Abstract
Background
Analysis of the distribution of reaction times (RTs) in behavioral tasks can illustrate differences attributable to changes in attention, even when no change in mean RT is observed. Detrimental attentional effects of both acute and chronic exposure to alcohol may therefore be revealed by fitting RT data to an ex-Gaussian probability density function which identifies the proportion of long-RT responses.
Methods
Adolescent male rhesus macaques completed a 5-choice serial reaction time task (5CSRT) after acute alcohol consumption (up to 0.0, 1.0 and 1.5 g/kg). Monkeys were next divided into chronic alcohol (N=5) and control groups (N=5); the experimental group consumed 1.5–3.0 g/kg alcohol for 200 drinking sessions. Unintoxicated performance in the 5CSRT task was determined systematically across the study period and the effect of acute alcohol was redetermined after the 180th drinking session. The effect of extended abstinence from chronic alcohol was determined across 90 days.
Results
Acute alcohol exposure dose-dependently reduced the probability of longer RT responses without changing the mean or the standard deviation of the RT distribution. The RT distribution of control monkeys tightened across 10 months whereas that of the chronic alcohol group was unchanged. Discontinuation from chronic alcohol increased the probability of long RT responses with a difference from control animals observed after 30 days of discontinuation.
Conclusions
Alcohol consumption selectively affected attention as reflected in the probability of long RT responses. Acute alcohol consumption focused attention, chronic alcohol consumption impaired the maturation of attention across the study period and alcohol discontinuation impaired attention.
1. Introduction
Acute alcohol consumption typically increases response times in humans at relatively high (Baylor et al. 1989; Cameron et al. 2001; King 1975; Krull et al. 1992; Lubin 1977) and low doses (Friedman et al. 2011; Schweizer et al. 2006). In such tasks there is often evidence that these changes in response time may be more attributable to cognitive impairment than motor impairment (Breitmeier et al. 2007; Hernández et al. 2006; Hernández et al. 2010; Hernández et al. 2007). Likewise, functional magnetic resonance imaging experiments with humans indicate that alcohol doses that increase response times also reduce activity in brain areas known to regulate attention, error detection and executive function (Anderson et al. 2011; Marinkovic et al. 2012). Inhibitory effects of alcohol on response time are most commonly observed on the ascending limb of the blood-alcohol concentration (BAC) curve and acute tolerance is thought to restore response times to pre-exposure levels during the descending limb of the BAC curve (Schweizer and Vogel-Sprott 2008). Similar to observations with humans, moderate and large doses of alcohol have been shown to slow response time in monkeys (Moody et al. 1980)
Despite evidence that alcohol increases response times under many conditions, there is also evidence that it can produce activating effects in some instances. Acute alcohol has been shown to decrease response times in humans performing choice reaction time tasks (McManus et al. 1983; Tiplady et al. 2001), moderate to low doses (0.35–2.8 μmol i.c.v.; 0.5 g/kg i.p.; 0.6–1.0 i.g.) of alcohol have been shown to increase operant response rates (Arizzi et al. 2003) and to stimulate locomotor behavior in rats (Pohorecky et al. 1989; Stodulka 1991); similar locomotor effects have been shown in mice (Kamens and Phillips 2008; Larsson A 2002). Taken together, these data do not support the “global-slowing” effect of alcohol advanced by some (Maylor and Rabbitt 1993). Instead, the existing data support the conclusion that the behavioral pharmacology of alcohol is complex, task-specific and that results can vary as methodology changes (Ryan et al. 1996).
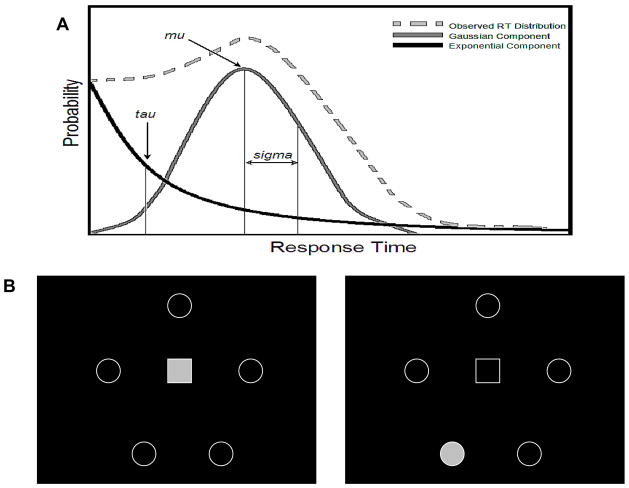
While alcohol can produce both stimulant and depressant effects (Lewis and June 1990), there are only a few examples of alcohol decreasing response times. The shape of the typical response time distribution may, however, obscure the activating effects of alcohol since response times are rarely distributed normally and are typically positively skewed (Heathcote et al. 1991). The unimodal shape of response time distributions are characterized by a large proportion of short response times, a mean value close to the lower limit of observed response times and a long right-handed tail that contains a few comparatively long response times (Lacouture and Cousineau 2008), see Figure 1A. When mean response times are near the lower limit, any further reduction in those already low values can be difficult to detect.
Figure 1.
(A) Illustration of response time probability density function (PDF). Response time PDF can be deconvolved to render estimates of mu (mean of Gaussian component), sigma (standard error of Gaussian component) and tau (mean of exponentially-distributed component). (B) Illustration of stimuli used in 5-choice serial reaction time task (5CSRT). Each 5CSRT trial began with an observing response on a centrally-located stimulus. To be successful, each observing response had to be maintained until a target stimulus was presented one of 5 possible target locations. Response time was defined as the latency to respond after the appearance of the target stimulus.
