Abstract
Background
Apathy is common in late-life depression and is associated with disability and poor antidepressant response. This study examined whether resting functional connectivity (FC) of the nucleus accumbens (NAcc) and the dorsal anterior cingulate (dACC) with other structures can distinguish apathetic depressed older patients from nonapathetic depressed patients and normal subjects.
Keywords: functional connectivity, apathy, late life depression
INTRODUCTION
The geriatric psychiatry literature is replete with descriptions of apathy syndromes of depression. Terms such as “masked depression” and “depression without sadness” have been used in clinical texts to describe depressive syndromes accompanied by apathy and often reference is made to their high frequency in late life (Fisch, 1987; Gallo et al., 1997).
Apathy in depression has been defined as “lack of motivation not attributable to diminished level of consciousness, cognitive impairment, or emotional distress” (Marin, 1990; Marin et al., 1991; Starkstein and Leentjens, 2008). Lack of motivation is the superordinate dimension of apathy that influences emotional, cognitive and behavioral functions. On an emotional level, apathy is expressed as diminished emotional responses to desirable or undesirable events and lack of concern about their consequences. On a cognitive level, apathy leads to loss of interest in novel activities and in planning. Finally, on a behavioral level, apathy presents as lack of engagement and effort in productive activities.
Apathy affects 19–88% of non-demented individuals with major depression, and is most prevalent in depressed older adults (Forsell et al., 1993; Lampe and Heeren, 2004; Mehta et al., 2008). An epidemiologic study showed that a motivational disturbance was more common in older adults than a mood disturbance (Forsell et al., 1993). In a clinical sample of older adults with unipolar major depression, loss of interest was most common in older patients with first episode in late than in early life (Krishnan et al., 1995).
Apathy can be distinguished from the rest of the symptoms and signs of the depressive syndrome. Scores of the Apathy Evaluation Scale (AES) were not significantly correlated with depressive symptoms and signs (Marin et al., 1993) in a mixed sample consisting of normal elders and of patients with major depression, hemispheric stroke, and Alzheimer’s disease. Further, different relationships were identified between apathy (AES) and depression (HAM-D) scores among elderly patients with different diagnoses (Marin et al., 1994). For example, patients with right hemisphere stroke had similar levels of apathy and depression, but apathy and depression scores were not correlated. Low apathy and high depression scores were reported in left hemisphere stroke. Finally, no correlation was found between apathy and depression in a study of patients with five neurodegenerative disorders using the Neuropsychiatric Inventory, whose depression subscale does not include apathy items (Levy et al., 1998).
The few available studies suggest that apathy is associated with poor treatment response of depression. In adults with major depression, apathy at entry predicted poor response of depressive symptoms to treatment (Chaturvedi and Sarmukaddam, 1986). Similarly, severe apathy at baseline predicted a poor remission rate of both depression and apathy in drug-resistant patients with major depression treated with deep transcranial magnetic stimulation targeting the nucleus accumbens and the ventral tegmentum (Levkovitz et al., 2011). Finally, in elderly patients with major depression apathy at treatment end was correlated with poor outcome of antidepressant treatment (Lavretsky et al., 1999).
Little is known about the neurobiology of apathy of depression. The current views on the neurobiology of apathy are based on studies of neurodegenerative disorders and stroke. Akinetic mutism, an extreme form of apathy, has been described in a variety of conditions involving the ventral striatum (nucleus accumbens, ventromedial caudate), dorsal anterior cingulate cortex, ventral globus pallidus, and medial thalamus (Bonelli and Cummings, 2007). In Parkinson’s disease, apathy was inversely correlated with dopamine and norepinephrine transporter binding (11C-RTI-32) in the ventral striatum (Remy et al., 2005). In frontotemporal dementia, apathy was associated with abnormal metabolism in the ventral striatum, ACC, orbitofrontal cortex, and caudate (Chase, 2011). In Alzheimer’s disease, apathy was correlated with neurofibrillary tangle density in the ACC (Marshall et al., 2006), while MRI studies found correlations of apathy with grey matter of the ACC, frontal cortex, head of caudate and putamen (Apostolova et al., 2007; Bruen et al., 2008; Chase, 2011). Functional imaging studies showed that apathy is associated with abnormal metabolism in the ACC and other frontal and orbital regions with less consistent abnormalities in temporal and medial thalamic areas. In stroke and traumatic brain injury, right sided lesions led to greater apathy than left sided lesions (Andersson et al., 1999). Overall, an average of 61% of patients with focal frontal lobe lesions had apathy while apathy was identified in 40% of patients with basal ganglia lesions (Chase, 2011). Among those with subcortical lesions, damage of the caudate nucleus, globus pallidus, and mediodorsal thalamic nuclei were most frequently associated with apathy. Taken together, these studies suggest that apathy may be associated with abnormalities of the ventral striatum, ACC, and basal ganglia.
