Abstract
Objective
This study aims to evaluate HPV viral load as a biomarker for triage into colposcopy and CIN2 therapy, in order to reduce the colposcopy referral rate and CIN2 over treatment in low resource settings.
Methods
In 1999, 1997 women aged 35–45 in Shanxi, China, received six cervical screenings with pathological confirmation. In 2005, 1461 histologically normal women, 99 with cervical intraepithelial neoplasia (CIN) grade 1 (CIN1), and 30 with CIN grade 2 or worse (CIN2+) were rescreened in a follow-up study. HPV was detected by Hybrid Capture 2. Viral load, estimated by the ratio of relative light units to standard positive control, was categorized into four groups: negative (<1.0), low (≥1.0, <10.0), moderate (≥10.0, <100.0) and high (≥100.0). We estimated cumulative incidence of CIN2+ by viral load subgroups and calculated adjusted hazard ratios (aHR) for CIN2+ using Cox proportional hazards regression.
Results
Cumulative incidence of CIN2+ increased with baseline HPV viral load among normal women and women with CIN1 at baseline (P-trend<0.001). Repeat moderate-high viral load was associated with the highest risk for CIN2+ (aHR=188.8, 95% confidence interval: 41.2–864.1). Raising the RLU/PC cutoff from 1.0 to 10.0 for colposcopy greatly reduced the referral rate from 18.1% to 12.9%. It also increased the specificity (84.8% vs. 90.4%), the positive predictive value (22.5% vs. 28.9%), and the positive likelihood ratio (6.4 vs. 8.9), yet with loss of the sensitivity by 12% (97.6% vs. 85.7%). Among women with CIN2 at baseline, 56% regressed to normal, 24% regressed to CIN1, 4% remained CIN2, and 16% progressed to CIN3+.
Conclusions
Locales using HPV testing as the primary screening method, and lacking high-quality cytology-based screening, should consider viral load as an alternative to colposcopy triage for women over age 35. Viral load may also predict CIN2 progression until additional biomarkers become available.
Keywords: Cervical Intraepithelial Neoplasia (CIN), Hazard Ratios (HR), Human Papillomavirus (HPV) viral load, Regression, Progression
Introduction
Persistent infection with carcinogenic human papillomavirus (HPV) types is the cause of virtually all cervical cancer. HPV DNA tests are highly sensitivity (97.5%) for detection of cervical intraepithelial neoplasia grade 3 or worse (CIN3+). 1 Randomized controlled trials have demonstrated that HPV testing, followed by cytological triage of HPV-positive women, is a good choice as a primary screening method in older women. 2 However, high-quality cytologic screening is unavailable in many locales and highly sensitive primary HPV screening could lead to excessive colposcopy referral and unnecessary health expenditures. Therefore, there is a need to develop triage methods for HPV positive women that are appropriate for low resource settings.
Under current clinical guidelines recommended by WHO, 3 women with mild cervical intraepithelial neoplasia (CIN1) are to be monitored while those with CIN grade 2 or worse (CIN2+) lesions are usually referred for immediate therapy. However, up to 40–68% of CIN2 may spontaneously regress, 4,5 suggesting overtreatment of some women. Novel biomarkers are needed for further triage of these women, to reduce the burden of overtreatment on individual women and the healthcare system as a whole. A previous cross-sectional study examined HPV viral load as a possible triage candidate.6 However, evidence from prospective studies is needed to support these findings.
Therefore, our objectives were to evaluate HPV viral load as a biomarker for triage into colposcopy and CIN2 therapy, in order to reduce the colposcopy referral rate and CIN2 over treatment in low resource settings. We conducted a population-based cohort study in Xiangyuan County, Shanxi Province, China — an area with high cervical cancer mortality (52/100,000).7 The rate of cervical lesion regression and progression, and the risk for developing precancerous cervical lesions in association with HPV viral load and baseline histological status, were evaluated at a six-year follow-up assessment.