Changes in response time performance are also difficult to confirm with common parametric techniques, largely because the data sets do not conform to the assumptions of those analyses (Heathcote et al. 1991; Lacouture and Cousineau 2008; Matzke and Wagenmakers 2009). One approach to dealing with data sets that are not distributed normally is to analyze the logarithmic transform of those data. Unfortunately, this technique also ignores important details about response time performance, such as the frequency with which extreme response times are emitted (Heathcote et al. 1991; Van Zandt 2000). As an alternative analysis, fitting RT data sets to an ex-Gaussian probability density function (PDF) can produce important insight into the behavioral pharmacology of alcohol. Fitting response time data to an ex-Gaussian PDF allows normally-distributed (Gaussian) components to be deconvolved from exponentially-distributed components (Luce 1986). This analysis renders the mean value of Gaussian component (sigma), the standard deviation of the Gaussian component (mu) and the mean of the exponential component (tau). By separating response time distributions into these constituent components, additional information about response time performance can be determined (Heathcote et al. 1991). In particular, changes in the probability of unusually long response times, can be detected by changes in tau.(Luce 1986) These analytical techniques have recently been applied to show that acute exposure to a moderate dose of alcohol (i.v.) could increase the probability of long response times in adult monkeys (Jedema et al. 2011). The present study sought to determine effects of acute and chronic alcohol exposure on the RT performance of adolescent monkeys using a more naturalistic oral consumption model (Katner et al. 2004). As we have previously discussed, alcohol is a commonly used recreational drug (Crean et al. 2011; Katner et al. 2007; Wright et al. 2012) with high potential to cause acute and lasting cognitive impairment in addition to other consequences (Crean et al. 2011; Marcondes et al. 2008; Taffe et al. 2010). Animal models are necessary to determine the specific effects of controlled alcohol exposure and to distinguish affected from spared cognitive domains. In this work the focus was on attentional properties indexed by skew in the RT distribution.
2. Material and Methods
2.1 Subjects
These experiments were conducted on adolescent male rhesus macaques (Macaca mulatta, Primate Products, Inc, Immokalee, FL, USA). At the onset of these studies, the median age of the monkeys was 48 months (range = 39 to 50 months) and 70% of the monkeys were born within 60 days of each other. The mean weight was 6.5 kg (range = 5.4 – 7.4 kg). Previous work in this lab indicates the rate of bodyweight gains begins to increase at around 32 months of age for male rhesus macaques. Likewise, experience in this lab indicates that male rhesus macaques do not reach stable mature weight of 12–16 kg until about 8–9 years of age. These observations are consistent with an increase in plasma testosterone observed in intact male monkeys across the 36–48 month interval (Rose et al. 1978) and observation of brain growth tapering off at about 40–50 months of age (Knickmeyer 2010). Thus, the age range of the monkeys used in these experiments is consistent with a start in the immediate peri-pubertal time point and then stretching into late adolescence, similar to the high school population of humans.
Monkeys were maintained on a diet of standard nonhuman primate chow (Harlan Teklad 15% Monkey Diet #8714, Harlan Laboratories Inc., Madison, WI USA). Each monkey was fed approximately 37 grams of chow per kilogram bodyweight. Monkey chow was supplemented with fresh fruit or vegetables and a multi-vitamin tablet (Kirkland Signature Sugar-free Children’s Chewable Vitamins, Seattle WA USA) each day. Monkeys were fed approximately 20% of their daily chow at least 1 hour before the morning testing sessions. The balance of their daily ration was divided across two subsequent feeding sessions. Water was available ad libitum. All of the monkeys were single-housed in a common colony room.
2.2 Testing environment
The colony room was maintained between 22O C and 25O C on a 12-hour light cycle (lights on at 6:00 am). Ethanol consumption and behavioral testing took place in the home cages between 9:00 am and 3:00 pm. These experiments followed guidelines adopted by the US National Institutes of Health (Clark et al. 1997) and took place in an AALAC-approved facility. The experimental protocol was approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
2.3 Apparatus
Monkeys responded to visual stimuli presented on touch-sensitive LCD panels housed in stainless steel consoles. The testing system was controlled by Monkey CANTAB computer software (Cambridge Neurological Test Automated Battery, Lafayette Instruments, Lafayette, IN, USA). The touch-sensitive LCD panels measured approximately 23 cm X 30 cm (~38 cm diagonally) and were positioned directly in front of the home cages. The home cages featured five strategically placed access ports that allowed the monkeys access to the touch-sensitive LCD panels and the collection cup into which rewards were dispensed.
2.4 Preparation of Alcohol Solutions
Alcohol (4% v/v) was added to a 6% (w/v) fruit-flavored solution (e.g, Tang ®, Kool-Aid, Country Time Lemonade; Kraft Foods, Glenville, IL, USA) to facilitate robust and consistent patterns of ethanol consumption. It has been demonstrated that adding alcohol to a fruit-flavored solution produces controlled and behaviorally relevant levels of alcohol consumption in rhesus macaques (Katner et al. 2004; Katner et al. 2007).
2.5 Chronic Alcohol Consumption
Monkeys in the experimental group (n = 5) were allowed to consume alcohol 5 days each week for approximately 10 months (200 drinking sessions). A separate group of monkeys (n=5) were allowed to consume only the fruit-flavored solution during the same period. Drinking sessions were conducted after daily training/testing sessions. Afternoon feeding sessions were scheduled at least 1 hour after the completion of the drinking sessions to facilitate uniform alcohol absorption. Maximum daily alcohol doses were capped at 1.5 g/kg until the 99th drinking session, when they were increased to 3.0 g/kg over 6 consecutive sessions. Maximum dose was achieved by varying drink volume while holding concentration steady. At the end of each drinking session, the dose of ethanol consumed by each monkey, the rate of ethanol consumption and the mean dose of ethanol consumed by the group were calculated.
