Abstract
Aim
To evaluate whether venlafaxine-extended release (VEN-XR) is an effective treatment for cannabis dependence with concurrent depressive disorders.
Design
This was a randomized, 12 week, double-blind, placebo-controlled trial of outpatients (n = 103) with DSM-IV cannabis dependence and major depressive disorder or dysthymia. Participants received up to 375 mg VEN-XR on a fixed-flexible schedule or placebo. All patients received weekly individual cognitive-behavioral psychotherapy that primarily targeted marijuana use.
Settings
The trial was conducted at two university research centers in the United States.
Participants
One hundred and three cannabis dependent adults participated in the trial.
Measurements
The primary outcome measures were 1) abstinence from marijuana defined as at least two consecutive urine-confirmed abstinent weeks and 2) improvement in depressive symptoms based on the Hamilton Depression Rating Scale.
Findings
The proportion of patients achieving a clinically significant mood improvement [50% decrease in Hamilton Depression score from baseline] was high and did not differ between groups receiving VEN-XR (63%) and placebo (69%) (X12=0.48, p-value= 0.49). The proportion of patients achieving abstinence was low overall, but was significantly worse on VEN-XR (11.8%) compared to placebo (36.5%) (X12=7.46, p-value<0.01; OR = 4.51, 95% CI: 1.53, 13.3). Mood improvement was associated with reduction in marijuana use in the placebo group (F1,179=30.49, p-value<0.01), but not the VEN-XR group (F1,186=0.02, p-value=0.89).
Conclusions
For depressed, cannabis-dependent patients, venlafaxine-extended release does not appear to be effective at reducing depression and may lead to an increase in cannabis use.
INTRODUCTION
Marijuana is the most commonly used illegal drug in the world (1). Marijuana dependence is more prevalent than stimulant or heroin dependence in most countries, including the United States (1-3), and is frequently the primary drug problem among both adolescents and adults seeking treatment (3, 4). However, it is difficult to treat. Behavioral methods have shown promise (5, 6), while as yet there are no effective medications for marijuana dependence.
Many cannabis-dependent adults suffer from additional psychiatric disorders, with depression being particularly common (7-9). Cannabis dependence doubles the odds of having a depressive disorder in the general population (8-10), and depression is prevalent among cannabis-dependent patients seeking treatment (11). This suggests that identification and treatment of depression might be an effective treatment strategy in the depressed subgroup of cannabis dependent patients.
Among alcohol, opioid and cocaine dependent patients, depressive disorders are associated with worse treatment outcome (12-15). Correspondingly, meta-analyses of placebo-controlled trials (16, 17) have suggested that, among alcohol dependent patients, treating depressive disorders with antidepressant medication is effective in reducing alcohol use, particularly in trials where the placebo response rate was low. Among trials with depressed opioid and cocaine dependent patients, the findings are less consistent (16-18). Interestingly, most of the positive trials in this literature involved tricyclic antidepressants or other medications with noradrenergic effects, while many of the negative trials tested selective serotonin-reuptake inhibitors (SSRIs). Although high placebo (PBO) response may explain these negative results, medications that enhance noradrenergic transmission might be more effective among depressed substance abusers.
Evidence on the treatment of co-occurring depression and cannabis dependence is limited. A secondary analysis of a PBO-controlled trial among depressed alcoholics (19) found the SSRI fluoxetine was also effective at reducing concurrent marijuana use (20). However, a recent trial with 70 depressed cannabis-dependent adolescents and young adults found a high PBO response rate and no advantage for fluoxetine over PBO on either depression or cannabis use outcomes (21). Venlafaxine was chosen for investigation for this trial because it is a well-tolerated broad spectrum antidepressant and there were some data suggesting that it might have greater efficacy than standard SSRIs because of its dual mechanism of action as both a serotonin and norepinephrine reuptake inhibitor (22-25). By improving mood, it was hypothesized that marijuana use would diminish.
We now report what is, to our knowledge, the largest PBO-controlled trial, to date, of an antidepressant medication for treatment of adults with cannabis dependence and co-occurring major depression or dysthymia. Venlafaxine-extended release (VEN-XR) rather than immediate release venlafaxine was chosen because it can be administered daily and improve adherence. Similar to specific serotonin reuptake inhibitors, it is generally well tolerated. It was hypothesized that VEN-XR would both reduce depressive symptoms and increase marijuana abstinence compared to PBO.
