Abstract
Human tumors progress despite the presence of tumor associated antigen (TAA)-specific T cells. Many different molecular and cellular mechanisms contribute to the failure of T cells to eradicate the tumor. These include immune suppressive networks that impair ongoing T cell function and enable tumor escape. Recent studies have started to reveal the nature of effector T cells in the tumor microenvironment. In this article we discuss T cell anergy, exhaustion, senescence and stemness, and review the phenotype of dysfunctional T cell subsets and the underlying molecular mechanisms in the tumor microenvironments. We suggest that targeting T cell dysfunctional mechanisms and introducing/promoting T cell stemness are important approaches to treat patients with cancer.
Keywords: T cell anergy, exhaustion, senescence, stemness, cancer, PD-1, Tim-3, B7-H1, HIF-1
Introduction
Throughout life, we encounter a multitude of antigens and pathogens that threaten our health and survival. To fend off this antigenic insult, the immune system has evolved and armed itself with an innate immune system for immediate immune attack against the inciting antigen and with an adaptive immune system for long-term protection. The major players in adaptive immunity are effector T cells. Despite tremendous advances in our characterization of effector T cells in infectious disease models in the last decade, the nature of effector T cells is not well understood in patients with cancer. As our current knowledge of effector T cells arises almost exclusively from studies of infectious disease models (particularly mouse models), the induction of memory and effector T cells in cancer is often inadvertently thought of as being analogous to that observed in chronic infections. Although we have identified many T cell effector mechanisms in mouse infectious disease model, it is evident that the phenotype and functional profile of effector T cells in cancer are dramatically impacted by the tumor microenvironment in patients with cancer. Over the past few years, immunologists have achieved important insights into cancer immunopathogenesis in patients [1–3], and therefore have started to dissect the nature of T cells in the tumor environment. In this review, we discuss the phenotype, functionality, and the underlying mechanisms of anergic T cells, exhausted T cells, senescent T cells and stem-like T cells in the tumor microenvironment.
T cell anergy
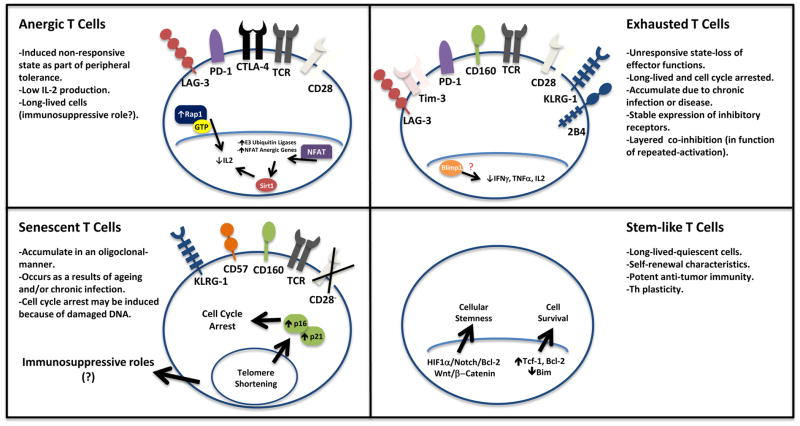
T cell anergy is generally described as the induced hyporesponsive state with low IL-2 production or incomplete activation, to which naïve T cells fall upon low co-stimulatory and/or high co-inhibitory stimulation (Fig. 1). It has been proposed that T cell anergy serves to induce tolerance in the periphery and protect the host from developing autoimmune disease [4–6]. Although this phenomenon has been the focus of many studies of human disease including cancer, it is unclear what causes and/or enforces their anergic state and phenotypic distinctions. Here, we review important observations on T cell anergy in the context of the tumor microenvironment.