2.6 5-Choice Serial Reaction Time Task (5CSRT)
Each trial began when monkeys emitted an observing response on a centrally-located visual stimulus (Image 1B). Monkeys were required to maintain the observing response continuously for between 0.75 and 2.5 seconds. The duration of the observing response varied pseudo-randomly within each session. If the monkey failed to maintain the observing response for the required duration, the trial was terminated. The trial was also terminated if the monkey failed to emit an observing response within 30 seconds.
When the required duration of the observing response elapsed, a single target stimulus appeared in 1 of 5 possible locations (Image 1B). The target stimulus remained illuminated for 2 s. Monkeys had up to 10 s to emit a target response before the trial ended. Correct target responses were reinforced with two 190 mg fruit-flavored nonhuman primate tablets (Product #5TUR, TestDiet, Richmond, IN USA). Each 5CSRT session consisted of 60 trials. Only monkeys that had satisfied the performance criterion in the terminal training phase were included in the alcohol trials.
Effects of acute alcohol on performance in the 5CSRT task were determined in monkeys (N=8) prior to the initiation of chronic (Experiment 1) and during the course of chronic alcohol (N=5) or vehicle (N=5) consumption (Experiment 3). Effects of chronic alcohol consumption on baseline performance in the 5CSRT task were determined in Experiment 3. The effect of the discontinuation chronic of alcohol consumption on (untreated) performance in the 5CSRT task was determined in Experiment 4.
In Experiments 1 and 3, all of the monkeys were given up to 30 minutes to drink the fruit-flavored alcohol solution (0 g/kg, up to 0.5 g/kg and up to 1.5 g/kg). The dose order was randomized (Latin squares method) and each monkey was presented with each dose. The 5CSRT task began 30 minutes after the end of the drinking session or 30 minutes after finishing the solution, whichever came first. In Experiment 2, performance in the 5CSRT task was determined 22 hours after the prior alcohol consumption. Experiment 4 began on the first day after chronic alcohol consumption was discontinued and examined animals after 30 and 90 days of discontinuation.
2.7 Statistical Analyses
Individual response time data from each 5CSRT session were fit to ex-Gaussian probability density functions as described in Lacouture & Cosineau (2008) using MATLAB (ver. R2012a, MathWorks, Inc., Natick, MA USA). This analysis allowed the Gaussian and exponential probability density functions to be deconvolved. The resulting measures (i.e., mu, sigma, and tau) were analyzed by one-way (Experiment 1) or two-way (Experiments 2, 3 and 4) repeated-measures analysis of variance (SigmaStat, ver. 3.5, Systat Software, Inc, Richmond, CA USA). Pre-planned comparisons between groups were used initially to determine effects of protracted withdrawal in the 1–30 day interval after discontinuing chronic ethanol. Post-hoc analyses were conducted using the Holm-Sidak method with all possible pairwise comparisons. The criterion for significance was p < 0.05 for all tests
3. Results
3.1 Alcohol Consumption
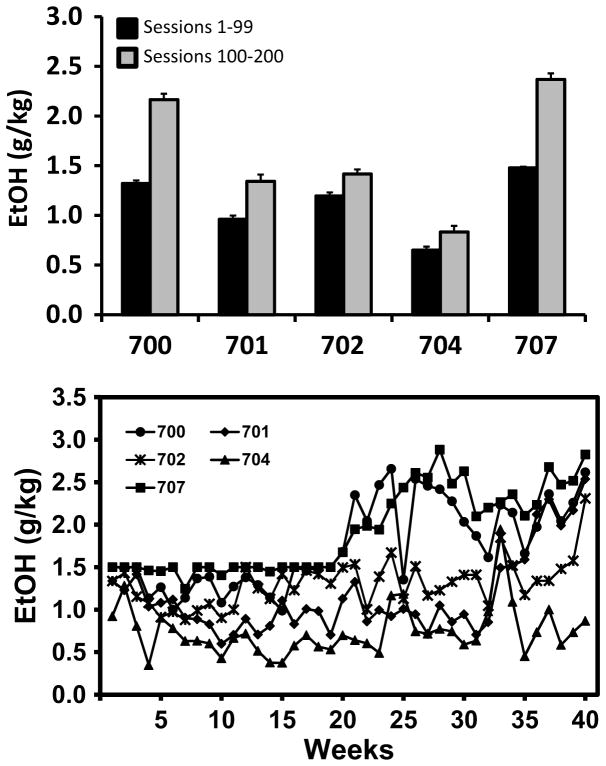
As reported previously (Wright et al. 2012), mean daily consumption across all drinking sessions was 1.38 g/kg (+/− 0.02 SEM). When tested during the 7th month of chronic alcohol consumption, a 1.58 g/kg (+/− 0.32 SEM) mean dose of alcohol produced mean blood-alcohol levels of 89.70 mg% (+/− 16.60 SEM) for these monkeys. Mean daily consumption for the entire interval ranged from 0.74 g/kg (+/− 0.04 SEM) to 1.93 g/kg (+/− 0.04 SEM) for the 5 monkeys in the experimental group; means for the intervals in which the daily limit was 1.5 (Sessions 1–99) and then raised (over 6 sessions) to 3.0 (Sessions 100–200) are depicted in Figure 2. The weekly average intakes are also shown to illustrate the stability of individual preferences and the inter-individual preference rankings, similar to what has been previously shown with this model (Katner et al. 2004). The volume of vehicle presented to each monkey in the control group was yoked to a corresponding member of the experimental group to control differences in caloric intake. When tested during the 7th month of chronic vehicle consumption, a 1.41 g/kg (+/− 0.46 SEM) mean dose of alcohol produced mean blood-alcohol levels of 81.94 mg% (+/− 22.36 SEM) for the monkeys in the control group.