METHODS
Study Participants
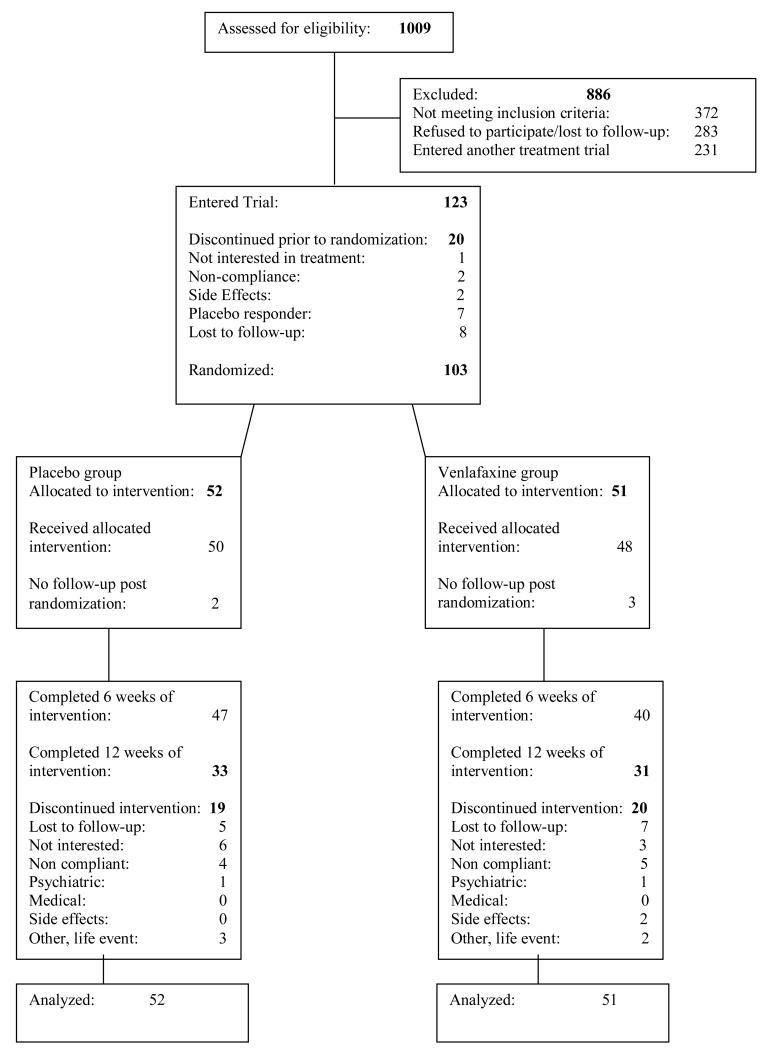
Treatment seekers for problems related to marijuana use were recruited by local advertising or clinical referrals. The CONSORT diagram is presented in Figure 1. The medical screening included a history and physical exam, an electrocardiogram, and laboratory testing. The psychiatric evaluation included the Structured Clinical Interview (SCID) for Diagnostic and Statistical Manual of Mental Disorders- Axis I disorders DSM-IV (26, 27), modified to relate the course of depressive symptoms and substance abuse history (28). In DSM-IV, a “primary” mood disorder is diagnosed if the mood syndrome antedates onset of substance abuse, persists during lengthy abstinent periods, or is “substantially in excess” of the usual toxic or withdrawal effects of substances. A “substance-induced mood disorder” may be diagnosed if there is depressed mood that has never been temporally independent of substance abuse but is “in excess” of the usual toxic or withdrawal effects and “warrants independent clinical attention” (26). Patients were eligible if they met current syndromal criteria for either Major Depression of at least 3 months duration or Dysthymia by SCID interview, and also met one of the above criteria, in an effort to exclude patients in whom mood symptoms were likely usual effects of substances (e.g. cannabis withdrawal).
Figure 1.
Flow diagram of participants recruited to trial.
Participants were treated at the Substance Treatment and Research Service (STARS) of Columbia University/New York State Psychiatric Institute (NYSPI; n=113) or at STARS- of Columbia University/North Shore-LIJ Medical Center (n=10).
Study inclusion required that participants 1) were between the ages of 18-60, 2) met DSM-IV-TR criteria for current cannabis dependence and reported that marijuana was their primary drug of abuse, 3) met DSM-IV criteria for current Major Depression or Dysthymic Disorder and received a total score of ≥ 12 on the Hamilton Depression Inventory (HAMD), and 4) had a depressive syndrome of at least 3 months duration in the current episode. Participants were excluded if they: 1) met DSM-IV criteria for past mania, schizophrenia, or any psychotic disorder other than transient psychosis due to drug abuse; 2) were physiologically dependent on any substances (other than nicotine) that would require a medical intervention/detoxification; 3) had significant risk for suicide; 4) had a history of a seizure disorder; 5) had an unstable medical condition; 6) had a history of allergic reaction to VEN; 7) failed to respond to a previous adequate trial of VEN of at least 300 mg for ≥ 6-week period; 8) were currently being prescribed psychotropic medication, except for acute treatment of insomnia; and 9) females who were nursing, pregnant and/or unwilling to use an effective method of birth control.
The study was approved by the Institutional Review Boards of NYSPI and the North Shore-LIJ Medical Center. After a complete description of the study was presented to the subjects, written informed consent was obtained. The study was conducted from January 2004 through September 2010.
Study Design
The study was a randomized, double-blind, parallel-group, 12-week clinical trial comparing placebo to VEN-XR. The trial included a one-week PBO lead-in phase, a three week medication titration phase, and an 8-week medication maintenance phase. Participants were scheduled to attend the research clinic twice per week. Patients were randomized at the end of the PBO lead-in phase using a computer generated fixed block size of 4, with a 1:1 allocation ratio, and stratified by joints used per week [<21 (n=41) versus ≥ 21 (n=62)] and severity of depression based on the HAMD score [≤ 20 (n=67) versus > 20 (n=36)]. A research pharmacist, who was independent of the research team, conducted the randomization and maintained the allocation sequence. Participants, care providers and outcome assessors were kept blinded to the allocation.
Medication
VEN-XR or matching PBO was prepared by the pharmacy at the New York State Psychiatric Institute, packaged in matching gelatin capsules with lactose filler. Participants were instructed to take the medication once per day in the morning. Study medication was provided to participants on a weekly basis. Each week, participants were asked to return all bottles and unused medication. The study staff documented any unused or missed medication.
PBO responders during the 1-week placebo lead in (N = 7), defined as a Clinical Global Impression (CGI) (29) rating of 1 or 2 (much, or very much improved) and a reduction in the HAMD score > 75% or total score ≤ 7, were not randomized.
Following the PBO lead-in week, participants were randomized into either the VEN-XR or PBO group. VEN-XR (or matching PBO) was titrated to the target dose of 225 mg/day (or the maximum tolerated dose) over the three weeks after randomization. After the fourth week post-randomization, patients with persistent depression who were not rated as having a CGI-Depression score of 1 (“very much improved’) and who were tolerating 225 mg/day had their dose increased to a maximum of 375 mg/day. Dose reductions were also allowed if 225 mg/day was not tolerated.