Figure 1. General characteristics for anergic, exhausted, senescent and stem-like T cells.
a) Anergic T cells are T cells stimulated with low co-stimulatory and/or high co-inhibitory signaling. These cells are unresponsive to subsequent activating conditions with limited IL-2 expression. (b) Exhausted T cells are effector T cells that have lost their effector functions including effector cytokine expression due to repeated stimulation. These cells express multiple regulatory receptors. (c) Senescent T cells are described as unresponsive/terminally differentiated T cells. The hallmark is their cell cycle arrest along with limited CD28 expression and/or high levels of regulatory receptor expression. (d) Stem-like T cells may show a naïve or memory phenotype. Importantly, these cells are capable of self-renewal, have enhanced anti-tumor responses and are long-lived effector cells.
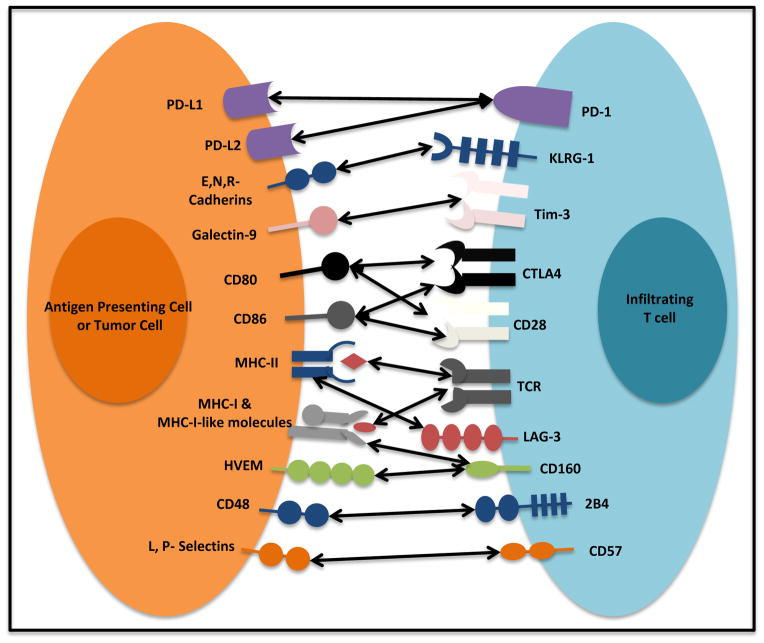
The first question is whether T cell anergy is an operative mechanism causing T cell dysfunction in the tumor microenvironment. The evidence for T cell anergy in the tumor context has been indirect. A core problem has been a lack of positive markers to characterize a loss of function state of T cells. Nonetheless, the following observations support that T cell anergy can be an important phenomenon in cancer: (a) There is an active imbalance between stimulatory and inhibitory B7 family members in the tumor microenvironment [1,3,7,8]. Human tumors and tumor associated antigen presenting cells (APCs) often express high levels of B7-H1 (CD274 or PD-L1), B7-H2 (CD275 or ICOS-L), B7-H3 (CD276), B7-H4 (B7S1 or B7x), and B7-DC (CD273 or PD-L2) with low-to-absent expression of B7-1 (CD80) and B7-2 (CD86) [7–10] (Fig. 2). This indicates a poor co-stimulatory, high co-inhibitory and therefore anergy-promoting environment. (b) Animal model studies have shown that introduction of B7-1 into tumors by transfection or blockade of inhibitory B7 family members can reduce tumor growth or result in spontaneous tumor rejection in vivo [7–13]. (c) Homeostatic proliferation of anti-tumor T cells in a lymphopenic host both reverses anergy and promotes tumor rejection in vivo [14]. (d) There is evidence indicating antigen specific, T cell-intrinsic dysfunction in the tumor microenvironment [15,16]. Therefore, T cell anergy may be a functional mechanism in patients with cancer. However, the relative impact of T cell anergy on tumor immunity remains to be defined.
Figure 2. Immunoregulatory receptors and their ligands.