Figure 2.
Mean daily dose of alcohol (+ SEM) for each monkey in the chronic alcohol group, separated by the intervals in which the daily limit was 1.5 (Sessions 1–99) and 3.0 (Sessions 100–200) g/kg. The lower panel depicts the mean weekly intake for each individual. Control monkeys drank flavored, calorie-matched solutions each day across same period.
3.2 Response time distributions
As is commonly the case, virtually all response time distributions were heavily skewed to the right; skewness values were between 4.59 (95% CI = 4.34–4.84) and 7.38 (95% CI = 7.38–7.62) after acute alcohol consumption.
3.2.1 Experiment 1 - Response time distribution after acute alcohol consumption
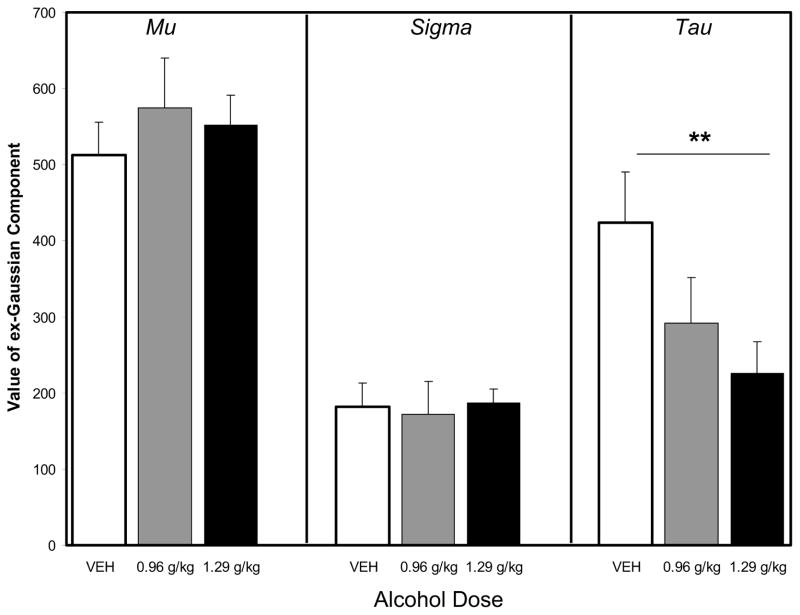
Acute alcohol consumption reduced the probability for unusually long response times, but did not alter mean response times. A one-way repeated measures ANOVA confirmed a statistically significant effect of alcohol dose on the mean of the exponential probability density function (tau), F2,14 = 6.705, p < 0.01 (Figure 3). Subsequent post-hoc tests (Holm-Sidak method, all pairwise multiple comparisons) confirmed that tau was reliably lower after consumption of a 1.29 g/kg dose of alcohol than under control conditions, p < 0.01. tau was also numerically lower after consumption of a 0.96 g/kg dose of alcohol than under control conditions, though the difference was not reliable. Separate one-way repeated measures ANOVAs failed to confirm a significant effect of acute alcohol consumption on either the mean (mu) or the standard deviation (sigma) of the Gaussian probability density function (Figure 3).
Figure 3.
The effect of acute alcohol consumption on response time distribution. Adolescent monkeys with minimal prior exposure to alcohol (n=8) were provided the opportunity to voluntarily consume the vehicle or up to 1.0 and 1.5 g/kg in a repeated measures design. Alcohol selectively and dose-dependently reduced RT skew (tau), but not the mean (Mu) or variance (Sigma). Post-hoc analysis confirmed that a mean 1.29 g/kg alcohol dose reduced tau as compared to vehicle conditions, p<0.01 (indicated by **). Error bars represent SEM.
3.2.2 Experiment 2 – The effect of chronic alcohol consumption on baseline response time distribution
The response time distribution was not altered during the course of chronic alcohol consumption these experiments. A two-way repeated measures ANOVA failed to confirm a significant effect of chronic alcohol consumption group on tau, mu or sigma (data not shown).
3.2.3 Experiment 3 – The effect of acute alcohol on reaction time distribution during chronic alcohol consumption
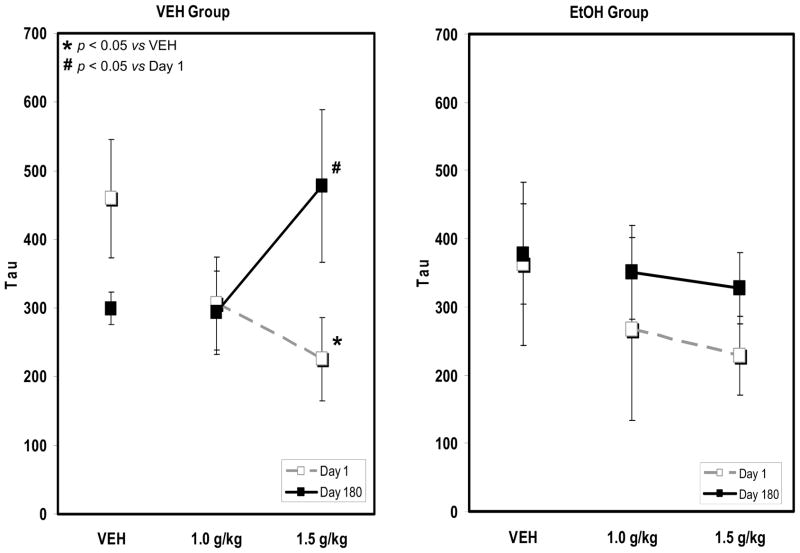
Acute alcohol consumption affected the probability of unusually long response times differently in each group when assessed after 180 days of the chronic drinking manipulation. A two-way repeated measures ANOVA confirmed a significant interaction of alcohol dose and day (i.e., Day 1 vs Day 180) on tau for the control group, F4,16 = 3.797, p = 0.023 (Figure 4). Subsequent post-hoc tests (Holm-Sidak method, all pairwise multiple comparisons) confirmed that, on the first test day, tau was reliably lower after consumption of the highest dose of alcohol than after consumption of the vehicle, p = 0.010. After 180 vehicle consumption sessions, the highest dose of alcohol reliably elevated tau above what was observed on the first test day, p < 0.05.