Manualized Psychotherapy
All participants received weekly Cognitive Behavioral Therapy/ Relapse Prevention Treatment(CBT/RP) (30). The first four weeks of treatment included techniques derived from motivational enhancement therapeutic techniques (31) that have been subsequently adapted for use among cannabis-dependent patients (32). Patients were encouraged to set a quit date at the onset of treatment, however, if a patient set a goal of reducing their use, therapy focused on this goal, and abstinence sampling was revisited during the study using motivational interviewing principles. The core therapy modules focused on the reduction and cessation of marijuana use by developing the skills necessary to manage thoughts and cravings for marijuana, implementing drug refusal skills, and managing environmental contexts that could increase the probability of relapse. In addition, modules were included to address the relationship between cognition and negative affect, developing strategies for managing negative mood, altering depressionogenic thinking patterns, and increasing the frequency of pleasant activities.
Procedures
Patients were asked to come to the clinic twice a week. Once a week patients met with a psychiatrist to administer clinical ratings of mood and marijuana use, assess side effects and clinical status, and adjust medication dosage as needed. Participants were compensated $5-$20 for transportation costs per visit. To better assess medication compliance, participants earned an additional $10 per week if they returned their pill bottles and any remaining medication.
Marijuana Use
At each visit self-reported marijuana use was assessed with the time line follow back (TLFB) calendar method, customized for tracking cannabis use (33, 34). Quantitative urine THC levels were obtained at each visit. The Analytical Psychopharmacology Laboratory of the Nathan Kline Institute tested each urine sample for the presence of 11-nor- Δ9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) using fluorescence-polarization immune analysis (FPIA). A cutoff of 100 ng/ml was used as the point between positive and negative to decrease the probability of false positives (35). All THC levels were creatinine-normalized to control for potential urine dilution.
Mood Symptoms
Mood outcome was evaluated with the Hamilton Depression Rating Scale (HAMD) (36-38) every two weeks.
Side Effects
Side effects were assessed weekly by the study psychiatrist using the Modified Systematic Assessment for Treatment and Emergent Events (SAFTEE)(39, 40).
Data Analysis
The sample size of 60 patients per group was chosen to afford sufficient statistical power (power > 0.80 at alpha < 0.05, two-tailed) to detect effect sizes of just under 0.50.
The primary outcome measure for marijuana use was a dichotomous abstinence response, defined as at least two consecutive urine-confirmed abstinent weeks. This outcome was chosen since continuous abstinence has been shown to predict long-term abstinence, albeit for cocaine (30, 41). Each week during the study, subjects were scored as urine-confirmed abstinent if both self-reported marijuana use for that week was negative, according to the quantitative substance use daily inventory (TLFB), and all urines collected for that week were negative for THC (i.e., quantitative THC <100 mg/ml normalized for creatinine). Patients who achieved the two consecutive abstinent weeks were classified as abstinent whether or not they subsequently dropped out of the study. Patients who dropped out of the study without achieving two continuous weeks of abstinence were classified as not abstinent.
The primary outcome measures for depression were two dichotomous variables: 1) at least a 50% reduction in the HAMD total score between randomization and end-of-study; 2) a score of less than 8 at end-of-study. End-of-study was defined as week 12, or the last measurement prior to dropout for patients completing less than 12 weeks of treatment. For secondary analysis purposes, the HAMD scores were used as continuous longitudinal data measured once a week.
Secondary outcomes were: THC urine level (measured once a week, longitudinal continuous), side effects and adverse events (dichotomous), and treatment compliance (continuous). While abstinence was the primary outcome, reduction in use may be a reasonable goal, particularly for patients who are ambivalent about stopping their use. Thus, quantitative THC urine levels normalized for creatinine during the study was assessed.
Logistic regression was used to analyze all dichotomous outcomes. The dichotomous primary outcome marijuana abstinence was modeled using independent predictors: treatment (VEN-XR vs. PBO) and baseline urine THC level. The initial analysis included an interaction between treatment and baseline urine THC levels which was deemed not significant and omitted from the final logistic model.
Side effects and adverse events were analyzed using Fisher’s exact test. T-tests were used to analyze treatment compliance. Longitudinal outcomes THC urine level normalized for creatinine and HAMD total score were analyzed using mixed effect models with a lognormal or identity link function with the patient defined as a random factor and within patient autoregressive correlation structure AR(1). The two-way interaction between treatment and time was assessed and was retained in the final models if found significant. All analyses were conducted based on the intent-to-treat principle unless noted otherwise. All statistical tests were two-tailed and employed an alpha significance level of 0.05, unless otherwise stated and all interaction terms were evaluated at the significance level of 0.15. PROC GLIMMIX in SAS was used to conduct these analyses.
RESULTS
Sample Description
Demographic and baseline clinical characteristics of the PBO (N = 52) and VEN-XR (N = 51) groups are shown in Table 1. There were no demographic or baseline clinical characteristics that were significantly different between the two groups. Notably, this was a heavy using population. The mean days of use was 27.4 [S.D.=5.6] and baseline mean grams used per using day was 2.6 [S.D.=2.8]. Because active suicidal ideation was an exclusionary criterion, a small percentage of the sample were rated as very severely depressed by the Hamilton Rating scores (36) although most were rated as moderately or severely depressed.
Table 1.