T cell activation relies on the T cell receptor (TCR) recognizing its cognate antigen in the context of MHC molecules from an antigen presenting cell (APC) or an APC-like cell (tumor cell). Interaction between co-stimulatory molecules CD80, and CD86 and CD28 is crucial for appropriate T cell activation. Immunoregulatory receptors such as CTLA-4 and PD-1 are to fine tune T cell activation. High levels of multiple immunoregulatory receptors (LAG-3, 2B4, CD160, KLRG1, Tim-3, CTLA-4, and CD57) or their ligands are found in the tumor microenvironment. Potent and lasting immunoregulatory signaling results in reduced T cell function and tolerance.
Cellular and molecular mechanisms controlling T cell anergy are insufficiently understood. It is generally accepted that T cells that are presented antigen along with suboptimal CD28 co-stimulation [4,5] and/or high co-inhibition [17] result in anergic phenotypes, as characterized by their low IL-2 production and cell cycle arrest at the G1/S phase. Early growth response gene 2 (Egr2) may be a central transcription factor that regulates T cell anergic state [*18]. It has been suggested that the anergy program is initiated by improper mTOR and Ras/MAPK signaling in the cell, a pathway which lies directly downstream of TCR/CD28 engagement. Specifically, sole binding of TCR by MHC promotes Ca2+ imbalance on T cells and retention of active-RAP-1 in the cytosol, an imbalance that would normally be corrected by co-stimulation through CD28 (Ras/MAPK) [**19,20]. The effects of this imbalance on the genetic reprogramming of these cells have been hypothesized to be mediated by NFAT homodimer formation and transcription of anergy-inducing genes [21,22]. The E3 ubiquiting ligase family can affect PI3K, mTOR, and Ras/MAPK signaling pathways and help to actively maintain anergy [21,23,24]. Epigenetic factors such as IKAROS (through acetylation) [25] and Sirt1 are involved in histone modifications that promote T cell anergy [26,27]. Thus, anergy is the combined result of factors that negatively regulate proximal TCR-coupled signal transduction, together with a program of active transcriptional silencing that is reinforced through epigenetic mechanisms [6].
In summary, tumor induced T cell anergy may be one of the immune evasion mechanisms in patients with cancer. Egr2 may be the potential transcriptional factor controlling T cell anergy. However, the downstream molecular mechanisms involved in the anergic state have been incompletely understood. The lack of surface marker(s) to define anergic T cells makes T cell anergy research a difficult challenge for immunologists.
T cell exhaustion
Exhausted T cells are described as effector T cells with decreased cytokine expression and effector function, and being resistant to reactivation [28](Fig. 1). T cell exhaustion occurs when T cells are chronically activated at sites of chronic inflammation, such as cancer, autoimmunity, and chronic infection. Dissecting the mechanism by which an exhaustive phenotype is ensured has been the focus of much research with the molecular enforcers just being revealed.
Initial mouse studies have proposed that B7-H1/PD-1 signaling pathway mediates CD8+ T cell functional exhaustion in the context of chronic infection, and PD-1 was proposed to be a marker for exhausted T cells [*29]. Interestingly, far before these mouse studies in chronic infectious disease models [29], it was demonstrated that human tumor cells and/or tumor associated APCs expressed B7-H1, and B7-H1/PD-1 pathway mediated immune suppression [**9], and blockade of B7-H1/PD-1 pathway was investigated as therapeutic targets in solid human tumors [9,30] (Fig. 2). Exhausted CD8+ T cells were found in patients with melanoma [*31], ovarian cancer [9] and hepatocellular carcinoma (HCC) [30]. Recent clinical trials have validated that blockade of B7-H1/PD-1 signaling is a meaningful immune therapeutic regimen [**32,**33]. Although the detailed molecular mechanism of T cell exhaustion is incompletely defined, it is suggested that recruitment of SH2-domain containing protein tyrosine phosphatases (SHP-1 and/or SHP-2) to the immunoreceptor tyrosine-based switch motif (ITSM) within the PD-1 cytoplasmic tail inhibits signaling events, particularly PI3K/AKT activation, downstream signals of the T-cell receptor [34], and in turn results in T cell dysfunction. Notably, activated T cells and effector T cells in the early stage may express PD-1 and remain functional [35,*36]. Given that members of the inhibitory B7 family are widely expressed by malignant cells and APCs within the human tumor microenvironment [7], the development of novel therapeutic strategies targeting the inhibitory B7 family members in malignancies is under active clinical investigations and show exciting clinical promise [32,33].