Figure 4.
The effect of alcohol on tau before and after chronic alcohol consumption. There was a reliable interaction of dose and day on tau in the control group (n=5). Post-hoc tests confirmed that a mean alcohol dose near 1.5 g/kg reliably reduced tau from vehicle on day 1 (p<0.05, indicated by *). At day 180, alcohol increased tau above the value observed on day 1 (p<0.05, indicated by #). In monkeys that consumed alcohol chronically (n=5), however, the effect of alcohol on tau did not change reliably across the study period. Error bars represent SEM.
A separate two-way repeated measures ANOVAs also confirmed a main effect of day (i.e., Day 1 vs Day 180) on sigma in the control group, F1,7 = 22.565, p = 0.007. Subsequent post-hoc tests (Holm-Sidak method, all pairwise multiple comparisons) confirmed that, after 180 vehicle consumption sessions, sigma was reliably lower under baseline conditions (172.2 +/− 39.2 SEM vs. 106.9 +/− 21.8 SEM) and after the highest dose of alcohol.(201.2 +/− 17.4 SEM vs. 108.4 +/− 36.0 SEM). There was no evidence that mu changed reliably over the course of the study
In contrast, a two-way repeated measures ANOVA failed to confirm that alcohol reliably altered tau in the group of monkeys that chronically consumed alcohol at any point during these trials (Figure 4). Likewise, separate two-way repeated measures ANOVAs failed to confirm an effect of either alcohol dose or duration of exposure on mu or sigma (data not shown).
3.2.4 Experiment 4 – Changes in response time distribution after the discontinuation chronic of alcohol consumption
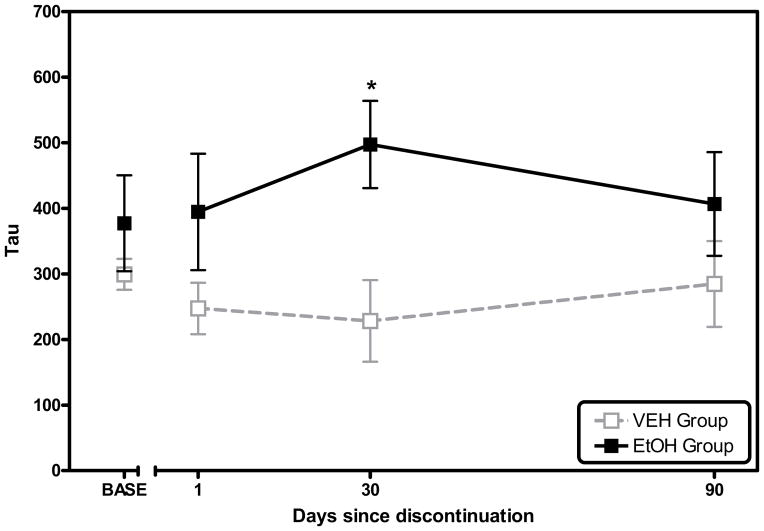
The group of monkeys with a history of chronic alcohol consumption exhibited an increased probability for unusually long response times during a period of extended abstinence. The initial planned comparison between groups for days 1 and 30 post-alcohol confirmed a difference at Day 30. The followup two-way repeated measures ANOVA including the baseline and Days 1–90 of discontinuation further confirmed a significant main effect of chronic alcohol consumption on tau when tested during extended abstinence, F1,8 = 7.107, p = 0.029 (Figure 5). Exploratory post-hoc analysis (Holm-Sidak method, all pairwise multiple comparisons) provided additional confirmation of the planned comparison, i.e., tau was reliably higher in the group of monkeys with a history of chronic alcohol consumption than in the control group after 30 days of abstinence. After 90 days of abstinence, the numerical difference in tau diminished and was no longer reliably different. Separate analysis failed to confirm a significant effect of chronic alcohol consumption on mu or sigma when tested during the period of extended abstinence.
Figure 5.
The effect of chronic alcohol consumption on tau during discontinuation of alcohol consumption. During discontinuation, tau was reliably higher in monkeys that consumed alcohol chronically than in the control monkeys. Subsequent post-hoc tests confirmed that this difference was statistically significant 30 days after the discontinuation alcohol consumption (p<0.05, indicated by *). Error bars represent SEM.
4. Discussion
In these studies, acute alcohol consumption selectively affected tau, the mean of the exponential component of the PDF (Figure 3), thus showing that in adolescent monkeys with minimal prior alcohol exposure, acute alcohol dose-dependently reduced the probability of long response times. This effect could be characterized as an improvement in response time performance, albeit in the absence of change in mean response times per se under these conditions. The outcome is consistent with an activating effect of alcohol leading to an improvement of sustained attention to the task.