Demographic characteristics of the patients randomized to PBO and VEN-XR (N=103)a
| PBO | VEN-XR | ||||
|---|---|---|---|---|---|
| Characteristic | (N=52) | (N=51) | p-value | ||
| Demographic Characteristics | Mean | SD | Mean | SD | |
| Age (years) | 35.9 | 9.3 | 34.2 | 10.8 | 0.40 |
| n | % | n | % | ||
| Male | 41 | 78.9 | 35 | 68.6 | 0.24 |
| Race/Ethnicity | 0.28 | ||||
| Hispanic | 14 | 26.9 | 13 | 25.5 | |
| Black | 12 | 23.1 | 10 | 19.6 | |
| White | 21 | 40.4 | 26 | 51.0 | |
| Asian | 1 | 1.9 | 2 | 3.9 | |
| Other | 4 | 7.7 | 0 | 0 | |
| Education | 0.46 | ||||
| ≤ High School | 12 | 23.5 | 17 | 33.3 | |
| Some College | 29 | 56.9 | 28 | 54.9 | |
| College & Graduate School | 10 | 19.6 | 6 | 11.8 | |
| Employment Status | 0.49 | ||||
| Full-Time | 19 | 37.3 | 22 | 43.1 | |
| Unemployed/Others | 32 | 62.8 | 29 | 56.9 | |
| Currently Married | 9 | 17.7 | 10 | 19.6 | 0.80 |
| Clinical Characteristics | n | % | n | % | |
| High Depression (>20 HAM-D score) | 19 | 36.5 | 17 | 33.3 | 0.73 |
| High MJ Use (≥ 21 joints per week) | 29 | 55.8 | 33 | 64.7 | 0.35 |
| Mean | SD | Mean | SD | ||
| Marijuana Use Days Per Month | 27.5 | 6.5 | 27.4 | 4.5 | 0.91 |
| Grams of Marijuana used per using day | 2.4 | 2.9 | 2.7 | 2.8 | 0.63 |
| Joints of Marijuana used per week | 36.3 | 40.6 | 38.2 | 36.6 | 0.81 |
| Marijuana Dollar Amount Per Month | 647.6 | 632.6 | 779.1 | 662.5 | 0.32 |
| Years of Regular Marijuana use | 16.0 | 9.0 | 15.1 | 10.6 | 0.63 |
| Baseline HAMD-21 Score | 19.0 | 4.6 | 17.9 | 4.2 | 0.21 |
| Baseline HAMD-17 Score | 17.3 | 4.0 | 16.3 | 3.7 | 0.19 |
| Baseline Creatinine-Corrected Urine (ng/mg) | 926 | 1165 | 1139 | 1530 | 0.43 |
Frequencies may not sum to N=103 due to missing values. One patient did not report her education level, one patient did not report his employment status, and one patient did not report his marital status. Four patients did not report their grams of marijuana use per day and three patients did not report their years of regular marijuana use. Percentages may not add up to 100 due to rounding.
Retention in the Trial
Sixty-two percent of the sample (64/103) completed the 12-week trial. Thirty-four of the 103 participants (33%) dropped out before week 11 without achieving two continuous abstinence weeks, thus would be considered non-abstinent. The frequency of those dropouts did not differ by treatment arm (X12=0.82, p-value=0.36). Out of all baseline characteristics, those patients who dropped out were significantly younger (X12=7.27, p-value<0.01) and less likely to be married (X12=3.93, p-value<0.05) compared to those that completed treatment based on logistic regression.
PRIMARY OUTCOMES
Two Consecutive Weeks of Abstinence
Nineteen patients of the 52 (36.5%) in the PBO group and 6 of the 51 (11.8%) in the VEN-XR group achieved at least two consecutive abstinent weeks post-randomization. In the logistic regression model, abstinence was significantly affected by 1) treatment group, indicating greater likelihood of abstinence on PBO, compared to VEN-XR, and 2) baseline urine THC level, indicating higher baseline THC urine level is associated with lower odds of achieving abstinence (see Table 2). The interaction between treatment group and baseline urine THC level was not significant (X12=2.03, p-value=0.15) and was omitted from the final analysis. Notably, a patient receiving PBO had 4.51 (95% CI: 1.53, 13.3) times the odds of achieving two weeks continuous abstinence than a patient receiving VEN-XR with comparable baseline urine THC levels. A higher baseline THC urine level is associated with lower odds of achieving two weeks continuous abstinence. For every 10 ng/ml increase in the THC urine level at baseline, there was a 1.5% decrease in the odds of abstinence (IRR=0.985, 95% CI: 0.974, 0.996).
Table 2.
Depression and marijuana use outcome results
| Outcome | Predictors | ||||
|---|---|---|---|---|---|
| Baseline (effect of baseline for the adjusted model) |
PBO | VEN-XR | Time (effect of time for the longitudinal outcomes) |
Time by Treatment Interaction (effect of time by treatment) |
|
| (effect of treatment) | |||||
|
50% reduction of
HAMD at end of study (unadjusted and adjusted by baseline) |
X12=0.04, p-value=0.84 |
36/52 69.2% |
32/51 62.7% |
N/Aa | N/Aa |
|
Unadjusted by baseline: X12=0.48, p-value=0.49 Adjusted by baseline: X12=0.44, p-value=0.51 | |||||
|
< 8 on the HAMD at end
of study (unadjusted and adjusted by baseline) |
X12=4.95, p-value=0.03 |
30/52 57.7% |
26/51 51.0% |
N/Aa | N/Aa |
|
Unadjusted by baseline: X12=0.47, p-value=0.49 Adjusted by baseline: X12=0.95, p-value=0.33 | |||||
|
At least two consecutive
abstinent weeks (unadjusted and adjusted by baseline THC urine level creatinine corrected) |
X12=7.65, p-value<0.01 |
19/52 36.5% |
6/51 11.8% |
N/Aa | N/Aa |
|
Unadjusted by baseline: X12=7.87, p-value<0.01 Adjusted by baseline: X12=7.46, p-value<0.01 | |||||
|
HAMD over time
(adjusted by baseline HAMD) |
F1,456=19.25, p-value<0.01 |
Week 12 mean=5.65 |
Week 12 mean=6.61 |
F6,456=43.45, p-value<0.01 |
N/Ab |
| F1,456=0.76, p-value=0.38 | |||||
|
THC urine levels
creatinine corrected over time |
N/A | Week 12 mean=439 |
Week 12 mean=1403 |
F6,372=2.94, p-value<0.01d |
F6,372=3.25, p-value<0.01 (See Figure 2) |
| F1,372=9.06, p-value<0.01d | |||||
|
Self reported use in
grams over time |
N/A | Week 12 mean=4.51 |
Week 12 mean=7.18 |
F6,340=5.84, p-value<0.01 |
N/Ac |
| F1,340=0.99, p-value=0.32 | |||||
|
Treatment (effect of treatment) |
HAMD (effect of HAMD score) |
HAMD by Treatment Interaction (effect of HAMD by treatment) |
|||
|
THC urine levels
creatinine corrected (repeated over time) |
F1,365=19.52, p-value<0.01d |
F1,365=17.47, p-value<0.01d |
F1,365=16.11, p-value<0.01 (See Figure 3) |
||
| PBO: | F1,179=30.49, p-value<0. 01e |
N/A | |||
| VEN-XR: | F1,186=0.02, p-value=0.89e |
N/A | |||
Time and Time by Treatment interaction were not predictors in non-longitudinal models.