T cell exhaustion may be a layered or progressive process to which T cells fall upon repeated activation. T cells acquiring multiple inhibitory surface molecules in persistent disease settings such as chronic infection [37,38] and malignancies [30,**39,40], which effectively prevent T cell activation. In the course of defining exhausted PD-1+ T cells, T cell immunoglobulin and mucin-domain-containing molecule-3 (Tim-3) [39,41], lymphocyte-activation gene (LAG)-3 [38], and the B and T-cell lymphocyte attenuator (BTLA, CD272) [42,43] were found to be co-expressed with PD-1, and the co-expression has been strongly correlated with immune dysfunction in patients with cancer. In these studies, T cells co-expressing these surface molecules show a significant decrease in IL-2, IFNγ, and TNFα expression as well as cell cycle arrest, which defines T cell exhaustion. In line with the concept that Tim-3 and PD-1 may define and maintain T cell exhaustion, blockade of these surface molecules allows rescue of their effector functions as shown by cell cycle progression and acquired effector cytokine expression and cytotoxicity [40,43,44]. Notably, exhausted T cells may highly express multiple “inhibitory” receptors, including PD-1, 2B4 (CD244), BTLA, CTLA-4, CD160, LAG-3 and Tim-3 [38,42,43,45]. However, exhausted T cells may not necessarily co-express these molecules. Furthermore, it is controversial if the co-expression of inhibitory molecules is functionally important to determine T cell functional state. For example, in patients with HCC, the expression of PD-1 and Tim-3 is minimally overlapping in tumor infiltrating T cells. HCC-associated Tim-3+T cells expressed reduced CD28, suggesting that these cells may be early senescence stage [40]. The question remains unanswered if these “inhibitory molecule” expressing T cells share similar molecular and genetic signature in patients with chronic infection and cancer.
Nonetheless, it is assumed that the tumor microenvironment provides the necessary conditions for effector T cells to become functionally exhausted as well as being able to maintain this state during disease progression. The detailed molecular signals remain undefined. A promising aspect is that clinical blockade of B7-H1/PD-1, the key T cell exhaustion pathway, may rescue T cell effector functions in vivo, and results in significant objective clinical responses [32,33].
T cell senescence
Senescent T cells are characterized by telomere shortenings, phenotypic change (loss of CD28 expression), and cell cycle arrest [46,47] (Fig. 1). Telomere shortening is an inherent byproduct of cellular division, which affects cellular function and leads to cell senescence [48]. Cell cycle controlling proteins p16, p21, and p53, normally inhibit cell cycle progression and have been shown to be accumulated in senescent cells [49–51]. In addition to phenotypic alteration, senescent T cells manifest defective killing abilities and the development of negative regulatory functions [52,53].
It is naturally thought that senescence is associated with physiological ageing process. Indeed, the cell has its natural life-span and proliferation exhaustion results in cell senescence. However, high levels of senescent T cells were found in younger patients with autoimmune disease and chronic viral infection [54]. This suggests that cells in younger patients may become senescent, and chronic activation and proliferation may still cause T cell senescence [55]. In line with this notion, tumor cells can induce T cell senescence in in vitro co-cultures [56]. Phenotypically, senescent CD28−/dimCD8+T cells are observed in patients with lung cancers [57], head and neck cancer [58]. DNA damage can cause mouse thymic precursor lymphocytes to withdraw from the cell cycle and undergo senescence [59]. This is sufficient to inhibit oncogenic chromosomal abnormality and suppress tumorigenesis. However, there is no direct evidence that similar mechanisms are applied for mature T cell senescence in the tumor microenvironment.