Materials and Methods
Baseline Study Procedures
In 1999, a cross-sectional comparative trial was carried out to evaluate multiple screening techniques for the detection of cervical neoplasia. 8,9 1997 non-pregnant women 35–45 years old, with no history of cervical screening or hysterectomy were enrolled in a cohort through the Women and Children’s Hospital in Xiangyuan County. After providing written informed consent, participants completed a standardized questionnaire regarding demographic information and risk factors. Participants also underwent a cervical examination consisting of cervical screening tests and a minimum of four biopsies. Cervical screening included vaginal swabs for a HPV-DNA testing, liquid-based cytology, visual inspection with acetic acid (VIA), and colposcopy. HPV infection was assessed using the Hybrid Capture 2 (HC2) assay (QIAGEN, Gaithersburg, Maryland). Abnormalities discovered via colposcopy were biopsied using a multiple quadrant protocol. If no abnormalities were observed in a given quadrant, a random biopsy was taken from that quadrant at either 2, 4, 8, or ten o’clock on the exocervix at the squamocolumnar junction (SCJ), as appropriate. Endocervical curettage (ECC) was also performed on each participant. Women with histologically confirmed CIN2+ lesions were offered immediate, affordable, standard therapy on a voluntary basis, specifically, the loop electrosurgical excision procedure, cone biopsy treatment or hysterectomy, according to local guidelines.
Follow-up Study Procedures
All women with baseline confirmed CIN1 or less and with baseline HPV and cytological results were invited to participate in a follow-up study in 2005 at the hospital where they were originally enrolled.9 Women with baseline confirmed CIN2+ were followed up as well, but only those without therapy during the past 6 years were included into this analysis. After obtaining written informed consent, we collected self-reported cervical treatment history since the baseline examination as well as updated sociodemographic and risk factor data. Women reporting cervical therapy nine months or more after baseline were administered a questionnaire gathering detailed treatment information including date, hospital, and type of therapy.
Screening tests included ThinPrep liquid-based cytology (Hologic, Marlborough, Massachusetts), HC2 HPV testing, VIA and colposcopy. Abnormalities found during colposcopy were biopsied at the lesion site. ECC was performed if the SCJ was not visualized. Cytological results were interpreted using the Bethesda classification system.10 Women were referred for a second colposcopy if they had either atypical squamous cells of undetermined significance (ASCUS) with HPV-positive results at the follow-up visit, or other worse cytological abnormalities. Cervical biopsies and treatment followed the same multiple quadrant protocol and therapy guidelines as at baseline.
Of the 1997 participants in the baseline study, 59 (2.9%) women were ineligible due to incomplete baseline testing data, 1831 (91.7%) women attended the follow-up study, and an additional 107 (5.4%) were lost to follow-up. Study personnel traced women lost to follow-up through home visits. General demographic information, reasons for nonparticipation, and history of cervical disease and related treatment were collected in brief interviews with the surviving women. For deceased subjects, the decedent’s close relatives or local doctors were interviewed for the cause and date of death and other important sociodemographic characteristics. Death certificates were not routinely completed in this area.
Institutional review board approval was obtained from the Cancer Institute/Hospital, Chinese Academy of Medical Sciences, the Cleveland Clinic, and the University of North Carolina.
Quality assurance procedures
Personnel were trained before the start of studies. Specimens were shipped to Cancer Institute, Chinese Academy of Medical Sciences (CICAMS) for blinded testing. Within two weeks after sampling, senior technicians at CICAMS assessed HPV viral load using the HC2 assay. Cervical biopsy slides were prepared and read by senior pathologists at CICAMS. Women reporting a history of treatment for suspicion of CIN or cancer during the past six years had their treatment records reviewed and the corresponding slides reread by CICAMS pathologists for further confirmation.9 Pathology was the gold standard endpoint measure, final classification was based on a combination of testing measures for women lacking pathological results.1
Statistical analysis
HPV viral load was measured using the ratio of relative light units to standard positive control (RLU/PC) as a surrogate. HPV viral load is typically divided into positive (≥1.0) and negative (<1) groups. Based on previous publications and the trisection cutoff of our positive viral load data in this study,6 we divided the positive group into three viral load subgroups for our analysis: low (≥1.0, <10.0), moderate (≥10.0, <100.0) and high (≥100.0).
Socio-demographic characteristics of participants were described by frequencies and proportions, stratified by baseline HPV viral load. One-way ANOVA and Pearson’s chi-square tests were used to compare continuous and categorical factors by viral load categories, respectively. Sensitivity, specificity, predictive values and likelihood ratios were calculated and compared among women with different HPV viral loads.