This study investigated functional connectivity at rest (FC) in depressed elderly patients with and without apathy and in psychiatrically normal subjects. FC is based on the observation that spontaneous blood oxygen level dependent (BOLD) signal fluctuations among brain regions similarly modulated by specific tasks tend to be correlated (Biswal et al., 1995; Cordes et al., 2001; De Luca et al., 2005; Fox and Raichle, 2007; Fox et al., 2009; Lowe et al., 1998). FC during rest is thought to reflect important interrelationships among structures with related functions. Most of the brain’s energy (>85%) is consumed to maintain a functionally differentiated state at rest (Fox and Raichle, 2007). Studies using different methodology suggest that BOLD activity during a resting state is mainly driven by “intrinsic activity”, which remains consistent across different resting conditions (Fransson, 2005; Raichle and Mintun, 2006), task performance (Fair et al., 2007; Fransson, 2006; Greicius et al., 2004; Sapir et al., 2005; Sun et al., 2007; Waites et al., 2005), sleep (Fukunaga et al., 2006; Horovitz et al., 2008), and anesthesia (Peltier et al., 2005; Vincent et al., 2007).
This study conceptualized apathy of late-life depression as the behavioral expression of abnormal FC of the nucleus accumbens (NAcc) and the dorsal anterior cingulate (dACC) with brain structures related to mood regulation. This view was based on apathy studies on neurodegenerative disorders and on current views on the neurobiology of depression, which postulate abnormal interactions between reward structures, including the NAcc, and prefrontal regions (Phillips et al., 2003). Accordingly, this study compared FC between apathetic depressed elders with depressed elders without apathy and with normal elderly control subjects.
METHODS
Subjects
We studied depressed and psychiatrically normal adults aged 60 years and older. The depressed group consisted of consecutively recruited subjects who met DSM-IV criteria for unipolar major depression without psychotic features and had a score of 18 or greater on the 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960). The normal comparison subjects were recruited through advertisement and were required to have no history or presence of any psychiatric disorder. The subjects signed written informed consent approved by the IRBs of Weill-Cornell Medical College and of Nathan Kline Institute. The consent form informed subjects that there would be a placebo phase during the study, but did not specify the time of the placebo phase.
Individuals were excluded if they had: 1) Mini-Mental State Examination score < 24 (Folstein et al., 1975) or met the Mild Cognitive Impairment (MCI) criteria of Petersen et al (Petersen et al., 1999) during the clinical interview; 2) presence of delirium, history of stroke, head trauma, multiple sclerosis, or brain degenerative diseases; 3) metastatic cancer, brain tumors, unstable cardiac, hepatic, or renal disease, myocardial infarction, or stroke within the 3 months preceding the study; 4) diseases frequently associated with depression, i.e. lymphoma, pancreatic cancer, or endocrinopathies other than diabetes; 5) treatment with drugs associated with depression, i.e. steroids, alpha-methyl-dopa, clonidine, reserpine, tamoxifen, or cimetidine; and 6) metal implants. Depressed subjects with history of other axis I psychiatric disorders prior to the onset of depression were excluded.
Assessment
DSM-IV diagnosis was based on the SCID-R (First, 2002), administered at entry to the study. Depressive symptoms were assessed using the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). Overall cognitive impairment was rated with the Mini Mental State Examination (MMSE) (Folstein et al., 1975) and the Dementia Rating Scale (Mattis, 1988). Memory was rated with the Hopkins Verbal Learning Test-Revised (Brandt and Benedict, 2001), response inhibition with the Stroop Color Word Test (Golden, 1978) and visual attention and task switching with Trails A and Trails B (Reitan and Wolfson, 1985). Apathy was assessed with the Apathy Evaluation Scale (Marin et al., 1991) and dysexecutive behavior with the Frontal Systems Behavior Scale (FrSBe) (Grace et al., 2001). Disability was evaluated with the World Health Organization Disability Assessment Scale (WHODAS-II) (Epping-Jordan and Ustun, 2000).