The influence of acute alcohol exposure on tau reported here for Experiment 1 contrasts with recently published work in which an increase in tau was reported in monkeys (Jedema et al. 2011), but there are important distinctions between these two studies. There is evidence from humans that response time increases as the number of choices increase and that alcohol exacerbates this effect (Maylor et al. 1992). Since the data reported here were collected using a 5-choice task, while Jedema et al. used a 9-choice task, there may have been an interaction of task complexity with the effects of acute alcohol which explains the difference. It was also the case that the route of alcohol administration differed between these studies. The data presented in here were collected 30 minutes after alcohol consumption; the data presented in Jedema et al. were collected while alcohol was administered intravenously. Though there is evidence from adult humans that inhibitory effects of alcohol are commonly observed on the ascending limb of the blood-alcohol concentration (BAC) curve (Schweizer and Vogel-Sprott 2008), there is a dearth of information regarding the effect of ascending blood-alcohol levels on behavior in adolescent animals. There is also evidence from rats that acute alcohol tolerance develops more rapidly and robustly in adolescents than in adults (Morales et al. 2011; Varlinskaya and Spear 2006). It is not impossible that the differences between the present study and that of Jedema et al are attributable to differences in acute tolerance.
Somewhat more likely to explain the differences between these two observations is the fact that the data reported here were collected from monkeys that were approximately 4 years of age when the study began whereas the monkeys used in Jedema et al. were between 7 and 8 years of age. This distinction could be relevant given the evidence that adolescent rats exhibit greater brain activity and less sedation than adult rats after acute exposure to alcohol (Pian et al. 2008). These results are also consistent with other previously published studies indicating that adolescent rats are less sensitive than adult rats to the sedating effects of alcohol (Hollstedt et al. 1980; Little et al. 1996; Moy et al. 1998). Interestingly, the control group transitioned from a beneficial effect of acute alcohol on tau to a slowing across the first 9 months of the chronic drinking experiment. Thus, it may be the case that the peri-adolescent monkeys exhibited brain maturation towards a more adult-like state which was reflected in the changing effect of acute alcohol.
As a minor caveat, it is the case that laboratory macaques are an outbred species and therefore individual trajectories in brain maturation cannot be precisely predicted. The sample size in this study is insufficient to precisely detect individual differences that might have been relevant since it was designed for group effects. In particular, although every effort was made to match the groups for the chronic alcohol phase it was done on multiple variables including spontaneous alcohol preference and performance in several behavioral tests other than the RT procedure. One thing that can be observed by comparison with other cohorts run in prior chronic alcohol studies is that the distribution of alcohol preferences in this study matched prior groups which differed in starting age (Crean et al. 2011; Katner et al. 2004; Katner et al. 2007). We have also trained animals on various behavioral tasks, including the RT procedure, at various ages and found more variability across individuals at a given age than across the range incorporated in this study (Crean et al. 2011; Taffe 2004; 2012; Taffe and Taffe 2011; Weed et al. 1999), although, as predicted, aged animals (over 20 years of age) show differences (Taffe et al. 2003). Thus it may be concluded that the age range in the present study didn’t account for the individual differences in either alcohol intake or baseline behavioral performance.
Interestingly, the probability of long response times (tau) after alcohol consumption did not evolve over the course of the study in the monkeys that consumed alcohol chronically (Figure 4); although there may have been a subtle tendency for the response to flatten over the course of the study, these changes were neither large nor statistically significant. The effect of alcohol on mu and sigma did not change over the course of the study, further emphasizing the sensitivity of tau over the traditional measures of response time tasks.
One interpretation of these data is that chronic alcohol consumption impaired the maturation of the response to alcohol. Specifically, acute alcohol initially produced a dose-dependent decrease in the probability for long response times in the adolescent monkeys. Over the successive 9 months, however, the effect of acute alcohol on the probability of long response times (tau) flattened and then reversed in the control group. Once reversed, the effect of alcohol on tau looked very similar to what was has been reported in adult monkeys (Jedema et al. 2011) which may have been due to maturational changes. This change in the response to alcohol did not occur in the monkeys that consumed alcohol chronically which would correspondingly be interpreted as interference with brain maturation during the critical adolescent epoch. In addition there was a trend for tau to decrease after consumption of the vehicle in the control group over the course of the study. This trend might further serve to strengthen the interpretation that tau evolved (to a more mature pattern) in the control monkeys, but not in monkeys that consumed alcohol chronically.
Finally, the monkeys that consumed alcohol chronically exhibited an increased probability for unusually long response times in the months that followed alcohol discontinuation (Figure 5). Baseline values of tau, that is values of tau observed 22 hours after alcohol/vehicle consumption, were stable for both groups across the last 16 weeks of consumption. In monkeys that consumed alcohol chronically, however, the probability of long response times began to rise after alcohol consumption was discontinued, i.e., tau was consistently higher in the experimental group than it was in controls. This difference peaked after 30 days of discontinuation, even though there were no overt behavioral manifestations of a withdrawal syndrome at this, or any other point, during discontinuation. (This was predictable given the relatively low daily dose during the chronic study.) These data suggest that the effect of chronic alcohol consumption on the probability of long response times persisted for at least 30 days beyond alcohol discontinuation.