For women with CIN1 or normal histological status at baseline, we calculated the cumulative incidence of CIN2+. Using Cox proportional hazards regression, the adjusted Hazard Ratios (aHR) of CIN2+ and 95% confidence intervals (CI) were analyzed according to different combinations of HPV viral load at baseline and follow-up, adjusted for age, number of lifetime full-term births, and number of lifetime sexual partners. The association between categories of HPV viral load and the incidence of CIN2+ lesions among women with CIN1 or less was calculated using the Chi-square test for trend.
All statistical tests were two-sided and the level of significance was set at P=0.05. The 95%CI for rates and proportions were calculated using Fisher type exact confidence limits with Episheet (Version June 11, 2008, Ken Rothman). All other analyses were conducted using SPSS 15.0 (SPSS, Inc., Chicago, Illinois).
Results
Socio-demographic Characteristics
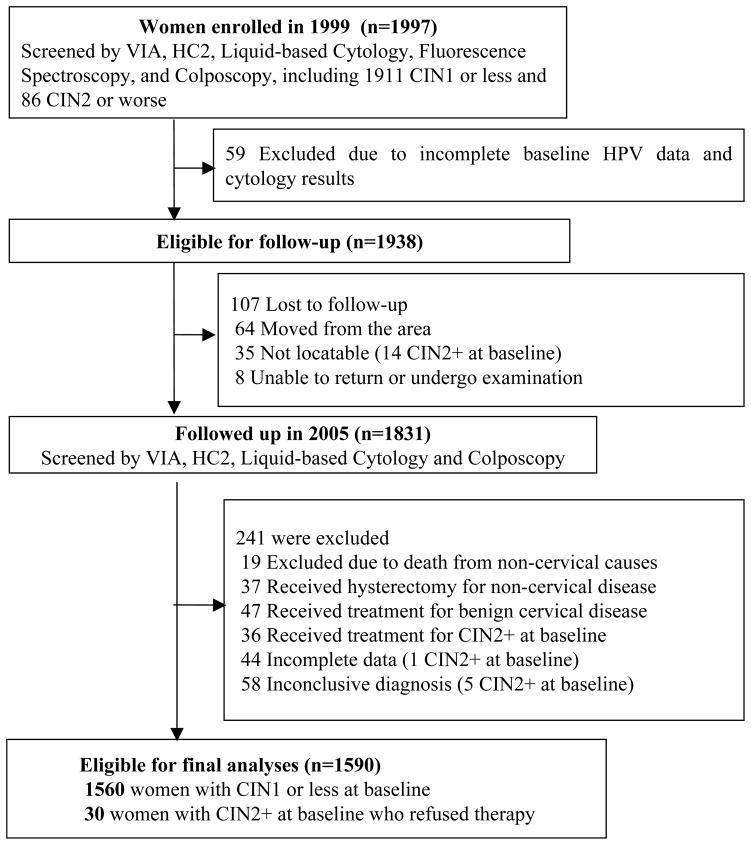
Of the total 1997 participants in the baseline study, 1911 were diagnosed with CIN1 or less and 86 were diagnosed with CIN2+. A total of 59 women were ineligible for follow-up due to incomplete baseline testing data, and an additional 107 were lost to follow-up. To evaluate the six-year outcomes of untreated cervical status, we excluded all women had any cervical therapy at baseline or during the past six years (n=120), women with incomplete data (n=44) or inconclusive diagnosis (n=58), and deaths from non-cervical causes (n=19) (Figure 1). Finally, a total of 1590 (80%) women were eligible for data analysis, including 1461 baseline normal women, 99 women with baseline CIN1, and 30 women with CIN2/CIN3 who refused therapy during the past six years.
Figure 1.
Study Population: Regression and Progression of Cervical Lesions of Different Human Papillomavirus Viral Load in Varied Histological stage: a six-year prospective study in Shanxi province, China, 1999–2005*.
Note: Unless specified, women lost to follow up belong to CIN1 or less group.
Most of the socio-demographic information were comparable between women who were included in the final analysis (n=1590) and those excluded (n=407) (P>0.05; data not shown). However, excluded women (n=407) had a higher rate of smoking and HPV infection, and a higher proportion of cytological abnormalities (P<0.001).
Among women included in the analysis, the mean age, age of menarche and age of sexual debut for all the participants was 39 years, 16 years, and 20 years, respectively (Table 1). Age of sexual debut differed marginally between HPV viral load subgroups (P=0.04). All women were married, and 39.7% of HPV-negative women reported two or more lifetime sexual partners compared to 52.5% among HPV-positive women (P=0.002). HPV negative women had less extramarital sex than HPV positive women (P<0.001). Most of the women were premenopausal, non-smokers and non-drinkers. All other baseline characteristics did not differ significantly by category of viral load (P>0.05).