In addition to low expression of CD28, high expression of Tim-3, CD57, killer cell lectin-like receptor subfamily G, member 1 (KLRG-1) are thought to be associated with T cell senescence [40,60–64](Fig. 2). For example, human HCC- associated T cells highly express Tim-3 and cyclin dependent kinase inhibitors, and interact with galectin-9+myeloid APCs. These cells fail to enter the cell cycle [40]. Similar findings were obtained in patients with melanoma [*63] and lymphoma [64]. It suggests that cancer associated Tim-3+ T cells may contain senescent cells and experience cell cycle arrest in G1/S phase.
A few studies have examined how Tim-3 carries out its function [65]. Galectin-9 is the Tim-3 ligand. Galectin-9 induces intracellular calcium flux, aggregation and death of Th1 cells in Tim-3-dependent manner [65]. Yet, it has been postulated that the human leukocyte antigen (HLA)-B-associated transcript 3 (Bat3) modulates Tim-3 function on T cells by interacting with Tim-3 cytoplasmic tail [66]. Bat3 localization at this protein region allows T cells continued proliferation and IFNγ production, an effect that was lost on Bat3 deficient cells and upon Tim-3 binding to galectin-9. However, how Tim-3 and Bat3 interaction links to T cell senescence remains to be elucidated.
T cell stemness
The self-renewal, expansion and multi-lineage developmental potential define the unique properties attributed to stem cells. Based on these properties, the concept of T cell stemness was proposed and “stem-like memory T cells” may encompass the capability to both self-renew and to generate more differentiated, memory T cells (Fig. 1).
The concept of T cell stemness was initially stemmed from the observations that mouse central memory T cells are arrested at a pre-differentiation stage by transcriptional inhibitors and retain replicative potential and long-term production of effector T cells after a second antigen challenge [67]. The capacity to continuously generate effector memory T cells will replenish the effector memory T cell pool, and help maintain a constant repertoire of memory T cells for a human lifetime, despite the finite lifespan of individual effector cells and reduced thymus function [68]. This notion has recently received certain experimental support. In a mouse model, CD44lowCD62Lhigh memory CD8+ T cells express high levels of stem cell antigen-1 (Sca-1), Bcl-2 and common IL-2 and IL-15 receptor β chain (CD122). Because these cells showed robust self-renewal and the multipotent capacity to generate central memory and effector memory T cells, they were designated T memory stem cells [69]. More recent mouse work has shown that by blocking T cell differentiation, Wnt signaling promoted the generation of this memory stem cell population [70]. These studies support the concept that certain subsets of memory T cells endow the capability both to self-renew and to generate more effector T cells. Therefore, by manipulating these “stemness properties” of memory T cells, it is possible to generate and maintain long-lived, self-renewing antigen-experienced memory T cells with stem cell-like properties for treating patients with cancer.
In line with these limited reports in non-tumor system, human tumor associated Th17 cells expressed stem cell markers and exhibited stem cell like features. When measured for their biological activity, mouse and human Th17 cells displayed greater survival potential, persistence as well as the ability of repopulating sub-lethally irradiated mice [**71,**72]. Furthermore, these cells achieved a higher anti-tumor response when compared to effector and central memory T cells. Interestingly, Th17 cells retain a stem cell-like phenotype through the coordinated effects of HIF1α/Notch/Bcl-2 and are also potent anti-tumor effectors [71], suggesting that stemness might correlate with better immune responses. Human Th17 cells were shown to give rise to distinct Th lineages, as measured through the expression of IFNγ, and Foxp3+ cells, heightened self-renewal, and survival capabilities [71,72].