Although tau is generally discussed as relevant to sustained attentional processes, some have suggested that changes in tau are reflective of alterations in decision processes, while changes in mu and sigma are reflective of alterations in perception and motor function (Hohle 1965). Indeed, there is evidence of a direct relationship between tau and the cognitive load of the task (Liu et al. 2012; Roelofs 2012; Spieler et al. 2000; Steinhauser and Hubner 2009). It is important to note that this view is controversial. There is evidence that the relationship between the constituent components of the ex-Gaussian PDF and psychological and physiological processes involved in response time performance is complex (Luce 1986; Spieler et al. 2000). Others have suggested that there are theoretical considerations that preclude possibility that the ex-Gaussian PDF corresponds to actual cognitive processes (Matzke and Wagenmakers 2009). Despite this, it has been noted that the ex-Gaussian PDF describes response time data quite well, even in the absence of a cogent theoretical underpinning (Spieler et al. 2000). Given these data, it is appropriate interpret the results of the ex-Gaussian analysis of response time data conservatively.
In conclusion, these data indicate that acute and chronic alcohol consumption during adolescence exerts a selective effect on the probability of long response times without altering response times per se. This identifies a beneficial, activating effect of acute alcohol drinking on selective attention which changes across development. The data further suggest that chronic alcohol drinking may interfere with the maturation of systems that are critical for tightening the RT distribution. Finally, the data show that the discontinuation of chronic alcohol can lead to an increase in the probability of long response times in the RT distribution in the absence of any change in mean RT. This suggests a profile of inattentiveness that persists up to 30 days after chronic drinking is discontinued. Given the age at which alcohol consumption commonly begins in the United States, these data highlight the need to better understand the impact of chronic alcohol consumption on the developing brain.
Acute alcohol reduced tau, the mean of exponential component of the distribution.
In the control group, the effect of alcohol on tau changed as the monkeys matured.
Chronic alcohol consumption impaired this maturation in the response to alcohol.
Alcohol discontinuation produced an increase in tau that peaked after 30 days.
Acknowledgments
This work was supported by USPHS grant AA016807. The authors would also like to acknowledge the support of the Pearson Center for Alcoholism and Addiction Research and USPHS grant AA007456. Finally, the authors would like to thank Dr. Yves Lacouture of Universite Laval for facilitating the analyses used in these experiments by making the DISTRIB toolbox code publicly available. This is publication #21937 from The Scripps Research Institute.
Abbreviations
- 5CSRT
5-Choice Serial Reaction Time
- BAC
Blood Alcohol Concentration
Probability Density Function
- RT
Reaction Time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizzi MN, Correa M, Betz AJ, Wisniecki A, Salamone JD. Behavioral effects of intraventricular injections of low doses of ethanol, acetaldehyde, and acetate in rats: studies with low and high rate operant schedules. Behavioural brain research. 2003;147:203–210. doi: 10.1016/s0166-4328(03)00158-x. [DOI] [PubMed] [Google Scholar]
- Baylor AM, Layne CS, Mayfield RD, Osborne L, Spirduso WW. Effects of ethanol on human fractionated response times. Drug Alcohol Depend. 1989;23:31–40. doi: 10.1016/0376-8716(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Breitmeier D, Seeland-Schulze I, Hecker H, Schneider U. The influence of blood alcohol concentrations of around 0.03% on neuropsychological functions--a double-blind, placebo-controlled investigation. Addict Biol. 2007;12:183–189. doi: 10.1111/j.1369-1600.2007.00056.x. [DOI] [PubMed] [Google Scholar]
- Cameron E, Sinclair W, Tiplady B. Validity and sensitivity of a pen computer battery of performance tests. J Psychopharmacol. 2001;15:105–110. doi: 10.1177/026988110101500207. [DOI] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. Ilar J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, Taffe MA. Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug Alcohol Depend. 2011;114:31–40. doi: 10.1016/j.drugalcdep.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman TW, Robinson SR, Yelland GW. Impaired perceptual judgment at low blood alcohol concentrations. Alcohol. 2011;45:711–718. doi: 10.1016/j.alcohol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Popiel AJ, Mewhort DJK. Analysis of response time distributions: An example using the Stroop Task. Psychological Bulletin. 1991;109:340–347. [Google Scholar]
- Hernández OH, Vogel-Sprott M, Huchín-Ramirez TC, Aké-Estrada F. Acute dose of alcohol affects cognitive components of reaction time to an omitted stimulus: differences among sensory systems. Psychopharmacology. 2006;184:75–81. doi: 10.1007/s00213-005-0237-7. [DOI] [PubMed] [Google Scholar]
- Hernández OH, Vogel-Sprott M, Huchín-Ramirez TC, Aké-Estrada F. Alcohol slows the brain potential associated with cognitive reaction time to an omitted stimulus. J Stud Alcohol Drugs. 2010;71:268–277. doi: 10.15288/jsad.2010.71.268. [DOI] [PubMed] [Google Scholar]
- Hernández OH, Vogel-Sprott M, Ke-Aznar VI. Alcohol impairs the cognitive component of reaction time to an omitted stimulus: a replication and an extension. J Stud Alcohol Drugs. 2007;68:276–281. doi: 10.15288/jsad.2007.68.276. [DOI] [PubMed] [Google Scholar]