Table 1.
Baseline characteristics of eligible women, stratified by baseline HPV viral load, Shanxi Province, China, 1999.
| Characteristic | HPV Negative N (%) n=1346 |
HPV Positive N (%)
|
P value† | |||
|---|---|---|---|---|---|---|
| Low Load n=82 |
Moderate Load n=66 |
High Load n=96 |
Subtotal HPV Pos N=244 |
|||
| Age (Mean±SD) | 39.1±3.2 | 39.3±3.3 | 39.6±3.3 | 39.0±3.0 | 39.2±3.2 | P>0.05 |
| Age of menarche (Mean±SD) | 16.0±1.7 | 16.0±1.7 | 16.2±1.5 | 16.2±1.7 | 16.1±1.7 | P>0.05 |
| Age of sex debut (Mean±SD) | 20.8±2.2 | 21.1±2.7 | 20.3±1.7 | 20.3±2.0 | 20.6±2.2 | P=0.04 |
| Married (Yes) | 1346(100.0) | 82(100.0) | 66(100.0) | 96(100.0) | 244(100.0) | P>0.05 |
| Junior High School Education and above (Yes) | 743 (55.2) | 48(58.5) | 32(48.5) | 56(58.3) | 136(55.7) | P>0.05 |
| Current smoker (No) | 1265(94.0) | 76(92.7) | 63(95.5) | 94(97.9) | 233(95.5) | P>0.05 |
| Alcohol consumption (No) | 1315(97.7) | 81(98.8) | 64(97.0) | 94(97.9) | 239(98.0) | P>0.05 |
| Family cancer history (Yes) | 387(28.8) | 21(25.6) | 13(19.7) | 26(27.1) | 60(24.6) | P>0.05 |
| Menopause (No) | 1326(98.5) | 81(98.8) | 65(98.5) | 94(97.9) | 240(98.4) | P>0.05 |
| Extramarital sexual relationships (Yes) | 501(37.2) | 44(53.7) | 32(48.5) | 50(52.1) | 126(51.6)* | P<0.001 |
| Lifetime no. of pregnancies | P>0.05 | |||||
| <4 | 956(71.0) | 58(70.7) | 46(69.7) | 63(65.6) | 167(68.4) | |
| ≥4 | 390(29.0) | 24(29.3) | 20(30.3) | 33(34.4) | 77(31.6) | |
| Lifetime no. of full-term births | P>0.05 | |||||
| <3 | 794(59.0) | 48(58.5) | 37(56.1) | 47(49.0) | 132(54.1) | |
| ≥3 | 552(41.0) | 34(41.5) | 29(43.9) | 49(51.0) | 112(45.9) | |
| Lifetime no. of sexual partners | P=0.002 | |||||
| 1 | 810(60.3) | 35(42.7) | 34(51.5) | 47(49.0) | 116(47.5)* | |
| ≥2 | 534(39.7) | 47(57.3) | 32(48.5) | 49(51.0) | 128(52.5) * | |
Comparison among four categories of HPV viral load.
Significant difference was found between HPV positive group and negative group, P<0.001
Validity of the Screening Test
Using moderate-to-high HPV viral load as the cut-point for colposcopy referral meant raising the RLU/PC cutoff from 1.0 to 10.0. Although the sensitivity for CIN2+ was reduced by 12% (97.6% vs. 85.7%), the specificity rose from 84.8% to 90.4%. Moreover, without loss of negative predictive value (NPV) (99.9% vs. 99.3%), the positive predictive value (PPV) also rose from 22.5% to 28.9%. The likelihood ratio (LR) was also improved, with the positive LR rising from 6.4 to 8.9, and negative LR reduced from 0.028 to 0.016. Furthermore, the referral rate was reduced from 18.1% to 12.9%.
