene. J Hum Evol 31: 93–128 doi:10.1006/jhev.1996.0053. [Google Scholar]
- 73. Egeland CP, Dominguez-Rodrigo M (2008) Taphonomic perspectives on hominid site use and foraging strategies during Bed II times at Olduvai Gorge, Tanzania. J Hum Evol 55: 1031–1052 doi:10.1016/j.jhevol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 74. Dominguez-Rodrigo M, Mabulla A, Bunn HT, Barba R, Diez-Martin F, et al. (2009) Unravelling hominin behavior at another anthropogenic site from Olduvai Gorge (Tanzania): new archaeological and taphonomic research at BK, Upper Bed II. J Hum Evol 57: 260–283 doi: 10.1016/j.jhevol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 75. Monahan CW (1998) The Hadza carcass transport debate revisited and its archaeological implications. J Archaeol Sci 25: 405–424 doi:10.1006/jasc.1997.0241. [Google Scholar]
- 76. Bunn HT, Ezzo JA (1993) Hunting and scavenging by Plio-Pleistocene hominids: nutritional constraints, archaeological patterns, and behavioural implications. J Archaeol Sci 20: 365–398 doi:10.1006/jasc.1993.1023. [Google Scholar]
- 77.Speth JD (2010) The Paleoanthropology and Archaeology of Big Game Hunting: Protein, Fat or Politics? New York: Springer. 233p.
- 78. Leonard WR, Robertson ML (1992) Nutritional requirements and human evolution: a bioenergetics model. Am J Hum Biol 4: 179–195 doi: 0.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- 79. Blumenschine RJ (1989) A landscape taphonomic model of the scale of prehistoric scavenging opportunities. J Hum Evol 18: 345–371. [Google Scholar]
- 80. Blumenschine RJ (1986) Carcass consumption sequences and the archaeological distinction of scavenging and hunting. J Hum Evol 15: 639–659 doi: 10.1016/S0047–2484(86)80002–1. [Google Scholar]
- 81. Capaldo SD, Peters CR (1995) Skeletal inventories from wildebeest drownings at Lake Masek and Ndutu in the Serengeti ecosystem of Tanzania. J Archaeol Sci 22: 385–408 doi: 10.1006/jasc.1995.0039. [Google Scholar]
- 82. Schaller GB, Lowther GR (1969) The relevance of carnivore behavior to the study of early hominids. Southwest J Anthropol 25: 307–341 Available: http://www.jstor.org/stable/3629426. [Google Scholar]
- 83. Faith JT, Behrensmeyer AK (2006) Changing patterns of carnivore modification in a landscape bone assemblage, Amboseli Park, Kenya. J Archaeol Sci 33: 1718–1733 doi:10.1016/j.jas.2006.03.004. [Google Scholar]
- 84. Cordain L, Watkins BA, Mann NJ (2001) Fatty acid composition and energy density of foods available to African hominids. World Rev Nutr Diet 90: 144–161. [DOI] [PubMed] [Google Scholar]
- 85. Milligan LA, Bazinet RP (2008) Evolutionary modifications of human milk composition: evidence from long-chain polyunsaturated fatty acid composition of anthropoid milks. J Hum Evol 55: 1086–1095 doi: 10.1016/j.jhevol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 86. Speth JD (1989) Early hominid hunting and scavenging: the role of meat as an energy source. J Hum Evol 18: 329–343 doi: 10.1016/0047–2484(89)900035–3. [Google Scholar]
- 87. Capaldo SD (1998) Simulating the formation of dual-patterned archaeofaunal assemblages with experimental control samples. J Archaeol Sci 25: 311–330 doi: 10.1006/jsac.1997.0238. [Google Scholar]
- 88. Milton K (2003) The critical role played by animal source foods in human (Homo) evolution. J Nutr 133: 3886S–3892S. [DOI] [PubMed] [Google Scholar]
- 89. Aiello LC, Wells JCK (2002) Energetics and the evolution of the genus Homo. Annu Rev Anthropol 31: 323–38 doi: 10.1146/annurev.anthro.31.040402.085403. [Google Scholar]
- 91. Aiello LC, Key C (2002) Energetic consequences of being a Homo erectus female. Amer J Hum Biol 14: 551–565 doi:10.002/ajhb.10069. [DOI] [PubMed] [Google Scholar]
- 92. Humphrey LT (2010) Weaning behaviour in human evolution. Sem Cell Devel Biol 21: 453–461 doi: 10.1016/j.semcdb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 93. Psouni W, Janke A, Garwicz M (2012) Impact of carnivory on human development and evolution revealed by a new unifying model of weaning in mammals. PLoS ONE 7(4): e32452 doi: 10.1371/journal.pone.0032452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Swedell L, Plummer TW (2012) A papionin multi-level society as a model for early hominin evolution. Int J Primat 33: 1165–1193 doi:10.1007/s10764–012–9600–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone surface modifications. Modifications detailed by long bone portion, bed, animal size group, and analyst.
(DOC)
East African Earlier Stone Age zooarchaeological assemblages. Surface modification data for bovid and taxonomically-indeterminate long bone specimens.
(DOC)
Minimum number of elements (MNE) for small and medium-sized bovids.
(DOC)
Skeletal element abundances and bone mineral densities.
(DOC)
Skeletal element abundances and standardized food utility indices.
(DOC)
Skeletal element abundances and within-bone nutrients.
(DOC)
Skeletal element abundances and within-bone resource extraction rates.
(DOC)
Skeletal element evenness. Evenness calculated using the Shannon evenness index. Bone frequencies modeled using a Bayesian multinomial model.
(DOC)