- Hohle RH. Inferred components of reaction times as functions of foreperiod duration. Journal of Experimental Psychology. 1965;69:382–386. doi: 10.1037/h0021740. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Jedema HP, Carter MD, Dugan BP, Gurnsey K, Olsen AS, Bradberry CW. The acute impact of ethanol on cognitive performance in rhesus macaques. Cereb Cortex. 2011;21:1783–91. doi: 10.1093/cercor/bhq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28:873–83. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Von Huben SN, Davis SA, Lay CC, Crean RD, Roberts AJ, Fox HS, Taffe MA. Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug Alcohol Depend. 2007;91:236–43. doi: 10.1016/j.drugalcdep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE. Ethanol induced slowing of human reaction time and speed of voluntary movement. J Psychol. 1975;90:203–214. doi: 10.1080/00223980.1975.9915777. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, et al. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. 2010;20:5. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Smith LT, Kalbfleisch LD, Parsons OA. The influence of alcohol and sleep deprivation on stimulus evaluation. Alcohol. 1992;9:445–450. doi: 10.1016/0741-8329(92)90046-d. [DOI] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4:35–45. [Google Scholar]
- Larsson ASL, Söderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, June HL. Neurobehavioral studies of ethanol reward and activation. Alcohol. 1990;7:213–219. doi: 10.1016/0741-8329(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Green CE, Cunningham KA, Moeller F. Increased intra-individual reaction time variability in cocaine-dependent subjects: role of cocaine-related cues. Addict Behav. 2012;37:193–197. doi: 10.1016/j.addbeh.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin RA. Influences of alcohol upon performance and performance awareness. Percept Mot Skills. 1977;45:303–310. doi: 10.2466/pms.1977.45.1.303. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response times: their role in inferring elementary mental organization. Oxford University Press, Inc; New York, New York: 1986. [Google Scholar]
- Marcondes MC, Watry D, Zandonatti M, Flynn C, Taffe MA, Fox H. Chronic alcohol consumption generates a vulnerable immune environment during early SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2008;32:1583–92. doi: 10.1111/j.1530-0277.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Human Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Wagenmakers EJ. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: a diffusion model analysis. Psychonomic Bulletin & Review. 2009;16:798–817. doi: 10.3758/PBR.16.5.798. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PM. Alcohol, reaction time and memory: a meta-analysis. Br J Psychol. 1993;84:301–317. doi: 10.1111/j.2044-8295.1993.tb02485.x. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PM, James GH, Kerr SA. Effects of alcohol, practice, and task complexity on reaction time distributions. Q J Exp Psychol A. 1992;44:119–139. doi: 10.1080/14640749208401286. [DOI] [PubMed] [Google Scholar]
- McManus IC, Ankier SI, Norfolk J, Phillips M, Priest RG. Effects on psychological performance of the benzodiazepine, loprazolam, alone and with alcohol. Br J Clin Pharmacol. 1983;16:291–300. doi: 10.1111/j.1365-2125.1983.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DB, Winger G, Woods JH, Stebbins WC. Effect of ethanol and of noise on reaction time in the monkey: variation with stimulus level. Psychopharmacology. 1980;69:45–51. doi: 10.1007/BF00426520. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–1624. doi: 10.1111/j.1530-0277.2011.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky L, Patel V, Roberts P. Effects of ethanol in an open field apparatus: modification by U50488H and WIN 44441–3. Physiology & behavior. 1989;45:273–287. doi: 10.1016/0031-9384(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Roelofs A. Attention, spatial integration, and the tail of response time distributions in Stroop task performance. Q J Exp Psychol (Hove) 2012;65:135–150. doi: 10.1080/17470218.2011.605152. [DOI] [PubMed] [Google Scholar]
- Rose RM, Gordon TP, Bernstein IS. Diurnal variation in plasma testosterone and cortisol in rhesus monkeys living in social groups. The Journal of endocrinology. 1978;76:67–74. doi: 10.1677/joe.0.0760067. [DOI] [PubMed] [Google Scholar]
- Ryan C, Russo K, Greeley J. Testing the global-slowing hypothesis: are alcohol’s effects on human performance process-specific or task-general? Acta Psychol (Amst) 1996;92:59–78. doi: 10.1016/0001-6918(94)00059-x. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacol. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Levels of selective attention revealed through analyses of response time distributions. J Exp Psychol Hum Percept Perform. 2000;26:506–526. doi: 10.1037//0096-1523.26.2.506. [DOI] [PubMed] [Google Scholar]
- Steinhauser M, Hubner R. Distinguishing response conflict and task conflict in the Stroop task: evidence from ex-Gaussian distribution analysis. J Exp Psychol Hum Percept Perform. 2009;35:1398–1412. doi: 10.1037/a0016467. [DOI] [PubMed] [Google Scholar]
- Stodulka J. Ethanol and physostigmine effects on open field behavior in Wistar rats. Acta Univ Palacki Olomuc Fac Med. 1991;131:39–81. [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques (vol 81, pg 59, 2004) Physiology & behavior. 2004;82:589–589. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Delta(9)tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol. 2012;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Bernot T, McKay H, Davis SA, Roberts J, Tuszynski MH. Adaptation of a computerized neuropsychological testing battery to the aged macaque. 33rd Annual Meeting of the Society of Neuroscience. Society for Neuroscience; New Orleans, LA, USA. 2003. p. Abstract No. 114.16. [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–9. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Taffe WJ. Rhesus monkeys employ a procedural strategy to reduce working memory load in a self-ordered spatial search task. Brain Res. 2011;1413:43–50. doi: 10.1016/j.brainres.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiplady B, Drummond GB, Cameron E, Gray E, Hendry J, Sinclair W, Wright P. Ethanol, errors, and the speed-accuracy trade-off. Pharmacol Biochem Behav. 2001;69:635–641. doi: 10.1016/s0091-3057(01)00551-2. [DOI] [PubMed] [Google Scholar]
- Van Zandt T. How to fit a response time distribution. Psychonomic Bulletin & Review. 2000;7:424–465. doi: 10.3758/bf03214357. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–1844. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cognitive Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Glavis-Bloom C, Taffe MA. Acute ethanol reduces reversal cost in discrimination learning by reducing perseverance in adolescent rhesus macaques. Alcohol Clin Exp Res. 2012 doi: 10.1111/acer.12050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]