Human Th17 cells have certain “stem cell properties” at the genetic, molecular and functional levels, and are long-lived cells. This property may be critically important for controlling Th17 cell biology. Manipulation of Th17 stemness may be therapeutically interesting for treating patients with Th17-associated chronic diseases.
Conclusions
Compelling evidence demonstrates the co-existence of T cell anergy, exhaustion, senescence and stemness in the tumor microenvironment. When we interpret the current literature, the following points may need to be taken into consideration: (a) T cell subset markers. Are there specific markers to phenotypically define anergic, exhausted, senescent and stem-like T cell subsets? It is arguable but experimentally operative that PD-1 may be a marker for exhausted cells, Tim-3 and KLRG-1 may be markers for senescent cells, and mouse stem-like T cells may express Sca-1 [70]. However, these markers are not mutually exclusive and inclusive in a given T cell subset. Our opinion is that these T cell subsets are functionally generated and defined. Therefore, genetic and functional pattern, but not specific surface phenotypes will define their nature and fate. For example, despite their phenotypic markers of terminal differentiation, Th17 cells have stem cell feature with powerful functionalities [71–73]. (b) Functional and phenotypic overlap. Although these cell subsets are conceptually distinct, they may be functionally and phenotypically overlapped. PD-1+ cells may express Tim-3 and LAG-3. Regardless of these different immunological concepts, it is evident that B7-H1/PD-1 and Tim-3/galectin-9 signaling pathways may synergistically and/or additively mediate T cell dysfunction, and simultaneous blockade of these pathways may result in improved T cell immunity. Preclinical and clinical studies suggest that T cell dysfunction may be functionally reversible. This paves the way for targeting cancer therapy. (c) Mechanistically intertwined. Although the underlying mechanisms causing T cell anergy, exhaustion and senescence are not well defined, compelling evidence indicate that dysfunctional T cells express in different degrees the “inhibitory” molecules including PD-1, Tim-3, LAG-3, 2B4, CD160, and KLGR-1. It suggests that different categories of T cell abnormalities may be mechanistically intertwined [28,74–76].
In conclusion, peripheral T cell tolerance mechanisms including regulatory T cells, T cell anergy, exhaustion, and senescence impair ongoing T cell immunity and enable tumor immune escape. Further clarification of their pathogenic mechanisms and roles will have significant implications for the development of novel therapeutic strategies targeting these mechanisms in malignancies. We suggest that targeting T cell dysfunctional mechanisms and introducing/promoting T cell stemness are important approaches to treat patients with cancer.
Highlights.
We provide an updated view on effector T cell compartment in the tumor microenvironment.
We describe dysfunctional T cell subsets in the tumor microenvironment.
We discuss co-inhibitory and co-stimulatory molecular networks in the tumor microenvironment.
We review stem like-memory T cell subsets (e.g. Th17) in the tumor microenvironment.