MRI
MRI Data Acquisition
MRI scans were acquired on a 1.5T Siemens Vision MR system at the Center for Advanced Brain Imaging (CABI) of the Nathan Kline Institute. Data were processed and analyzed at the Weill-Cornell Brain Imaging Analysis Laboratory. Anatomic imaging included a turbo dual echo scan and high-resolution whole brain images acquired using a 3D T1-weighted MPRAGE for coregistration with fMRI data. fMRI data were acquired using BOLD contrasts in a single-shot multi-slice echo planar image (EPI; TR = 2000 ms, TE = 50 ms, flip-angle = 90 degrees, matrix=64×64, FOV = 224mm, 22 5mm slices, no gap), which allowed whole brain coverage.
Image Processing
Resting state (awake, closed eyes) data were processed following the procedure of Biswal et al (Biswal et al., 2010). To eliminate T1 relaxation effects, the first 10 images were discarded. Images were then motion corrected and time shifted using AFNI (Cox, 1996). Next, time series were smoothed using a 6-mm full width-half maximum (FWHM) Gaussian kernel, temporally filtered (.005Hz–.1Hz), and normalized to MNI152 stereotaxic space using FSL (http://www.fmrib.ox.ac.uk/fsl). MPRAGEs were segmented using FSL’s FAST software, and these segmentations were normalized to MNI152 space using the transformations for the MPRAGE.
FC analysis
To calculate timeseries we used FSL’s FLIRT program to transform each subject’s resting-state data into MNI152 space using a 12 DOF linear affine transformation. Next, we calculated the spatial mean time series for each seed ROI. Using seeds that were anatomically defined, we placed one seed in each hemisphere of the NAcc and one seed was placed in each hemisphere in the dACC.
For each seed, individual participant analyses was carried out with GLM of FSL’s FEAT toolbox using the seed-based regression approaches (Biswal et al., 2010; Hoptman et al., 2010). The timeseries for each seed as well as CSF, white matter, total brain matter, and motion parameters were entered as predictors. Next, individual subject-level, Z-statistic images were extracted.
Data Analysis
Initially, chi square and Mann-Whitney tests were used to identify demographic and clinical variables distinguishing depressed from normal subjects and depressed subjects who achieved remission from those who remained symptomatic. Group level analyses of FC were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects), to produce thresholded z-score maps of activity associated with each seed. Images were thresholded using clusters determined by z > 2.3 and a corrected cluster significance threshold of p < 0.05 (Worsley et al., 1992). These maps revealed networks for each group (controls (N = 10), depressed patients with apathy at baseline (N = 7), depressed patients without apathy at baseline (N = 9). In addition, difference maps for direct group comparisons (e.g., depressed patients with apathy vs. depressed patients without apathy were performed; controls vs. depressed patients with apathy; controls vs. depressed patients without apathy). Two tailed significance tests were used in all comparisons.
RESULTS
Subjects
Twenty six older adults were studied. Of these, 16 were non-demented, non-MCI subjects (mean: 69 years, SD: 5.5) with non-psychotic, unipolar major depression (baseline MADRS mean: 23.5, SD: 4.0) and 10 were normal subjects (mean age: 68.6 years, SD: 7.0). There were no statistically significant differences in education (years), overall cognitive impairment (MMSE, DRS), memory (HVLT-R), and response inhibition (Stroop) between depressed and normal subjects. However, depressed subjects had higher depression (MADRS: z=4.2, p<0.0001) scores than normal subjects. Depressed subjects divided into an apathetic (AES ≥ 36.5) and a non-apathetic (AES<36.5) group. There were no statistically significant differences in demographics, severity of depression, and cognitive functions between apathetic and non-apathetic depressed subjects at baseline with the exception of apathy (AES) in which apathetic patients had had more abnormal scores (Table 1). Depressed subjects were scanned at the end of a 2-week psychotropic washout phase. None of the normal subjects was on psychotropic agents.
Table 1.