Time by Treatment interaction was not significant (F6,450 = 0.99, p-value =0.43) and was left out of the final model.
Time by Treatment interaction was not significant (F6,334 = 1.54, p-value =0.17) and was left out of the final model.
Part of the model, but not interpretable. The results must be interpreted through the interaction term.
Separate analyses for the PBO an VEN-XR groups to investigate a effect of HAMD on THC urine level creatinine corrected. In the VEN-XR group there was not a significant relationship between HAMD score and THC urine levels; For the PBO group, THC urine levels were significantly associated with HAMD score.
The above findings were not altered when the model was adjusted for baseline HAMD scores, age at first marijuana use, age of onset of regular marijuana use, and a dichotomous variable indicating heavy marijuana use (defined as 21 joints or more per week at baseline).
Depression response
Proportions of subjects who achieved 50% reduction of HAMD score at the end of the study were not significantly different between the two groups, and there were no significant differences in proportions of subjects who scored <8 on the HAMD (See Table 2).
When adjusted for baseline HAMD score we found 1) no significant effect of treatment and no significant effect of baseline HAMD on 50% reduction of HAMD; and 2) no significant effect of treatment, but a significant effect of baseline HAMD score on the proportion of subjects with < 8 HAMD, where lower baseline scores increased the odds of <8 HAMD at the end of study (see Table 2).
There were no significant longitudinal differences in HAMD score between the treatment groups. However a significant time effect indicates that both groups improved on HAMD scores over the course of the study (see Table 2).
SECONDARY OUTCOMES
THC urine levels (longitudinal)
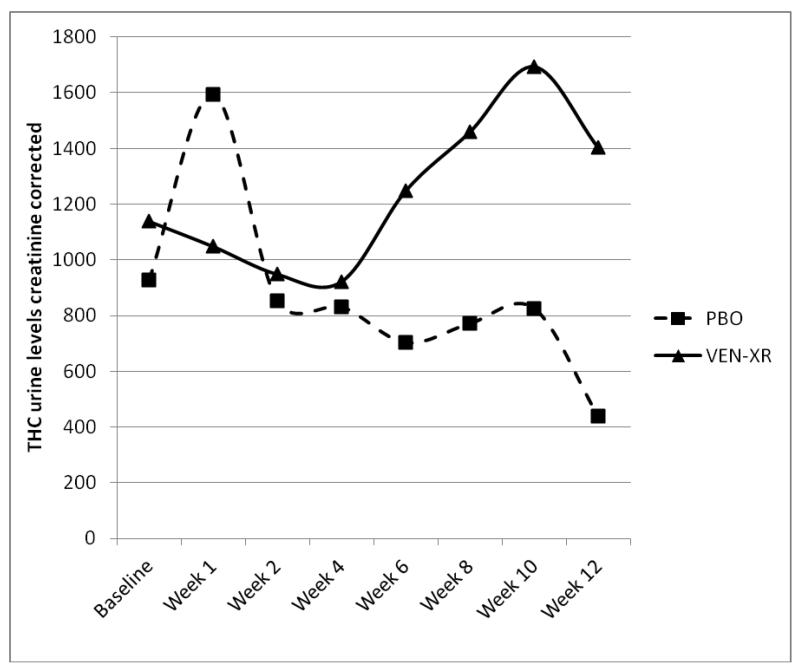
Figure 2 displays the observed average THC urine levels by group and by week in treatment. When analyzed longitudinally, there was a significant interaction between week and treatment, indicating higher THC levels in the VEN-XR group throughout the second half of the study (see Figure 2, Table 2).
Figure 2.
Observed MJ urine levels by week and by treatment assignment, VEN-XR (N=51) vs. PBO (n=52).
* When analyzed longitudinally, there was a significant interaction between week and treatment (see Table 2). The effect of treatment was not significant in weeks 1, 2, and 4. The VEN-XR group had significantly higher THC urine levels creatinine corrected in week 6 (T372=−2.71, p-value < 0.01), week 8 (T372=−2.59, p-value = 0.01), week 10 (T372=−2.62, p-value < 0.01) and week 12 (T372=−3.84, p-value <0.01) compared to the PBO group.