Regression and Progression of Cervical Lesions
Baseline Normal or CIN1
Among the 1560 women with CIN1 or normal status at baseline, 19 incident cases of CIN2+ were recorded. This includes 13 cases detected through follow-up and six cases (one CIN2, three CIN3, and two invasive cervical cancers) confirmed by post-operation histological diagnosis between enrollment and follow-up. Women who were HPV-negative at baseline had a six-year CIN2+ cumulative incidence of 0.1% (Table 2). However, CIN2+ cumulative incidence increased to 7.9% for women who were HPV-positive baseline. We observed a dose response relationship, with cumulative incidence of CIN2+ lesions increasing in proportion to HPV viral load at baseline (negative: 0.1%, low: 1.3%, moderate: 11.1%, and high: 11.7%) (P-trend<0.001). The aHR also increased in association with viral load (P<0.001) (negative: 1, low: 10.0 (95%CI: 0.9–110.7), moderate: 75.1 (95%CI: 15.5–364.2) and high: 83.5 (95%CI: 18.0–387.5)). In general, women with CIN1 had a higher progressive rate than normal women (8.1% vs. 0.8%).
Table 2.
Cumulative incidence of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and risk for CIN2+ among normal women or women with CIN1 at baseline, stratified by HPV viral load at baseline.
| Group | Baseline HPV Viral Load | No. CIN2+/No. women | Cumulative Incidence of CIN2+ | aHR† | 95% CI |
|---|---|---|---|---|---|
| Normal n=1461 |
Negative | 2/1302 | 0.2% | 1 | - |
| Low | 1/68 | 1.5% | 11.0 | 1.0–123.1 | |
| Moderate | 5/49 | 10.2% | 66.3 | 12.6–347.3 | |
| High | 3/42 | 7.1% | 49.0 | 8.2–294.0 | |
| CIN1 n=99 |
Negative | 0/42 | - | - | - |
| Low | 0/8 | - | - | - | |
| Moderate | 2/14 | 14.3% | - | - | |
| High | 6/35 | 17.1% | - | - | |
| CIN1 or Normal n=1560 |
Negative | 2/1344 | 0.1% | 1 | - |
| Low | 1/76 | 1.3% | 10.0 | 0.9–110.7 | |
| Moderate | 7/63 | 11.1% | 75.1 | 15.5–364.2 | |
| High | 9/77 | 11.7% | 83.5 | 18.0–387.5 |
aHR=Hazard Ratio adjusted for age, number of full-term births and number of lifetime sexual partners.
Women with moderate or high viral load at baseline, but with negative or low viral load at follow-up, experienced a cumulative incidence 3.0% (95%CI: 0.6–8.8), with a 19-fold increased risk for CIN2+ compared to those negative at both visits (aHR=19.1, 95%CI: 3.2–114.5) (Table 3). Women with negative or low viral load at baseline, but having moderate or high viral load at follow-up, experienced a lower cumulative incidence 1.0% (95%CI: 0.03–5.7). However, women with moderate or high viral load at both time points experienced a 29.0% (95%CI: 14.5–51.8) cumulative incidence of CIN2+ and an aHR of 188.8 (95%CI: 41.2–864.1).
Table 3.
Cumulative incidence and risk of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in association with varied HPV viral load among normal women or women with CIN1 at baseline, stratified by combinations of HPV viral load at baseline and follow-up.
| HPV Viral Load | No. CIN2+/No. women | Cumulative Incidence of CIN2+ | aHR‡ | 95% CI |
|---|---|---|---|---|
| Repeat Negative | 2/1199 | 0.2% | 1 | - |
| Single/ Repeat Low load† | 0/124 | - | - | - |
| Single Moderate/ High load at baseline | 3/100 | 3.0% | 19.1 | 3.2–114.5 |
| Single Moderate/ High load at follow up | 1/97 | 1.0% | 6.8 | 0.6–74.8 |
| Repeat Moderate/ High load† | 11/38 | 29.0% | 188.8 | 41.2–864.1 |
Viral load categories represent the maximum measured viral load for a given woman for either time point.
aHR=Hazard Ratio adjusted for age, number of full-term births and number of lifetime sexual partners.
Baseline CIN2+
In a sub-analysis of the women with CIN2 at baseline (n=25), the percentage of women who regressed to normal, regressed to CIN1, remained CIN2, and progressed to CIN3 was 56.0% (14/25), 24.0% (6/25), 4.0% (1/25), and 16.0% (4/25), respectively. None progressed to cancer. Among women with CIN2 and repeat moderate or high HPV viral load (n=9), one woman remained CIN2, four regressed to CIN1, and four progressed to CIN3. Among those with repeat high viral load, the progressive rate of CIN3 increased to 80.0% (4/5). Among the five women with baseline CIN3, two regressed and three remained as CIN3. None progressed to cancer.