We suggest anti-cancer modality by targeting T cell dysfunction and stemness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 2.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 6.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 7.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 8.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- **9.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. The paper showed the expression, regulation and function of B7-H1 (PD-L1) in human cancer microenvironment, and indicated that B7-H1/PD-1 pathway blockade is therapeutically meaningful in treating human cancer. [DOI] [PubMed] [Google Scholar]
- 10.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 12.Gajewski TF. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996;156:465–472. [PubMed] [Google Scholar]
- 13.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 14.Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 15.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 16.Broderick L, Brooks SP, Takita H, Baer AN, Bernstein JM, Bankert RB. IL-12 reverses anergy to T cell receptor triggering in human lung tumor-associated memory T cells. Clin Immunol. 2006;118:159–169. doi: 10.1016/j.clim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- *18.Zheng Y, Zha Y, Driessens G, Locke F, Gajewski TF. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J Exp Med. 2012 doi: 10.1084/jem.20120342. By focusing on DGK expression, a focal protein of T cell anergy, the authors demonstrated that Egr2, a transcription factor, mediates anergy establishment on T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. This is one of the early studies on the molecular pathway leading to T cell anergy. Active Rap-1 is found to be constitutively present in anergic T cells as well as mediating low expression of IL2 and inhibiting normal TCR signaling. [DOI] [PubMed] [Google Scholar]
- 20.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 21.Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 22.Soto-Nieves N, Puga I, Abe BT, Bandyopadhyay S, Baine I, Rao A, Macian F. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J Exp Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 25.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 26.Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc Natl Acad Sci U S A. 2012;109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- *29.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. The authors use PD-1 to describe exhausted T cells in mice with LCMV infection and further target the PD1/PDL1 to recover their dysfunctional state. [DOI] [PubMed] [Google Scholar]
- 30.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. This study elucidated the molecular pathways involved in human tumor associated T cell dysfunction. The authors found the expression of various co-inhibitory surface molecules (KLRG-1, 2B4, PD-1, and Tim-3) that may mediate and establish a non-responsive state from T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200694. These clinical trials showed that anti-B7-H1 (PD-L1) [32] or anti-PD-1 [33] treatments resulted in important objective clinical responses in patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. These clinical trials showed that anti-B7-H1 (PD-L1) [32] or anti-PD-1 [33] treatments resulted in important objective clinical responses in patients with cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haymaker C, Wu R, Bernatchez C, Radvanyi L. PD-1 and BTLA and CD8(+) T-cell “exhaustion” in cancer: “Exercising” an alternative viewpoint. Oncoimmunology. 2012;1:735–738. doi: 10.4161/onci.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. The authors found that B7-H1 is important in CD8+ T cell tolerance, and PD-1 expressed in early effector T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. The authors found that both PD-1 and Tim-3 pathways are involved in mediating CD8+ T cell dysfunction in anti-tumor immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adibzadeh M, Pohla H, Rehbein A, Pawelec G. Long-term culture of monoclonal human T lymphocytes: models for immunosenescence? Mech Ageing Dev. 1995;83:171–183. doi: 10.1016/0047-6374(95)01625-a. [DOI] [PubMed] [Google Scholar]
- 47.Effros RB. Replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. Am J Hum Genet. 1998;62:1003–1007. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 49.Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 54.Pawelec G, Solana R. Immunoageing - the cause or effect of morbidity. Trends Immunol. 2001;22:348–349. doi: 10.1016/s1471-4906(01)01956-1. [DOI] [PubMed] [Google Scholar]
- 55.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 57.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, Ferrari M, Cosentino M, Marino F, Pozzi E, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(−) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Nguyen T, Puebla-Osorio N, Pang H, Dujka ME, Zhu C. DNA damage-induced cellular senescence is sufficient to suppress tumorigenesis: a mouse model. J Exp Med. 2007;204:1453–1461. doi: 10.1084/jem.20062453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- *61.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci U S A. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. The authors showed that Bmi-1 is an important regulator of T cell survival and senescent KLRG-1+ CD8+ T cells do not readily express Bmi-1 upon TCR engagement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- *63.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. This paper described an NY-ESO-1– specific CD8 T cell subset that is rendered dysfunctional upon expression of Tim3 and PD-1 on their cell surface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang X, Bai X, Cao Y, Wu J, Huang M, Tang D, Tao S, Zhu T, Liu Y, Yang Y, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 66.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 68.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 70.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **71.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. Human TH17 Cells Are Long-Lived Effector Memory Cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. References 71 and 72 show how Th17 cells may be long-lived effector cells in both humans [71] and mice [72], and have stem cell characters and mediate potent anti-tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **72.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. References 71 and 72 show how Th17 cells may be long-lived effector cells in both humans [71] and mice [72], and have stem cell characters and mediate potent anti-tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei S, Zhao E, Kryczek I, Zou W. Th17 cells have stem cell-like features and promote long-term tumor immunity. Onco Immunology. 2012:1. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 75.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]