Relationship of Cannabis Outcome to Depression Outcome
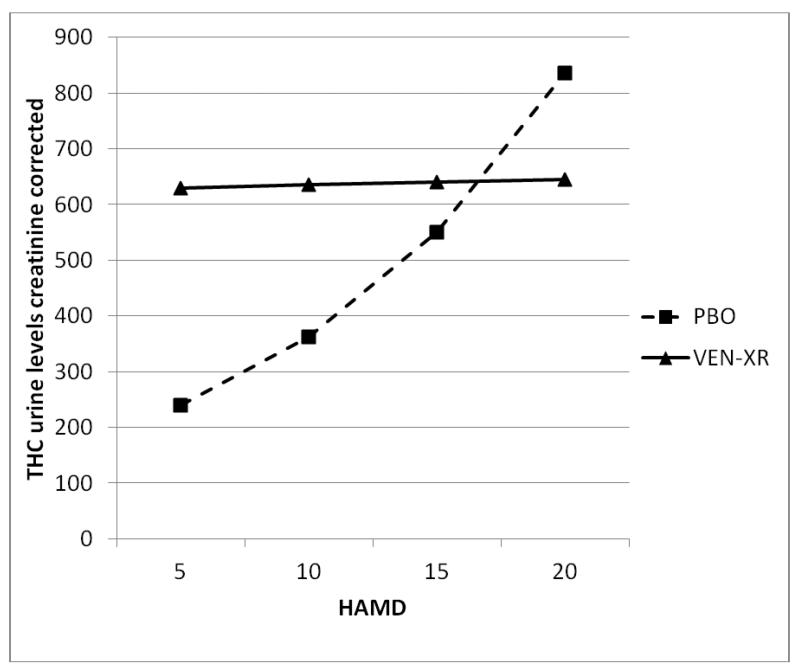
Because mood improvement has often been associated with reductions in substance use in antidepressant trials among depressed alcoholics or other types of drug users (16), we examined this association in the present trial. When urine THC levels were modeled as a function of HAMD scores over weeks in the trial and treatment group, there was a significant interaction between HAMD and treatment (see Figure 3). As can be seen in Figure 3, in the PBO group the expected association between THC improvement and depression improvement was observed, with lower HAMD scores associated with lower THC levels. In contrast, in the VEN-XR group THC levels remained high even when the HAMD scores at outcome were low.
Figure 3.
Modeled THC urine levels creatinine corrected based on the treatment assignment (VEN-XR, n=51; PBO, n=52) and HAMD score.
* When analyzed longitudinally, THC urine levels creatinine corrected were associated with HAMD score but the interaction between week and HAMD score was not significant (F6,354=0.96, p-value=0.45) so it was omitted from the final model. Additionally, week as a main effect was not significant (F6,354=0.96, p-value=0.45) and thus was omitted suggesting that the effect of HAMD on the THC urine levels is the same throughout the study.
** For each treatment group, THC urine levels were differently associated with HAMD score, demonstrated by significant interaction between HAMD and treatment (see Table 2).
*** When the groups were analyzed separately, in the VEN-XR group there was not a significant relationship between HAMD score and THC urine levels creatinine corrected (see Table 2). However, for the PBO group, THC was associated with HAMD score (see Table 2).
Side effects and adverse events
Side effects that were present in at least 5% of patients in at least one of the treatment arms are presented in Table 3. Loss of libido was the only side effect that showed a significant difference, being greater in the VEN-XR than PBO groups (see Table 3).
Table 3.
Moderate to severe adverse events experienced by patients randomized to placebo and VEN during the trial (N=103)
| PBO | VEN-XR | ||||
|---|---|---|---|---|---|
| (N=52) | (N=51) | p-valuea | |||
| Adverse Effect | n | % | n | % | |
| Anxiety | 1 | 1.9 | 6 | 11.8 | 0.060 |
| Diarrhea | 3 | 5.8 | 4 | 7.8 | 0.715 |
| Dizziness | 2 | 3.8 | 8 | 15.7 | 0.052 |
| Fatigue | 1 | 1.9 | 6 | 11.8 | 0.060 |
| GI Upset | 2 | 3.8 | 6 | 11.8 | 0.160 |
| Headache | 4 | 7.7 | 2 | 3.9 | 0.678 |
| Insomnia | 4 | 7.7 | 7 | 13.7 | 0.358 |
| Loss of Libido | 0 | 0 | 6 | 11.8 | 0.013 |
| Muscle Aches | 4 | 7.7 | 2 | 3.9 | 0.678 |
| Nausea | 4 | 7.7 | 6 | 11.8 | 0.526 |
| Syncopy or Light Headed | 4 | 7.7 | 2 | 3.9 | 0.678 |
P-values were obtained using Fisher exact test.
Side effects encountered exclusively in the VEN-XR group include constipation, depression, fever, gas, and loss of libido.
Treatment compliance
For all patients, the average compliance with medication as tested by the percentage of pills taken was 88.9%, and the average compliance with behavioral therapy was 79.2%. There were no significant differences in either medication (PBO: 90.3% versus VEN-XR: 87.5%, T100=0.93, p-value=0.35) or behavioral therapy (PBO: 82.3% versus VEN-XR: 76.0%, T101=1.5, p-value=0.14) compliance between the PBO and the VEN-XR group.
Mean medication blood levels for patients on VEN-XR were 299.6 ± 233.4 ng/ml. Five participants in the VEN-XR group had bloods level of 0, indicating clear non-compliance. Ten percent of blood tests done for VEN patients (9/90) were negative, and 7 of those 9 tests (77.8%) were for the 5 subjects who never tested positive for VEN-XR.
Mean sustained dose (standard deviation) for patients on VEN–XR was 4.4 (± 1.3) 75 mg capsules (330.3 mg ± 95.1) or 3.3 (± 1.8) capsules of placebo.
DISCUSSION
In this controlled trial among depressed cannabis dependent adults, VEN-XR was no better than PBO in reducing depressive symptoms. Both medication and PBO groups had large improvements in depressive symptoms. This resembles a prior controlled trial among cannabis dependent adolescents and young adults, which failed to find an antidepressant effect (21). The low overall abstinence rate in this study resembles the low abstinence rates found in other clinical trials for cannabis dependence (6, 35, 42-47), and reinforces that cannabis dependence is difficult to treat. Surprisingly, those receiving VEN-XR were less likely to become abstinent or reduce their marijuana use. While unexpected, this is an important finding, and has both theoretical and clinical relevance.