Discussion
Over the course of six years, the cumulative incidence of CIN2+ and the hazard ratio for CIN2+, among women with CIN1 or less at baseline, increased with increasing HPV viral load. Compared to women with repeat negative HPV test results, women with repeat moderate to high HPV viral loads had nearly a 190-fold increased risk for CIN2+. Over the same time period, 56% of women with CIN2 regressed to normal, 24% regressed to CIN1, 4% remained CIN2, and 16% progressed to CIN3.
Though persistent HPV infection is considered a necessary risk factor for CIN2+ lesion development, the use of HPV viral load as a short-term predictor for progression to cervical precancerous lesions has been controversial.11 Some studies have reported that a single measurement of HPV copy number does not reliably predict the risk of developing cervical neoplasia.12–14 However, others report that HPV viral load is a potentially valuable clinical predictor for development of CIN2+ lesions.15,16 Differences may be due to HPV testing methods; negative studies have tended to use real-time PCR as opposed HC2. However since HC2 is used in clinical settings worldwide, predictive implications based on the HC2 viral load surrogate (RLU/PC) are more relevant to clinical practice.
Shi’s 2009 analysis of this dataset called attention to the importance of repeat positive HPV results as a predictor of cervical precancerous lesion development.9 However, the role of HPV viral load in the development of these lesions was not considered. Our viral load results cast light on this issue. Based on our data, among women with repeat positive HPV results, the true high-risk individuals are those with moderate to high viral load, especially those with repeat moderate to high viral load. Due to the small number of cases, we reported a relatively large loss in sensitivity for detecting CIN2+ compared with prior studies.17 However, we also provided evidence that, for a single HPV test, using moderate to high viral load (>10.0 RLU/PC) as the triage cut-off greatly improved the PPV and specificity, and reduced the referral rate for colposcopy.
A comparable long-term Swedish study reported 54% of CIN2 regressing to normal, 16% having persistent dysplasia, and 30% progressing to CIN3+.18 Though the proportion regressing to normal was nearly identical to that in our study (54% vs. 56%), we found a substantially smaller percentage of progression to CIN3+ (16% vs. 30%). Our results also differed from another long-term Nordic study, which reported 53% regression, 24% persistence, and 21% progression among women with baseline CIN2.19 More recent studies reported 28% of CIN 2/3 lesions regressing to CIN1 or less over 15 weeks and 68% of CIN2 lesions regressing completely (negative cytology and biopsy) by three years.5,20 There are multiple possible reasons for these different rates. First, due to our limited sample, our estimates may be subject to significant random variation. Second, there are almost certainly important differences between the Western women and Chinese women including, but not limited to, genetics, nutritional status, age, and HPV type distribution of participants. Third, length of follow-up was not consistent and limits comparability. Finally, with regard to pathology, poor reliability in CIN2 diagnosis may also contribute to the discrepancies between studies. 21
Given the high probability of transient HPV infection and over-diagnosis of CIN2, which may spontaneously regress among women younger than 35 years, HPV-based screening has not been recommended as a primary screening method below a certain age.22 Furthermore, given that half of women with CIN2 regressed to normal over the course of six years, new biomarkers are crucial for triage of patients at the clinical threshold of therapy. Previous studies report that HPV16, HPV18, p16INK4a, Ki67, p53 and high methylation levels of specific sites in the HPV L1 gene could serve as potential biomarkers for progression to high-grade CIN lesions and cervical cancer.23,24 However, in the absence of consensus regarding definitive new predictive biomarkers, our study shows that moderate to high HPV viral load can act as an alternative predictor for progression of high-grade CIN among women over age 35 years. Furthermore, as HPV viral load could be read simultaneously with the HC2 result, this triage method is especially well-suited for areas using HC2 as the primary screening method and lacking financial support for additional biomarker testing.
Our study was the first Chinese prospective study investigating the natural history of different CIN lesions associated with varied HPV viral load. A key strength of our study is the rigorous assessment of each woman’s baseline pathological status. In 1999, no gold standard screening method for cervical cancer was identified and Xiangyuan County was a high-risk area with a high mortality of cervical cancer. For the purpose of evaluation of different screening methods, each woman had her pathological status determined by biopsies and ECC. A second strength was the ability to study the natural progressive and regressive rates of CIN2+, which is not readily replicable. Though current clinical guidelines recommend that women with CIN2+ lesions should be referred for immediate therapy,3 our study was conducted in a rural county in 1999 where such therapy was provided on a voluntary basis and were not readily acceptable for women without visible lesions or obvious pain. Third, the pathological slides of the baseline and follow-up study were read by the same pathological panel, which made the results more comparable and reliable.