There are several reasons why VEN-XR, an effective antidepressant agent, may not have demonstrated superiority to PBO in improving depression here. The most likely factor was the high PBO response rate for depressive symptoms. High PBO response is typically associated with lack of medication effect in clinical trials testing antidepressants for co-occurring depression and substance use (16). All patients in the present trial received cognitive behavioral therapy, and the psychotherapy may have overwhelmed any potential antidepressant effect of the medication. Patients with mild or moderate depression are likely to respond well to psychotherapy, without the addition of medication (48), and the majority enrolled in this study were not considered to have “very severe” depression, but rather had “moderate to moderately severe” based on the Hamilton Scale scores. While we did not specifically assess for functionality, much lower scores have been shown to be associated with poor functioning in other patient populations (49).
There are several possible explanations for the observed worse marijuana outcome on VENXR. VEN-XR is a serotonin-norepinephrine reuptake inhibitor, and prior work with monoamine reuptake inhibitors, suggest that they either worsen marijuana withdrawal (50) or are poorly tolerated (51). Although highly speculative, it is possible that when patients attempted to reduce or cease using marijuana, venlafaxine produced uncomfortable side effects or exacerbated withdrawal symptoms such that improvements in marijuana use were not observed. Reminiscent of this concept, serotonin re-uptake inhibitors have produced worse drinking outcome than PBO among alcohol dependent patients with early onset (52, 53). Cannabis dependence typically begins in adolescence. Thus, there may be some aspect of the pathophysiology of early onset substance dependence that sets up poor responsivity to serotonergic antidepressants. It is striking that in the present trial, VEN-XR seemed to prevent the typical association between improvement in depressive symptoms and improvement in substance use (Figure 3)(16). This suggests the mechanism at work directly influences cannabis use, independent of the outcome of depression.
There are several limitations of the study. This was an outpatient study and excluded patients with very severe depression. Thus we cannot generalize the findings to individuals with more severe depressive symptoms, who might be more likely to benefit from antidepressant medication. The study length was relatively brief. Longer treatment regimens may be needed to have an impact on cannabis use.
In conclusion, in this moderately depressed sample of cannabis dependent patients, depressive symptoms responded well to psychotherapy plus placebo, while VEN-XR was not helpful for depression and reduced the already low likelihood of achieving abstinence. Thus, VEN-XR was not shown to be effective in treating cannabis-dependent patients with depressive disorders. Clinicians managing depressed, cannabis-dependent patients, who are not responding to outpatient counseling, might consider more intensive psychosocial interventions or antidepressants, although to date there are few data supporting the efficacy of antidepressants in cannabis-dependent individuals with depression. The low abstinence rates for both treatment arms suggest the need for further treatment development efforts for this population.
ACKNOWLEDGMENTS
This research was supported by the National Institute on Drug Abuse (NIDA) grants R01DA15451, KO2 000465, K24 DA029647 and K24 DA022412.
We want to thank the staffs of the Substance Treatment and Research Service (STARS) of Columbia University/New York State Psychiatric Institute and Columbia University/North Shore-LIJ Medical Center for their clinical and administrative support.
This research was supported by the National Institute on Drug Abuse (NIDA) grants R01DA15451, KO2 000465, K24 DA029647 and K24 DA022412.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, no editorial direction or censorship. Dr. Levin had full access to all of the data of the study (both what is reported and what is unreported) and takes full responsibility for the integrity of the data and for the accuracy of the data analysis.
Footnotes
Declaration of Interests
Dr. Levin is a past consultant for Eli Lily and Company, Shire Pharmaceuticals Group, AstraZeneca, and OrthoMcNeil Pharmaceutical Inc. Also she has received research support from Eli Lily and Company, UCB PharmaInc, Shire Pharmaceuticals Group, AstraZeneca and OrthoMcNeil Pharmaceutical Inc. She currently receives medication from WorldMed for an ongoing study that is sponsored by the National Institute on Drug Abuse and served as a consultant to GW Pharmaceuticals in 2011. Dr. Nunes served on an advisory board for Eli Lily and Company in January 2012. Drs. Nunes, Bisaga, and Sullivan receive medication from Alkermes for ongoing studies that are sponsored by the National Institute on Drug Abuse.
Drs Mariani, Pavlicova, Carpenter, and Mr. Brooks and Mr. Agosti report no competing interests and no financial relationships with commercial interests.