Our study also has significant limitations. First, our conclusions are directly applicable to women 35–45 years old living in a high incidence areas and do not speak to the situation of younger women.Second, although a positive trend was found between the aHR for CIN2+ and HPV viral load subgroups, the confidence intervals overlapped among different HPV viral load subgroups due to the small sample size of endpoints. Third, women who refused treatment at baseline may have had smaller lesions than those who received therapy, thus we may have overestimated the regression rate. We also used CIN2 other than the more reliable CIN3 diagnosis as the study endpoint due to the small number of cases.25 Additionally, since we only tested women at the beginning and end of the six-year period, we were unable to capture incident CIN2+ lesions that spontaneously regressed between visits. Similarly, we were unable to determine if women with repeat positive HPV test results had persistent infection or were re-infected subsequent to clearing the infection. Finally, we lacked genotyping data which have allowed us to examine HPV type specific differences in rates of progression and regression.
In conclusion, based on our data, women with a negative baseline HPV test could be protected for at least six years.9 In contrast, women with moderate to high viral load, especially those with repeat high viral load, had extremely high risk for developing CIN2+ lesions. To reduce CIN2 overtreatment, novel biomarkers are needed for triage. Locales using HC2 HPV testing as the primary screening method, and lacking high-quality cytology-based screening systems, should consider viral load as an alternative method to triage women over age 35 years for colposcopy. Viral load may also serve as a predictor for CIN2 progression until additional biomarkers become available. Long-term studies with larger sample sizes and broader age distributions are needed to validate our findings.
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (China MOE #20030023014), the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University [R24 TW007988 to S-M.W and D.V.C.], the National Cancer Institute [R25 CA94880 to D.V.C.], and the American Relief and Recovery Act. Dr. Gwen Zahner is gratefully acknowledged for reviewing this manuscript. Qiagen Corporation is also gratefully acknowledged for the donation of HC2 kits. We also extend our gratitude to the women who participated in this study, as well as our collaborators at the Xiangyuan Women and Children’s Hospital and our colleagues at the Cancer Institute of the Chinese Academy of Medical Sciences.
Abbreviations
- aHR
age-adjusted hazard ratios
- ASC-H
atypical squamous cells: cannot exclude HSIL
- ASCUS
atypical squamous cells of undetermined significance
- CI
confidence intervals
- CICAMS
Cancer Institute, Chinese Academy of Medical Sciences
- CIN
cervical intraepithelial neoplasia
- CIN2+
CIN grade 2 or worse
- CIN3+
CIN grade 3 or worse
- ECC
endocervical curettage
- HC2
Hybrid Capture 2
- HPV
human papillomavirus
- HSIL
high-grade squamous intraepithelial lesions
- LSIL
low-grade squamous intraepithelial lesions
- RLU/PC
the ratio of relative light units to standard positive control
- SCJ
squamocolumnar junction
- VIA
visual inspection with acetic acid
Footnotes
Disclosure Statement
All authors declare that they have no conflicts of interest.
Contributor Information
Shao-Ming WANG, Department of Cancer Epidemiology, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Danny COLOMBARA, 1Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China 2Department of Epidemiology, School of Public Health, University of Washington, 1959 Northeast Pacific Street, Seattle, Washington 98195, USA.