Clinicaltrials.gov: Title “Free Venlafaxine Treatment for Marijuana Addiction and Depression”, NCT00131456, http://clinicaltrials.gov/show/NCT00131456
REFERENCES
- 1. [Accessed: 2012-11-16];World Drug Report. 2011 http://www.unodc.org/documents/data-and-analysis/WDR2011/World_Drug_Report_2011_ebook.pdf. Archived by WebCite® at http://www.webcitation.org/6CDmv9zDF)
- 2.Degenhardt L, Bohnert KM, Anthony JC. Assessment of cocaine and other drug dependence in the general population: “gated” versus “ungated” approaches. Drug Alcohol Depend. 2008;93(3):227–32. doi: 10.1016/j.drugalcdep.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SAMHSA . Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. Rockville, MD. [Google Scholar]
- 4.TEDS . National Admissions to Substance Abuse Treatment Services, DASIS Series: S-56, HHS Publication No. (SMA) 11-4646. Substance Abuse and Mental Health Services Administration; Rockville, MD: [Accessed: 2012-11-16]. 2011. Substance Abuse and Mental Health Services Administration, Treatment Episode Data Set (TEDS). 1999 - 2009. http://wwwdasis.samhsa.gov/teds09/teds2k9nweb.pdf. Archived by WebCite® at http://www.webcitation.org/6CDlQIa0V) [Google Scholar]
- 5.Budney AJ, Fearer S, Walker DD, Stanger C, Thostenson J, Grabinski M, Bickel WK. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16(5):331–42. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- 7.Grant BF, Pickering R. The relationship between cannabis use and DSM-IV cannabis abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10(3):255–64. doi: 10.1016/s0899-3289(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 8.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Jama. 1990;264(19):2511–8. [PubMed] [Google Scholar]
- 9.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36(10):1447–60. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- 10.Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28(4):643–52. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- 11.Ross HE, Glaser FB, Germanson T. The prevalence of psychiatric disorders in patients with alcohol and other drug problems. Arch Gen Psychiatry. 1988;45(11):1023–31. doi: 10.1001/archpsyc.1988.01800350057008. [DOI] [PubMed] [Google Scholar]
- 12.Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry. 2002;59(4):375–80. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- 13.Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R. Depression among methamphetamine users: association with outcomes from the Methamphetamine Treatment Project at 3-year follow-up. J Nerv Ment Dis. 2009;197(4):225–31. doi: 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 1998;55(3):259–65. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- 15.Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis. 1993;181(2):71–9. doi: 10.1097/00005053-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887–96. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 17.Torrens M, Fonseca F, Mateu G, Farre M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug Alcohol Depend. 2005;78(1):1–22. doi: 10.1016/j.drugalcdep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56(10):793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Perel JM, Thase ME, Black A. Fluoxetine in depressed alcoholics. A double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54(8):700–5. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- 20.Cornelius JR, Salloum IM, Haskett RF, Ehler JG, Jarrett PJ, Thase ME, Perel JM. Fluoxetine versus placebo for the marijuana use of depressed alcoholics. Addict Behav. 1999;24(1):111–4. doi: 10.1016/s0306-4603(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 21.Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, Brown SJ. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1-2):39–45. doi: 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weilburg JB, Rosenbaum JF, Biederman J, Sachs GS, Pollack MH, Kelly K. Fluoxetine added to non-MAOI antidepressants converts nonresponders to responders: a preliminary report. J Clin Psychiatry. 1989;50(12):447–9. [PubMed] [Google Scholar]
- 23.Seth R, Jennings AL, Bindman J, Phillips J, Bergmann K. Combination treatment with noradrenalin and serotonin reuptake inhibitors in resistant depression. Br J Psychiatry. 1992;161:562–5. doi: 10.1192/bjp.161.4.562. [DOI] [PubMed] [Google Scholar]
- 24.Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biol Psychiatry. 2000;48(9):894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- 25.Entsuah R, Gorman JM. Global benefit-risk assessment of antidepressants: venlafaxine XR and fluoxetine. J Psychiatr Res. 2002;36(3):111–8. doi: 10.1016/s0022-3956(01)00055-3. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Nunes EV, Deliyannides D, Donovan S, McGrath PJ. The management of treatment resistance in depressed patients with substance use disorders. Psychiatr Clin North Am. 1996;19(2):311–27. doi: 10.1016/s0193-953x(05)70290-2. [DOI] [PubMed] [Google Scholar]
- 29.Guy W. ECDEU Assessment Manual for Psychopharmacology, in National Institute of Mental Health. US Dept of Health, Education, and Welfare; Washington, DC: 1976. pp. 76–338. [Google Scholar]
- 30.Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry. 1994;51(3):177–87. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- 31.Miller WR, Zweben JE, DiClemente CC, Rychtarik RG. NIAAA Project Match Monograph. Vol. 2. National Institute of Health; Rockville, Maryland: 1999. Motivational Enchancement Therapy Manual: A clinical reasearch guide for therapists treating individuals with alcohol abuse and dependence. Series. [Google Scholar]
- 32.Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M. Brief Counseling for Marijuana Dependence. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2005. Vol DHHS Publication No. (SMA) 05-4022. [Google Scholar]
- 33.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption in Measuring Alcohol Consumption. Humana Press; Totowa, New Jersey: 1992. [Google Scholar]
- 34.Mariani J, Brooks D, Haney M, Levin FR. Quantification and Comparison of Marijuana Smoking Practices: Blunts, Joints, and Pipes. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74(2):307–16. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–7. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 38.Thase ME. Treatment of severe depression. J Clin Psychiatry. 2000;61(Suppl 1):17–25. [PubMed] [Google Scholar]
- 39.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl. 2005;(15):157–67. doi: 10.15288/jsas.2005.s15.157. discussion 140. [DOI] [PubMed] [Google Scholar]
- 40.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343–81. [PubMed] [Google Scholar]
- 41.Kosten TR. Can cocaine craving be a medication developement outcome? Drug craving and relapse in opioid and cocaine dependence. Am J Addict. 1992;1:230–237. [Google Scholar]
- 42.Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18(1):53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27(3):197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Hughes JR, Peters EN, Callas PW, Budney AJ, Livingston AE. Attempts to stop or reduce marijuana use in non-treatment seekers. Drug Alcohol Depend. 2008;97(1-2):180–4. doi: 10.1016/j.drugalcdep.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13(1):21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- 46.McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105(1-2):132–8. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin FR, Mariani J, Brooks DJ, M. P, Cheng C, Nunes E. Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. 2011 doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf NJ, Hopko DR. Psychosocial and pharmacological interventions for depressed adults in primary care: a critical review. Clin Psychol Rev. 2008;28(1):131–61. doi: 10.1016/j.cpr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Romera I, Perez V, Menchon JM, Polavieja P, Gilaberte I. Optimal cutoff point of the Hamilton Rating Scale for Depression according to normal levels of social and occupational functioning. Psychiatry Res. 2011;186(1):133–7. doi: 10.1016/j.psychres.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155(2):171–9. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- 51.Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP. Atomoxetine for treatment of marijuana dependence: a report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend. 2008;94(1-3):254–7. doi: 10.1016/j.drugalcdep.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041–9. [PubMed] [Google Scholar]
- 53.Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534–41. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]