Ju-Fang SHI, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Fang-Hui ZHAO, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Jing LI, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Feng CHEN, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Wen CHEN, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Shu-Min LI, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Xun ZHANG, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Qin-Jing PAN, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
Jerome L. BELINSON, Women’s Health Institute, Cleveland Clinic, 9500 Euclid Avenue Cleveland, Ohio 44195, USA
Jennifer S. SMITH, Gillings School of Global Public Health, University of North Carolina, 135 Dauer Drive, Chapel Hill, North Carolina 27599, USA
You-Lin QIAO, Cancer Institute/Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Panjiayuan Lane, Beijing 100021, China
References
- 1.Zhao F-H, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet oncol. 2010;11:1160–71. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbyn M, de Sanjosé S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J cancer. 2012;131:1969–82. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. A Guide to Essential Practice. Geneva: World Health Organization; 2006. Comprehensive Cervical Cancer Control. [PubMed] [Google Scholar]
- 4.Ovestad IT, Gudlaugsson E, Skaland I, et al. The impact of epithelial biomarkers, local immune response and human papillomavirus genotype in the regression of cervical intraepithelial neoplasia grades 2–3. Journal of clinical pathology. 2011;64:303–7. doi: 10.1136/jcp.2010.083626. [DOI] [PubMed] [Google Scholar]
- 5.Moscicki A-B, Ma Y, Wibbelsman C, et al. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstetrics and gynecology. 2010;116:1373–80. doi: 10.1097/AOG.0b013e3181fe777f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao F-H, Hu S-Y, Wang S-M, et al. Association between high-risk human papillomavirus DNA load and different histological grades of cervical neoplasia [article in Chinese] Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2009;43:565–70. [PubMed] [Google Scholar]
- 7.Li L, Lu F, Zhang S, et al. Cancer mortality trends in the People’s Republic of China: 1973–1992. Chinese J Oncol. 1997;19:3–9. [Google Scholar]
- 8.Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecologic oncology. 2001;83:439–44. doi: 10.1006/gyno.2001.6370. [DOI] [PubMed] [Google Scholar]
- 9.Shi J-F, Belinson JL, Zhao F-H, et al. Human papillomavirus testing for cervical cancer screening: results from a 6-year prospective study in rural China. Am J Epidemiol. 2009;170:708–16. doi: 10.1093/aje/kwp188. [DOI] [PubMed] [Google Scholar]
- 10.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 11.Gravitt PE, Kovacic MB, Herrero R, et al. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J cancer. 2007;121:2787–93. doi: 10.1002/ijc.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constandinou-Williams C, Collins SI, Roberts S, et al. Is human papillomavirus viral load a clinically useful predictive marker? A longitudinal study. Cancer Epidemiol Biomarkers Prev. 2010;19:832–7. doi: 10.1158/1055-9965.EPI-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi LF, Koutsky La, Castle PE, et al. Human papillomavirus type 18 DNA load and 2-year cumulative diagnoses of cervical intraepithelial neoplasia grades 2–3. J Natl Cancer Inst. 2009;101:153–61. doi: 10.1093/jnci/djn461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesselink AT, Berkhof J, Heideman DaM, et al. High-risk human papillomavirus DNA load in a population-based cervical screening cohort in relation to the detection of high-grade cervical intraepithelial neoplasia and cervical cancer. Int J cancer. 2009;124:381–6. doi: 10.1002/ijc.23940. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Chen Y, Li L, et al. Associations of high-risk HPV types and viral load with cervical cancer in China. Journal of clinical virology. 2006;35:264–9. doi: 10.1016/j.jcv.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Dalstein V, Riethmuller D, Prétet J-L, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 17.Rebolj M, Bonde J, Njor SH, et al. Human papillomavirus testing in primary cervical screening and the cut-off level for hybrid capture 2 tests: systematic review. BMJ. 2011;342:d2757–d2757. doi: 10.1136/bmj.d2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasiell K, Nasiell M, Vaćlavinková V. Behavior of moderate cervical dysplasia during long-term follow-up. Obstetrics and gynecology. 1983;61:609–14. [PubMed] [Google Scholar]
- 19.Syrjänen K, Kataja V, Yliskoski M, et al. Natural history of cervical human papillomavirus lesions does not substantiate the biologic relevance of the Bethesda System. Obstetrics and gynecology. 1992;79:675–82. [PubMed] [Google Scholar]
- 20.Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clinical cancer research. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carreon JD, Sherman ME, Guillén D, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007;26:441–6. doi: 10.1097/pgp.0b013e31805152ab. [DOI] [PubMed] [Google Scholar]
- 22.Ronco G, Giorgi-rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–57. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 23.Sun C, Reimers LL, Burk RD. Gynecologic Oncology Methylation of HPV16 genome CpG sites is associated with cervix precancer and cancer. Gynecologic Oncology. 2011;121:59–63. doi: 10.1016/j.ygyno.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pino M, Landolfi S, Torne A, et al. p16 INK4a Immunostaining Identifies Occult CIN Lesions in HPV-positive Women. Int J Gynecol Pathol. 2008;28:90–7. doi: 10.1097/PGP.0b013e31817e9ac5. [DOI] [PubMed] [Google Scholar]
- 25.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16:205–42. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]