Characteristics of Older Patients with Major Depression with High (AES1≥36.5) and Low (AES<36.5) Apathy Scores and Normal Elders.
| Variable | Apathetic Depressed (N=7) | Non-Apathetic Depressed (N=9) | Normal Elders (N=10) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 69.9 (4.9) | 68.3 (6.1) | 68.6 (7.0) |
| Education | 17.0 (2.8) | 17.3 (2.1) | 16.3 (3.8) |
| AES1-Patient Self-Rated | 43.6 (5.3)* | 30.1 (4.2) | 22.5 (2.7)** |
| MADRS2 | 25.6 (4.4) | 21.9 (3.0) | 2.1 (1.3)*** |
| MiniMental State Exam | 28.9 (1.1) | 29.3 (0.7) | 28.5 (0.97) |
| DRS3 Total | 134.9 (5.3) | 137.8 (3.3) | 137.3 (3.9) |
| DRS Initiation/Perseveration | 33.9 (4.4) | 36.0 (0.7) | 35.9 (1.1) |
| Stroop Color Word | 33.9 (11.5) | 36.4 (8.3) | 38.5 (6.8) |
| Trails A | 38.3 (7.7) | 33.5 (9.9) | 30.0 (11.1) |
| Trails B | 96.8(44.1) | 81.2 (18.2) | 75.7 (25.3) |
| HVLT-R4 Immediate Recall | 25.4 (5.2) | 28.2 (4.0) | 25.5 (5.1) |
| HVLT-R Delayed Recall | 9.1 (2.2) | 9.1 (2.2) | 9.5(2.2) |
Apathy Evaluation Scale;
Montgomery Asberg Depression Rating Scale;
Dementia Rating Scale;
Hopkins Verbal Learning Test-Revised;
Mann-Whitney comparisons:
Apathetic Depressed vs. Non-Apathetic Depressed p<0.001,
Normal Elders vs. Non-Apathetic Depressed p = 0.002,
Normal elders vs. Apathetic Depressed p = 0.001, Normal Elders vs. Non-Apathetic Depressed p < 0.001.
Functional Connectivity
NAcc Seeds
For both the left and the right NAcc seeds, both depressed and non-depressed subjects demonstrated significant FC within a network that included the striatum, the caudate, the amygdala, the thalamus, and the orbitofrontal cortex (OFC). Overall, apathetic depressed patients had lower FC of the NAcc with the right amygdala, basal ganglia (caudate, putamen), globus pallidus, and thalamus and higher FC with the dACC and dorsomedial prefrontal cortex (dmPFC) than non-apathetic patients. Voxel and MNI stereotaxic space coordinates appear in the Appendix.
Left NAcc
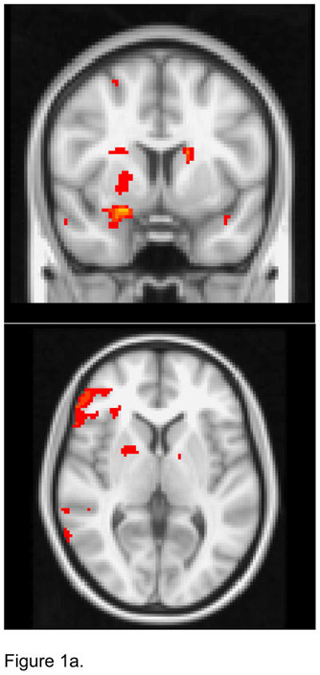
For the left NAcc seed, apathetic patients exhibited lower FC than non-apathetic patients with the right amygdala, putamen, and globus pallidus, and the left caudate (Figure 1a). Apathetic patients had higher FC than non-apathetic patients with the left dorsal ACC and dmPFC (Figure 1b).
Figure 1.
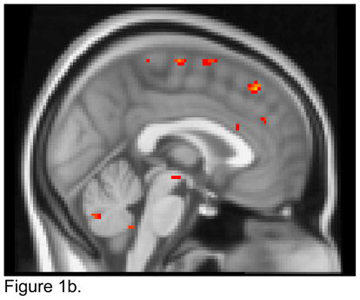
T maps of the left nucleus accumbens resting state connectivity: 1a. Apathetic Patients <NonApathetic Patients; 1b. Apathetic Patients > NonApathetic Patients. Images were thresholded using clusters determined by z>2.3 and a corrected cluster significance threshold of p<0.05.
Figure 1a. Seed in the Left Nucleus Accumbens:
Apathetic Patients <NonApathetic Patients
Figure 1b. Seed in the Left Nucleus Accumbens:
Apathetic Patients > NonApathetic Patients
When apathetic patients and healthy control subjects were directly compared, apathetic patients had lower FC with the right inferior gyrus, right amygdala, bilateral putamen and globus pallidus, and left hippocampus and superior frontal gyrus (Figure). Apathetic patients had higher FC with the left OFC and middle frontal gyrus. Non-apathetic patients had less pronounced differences in FC from healthy controls, i.e. lower FC with the dACC and higher FC with the inferior frontal gyrus.
Right NAcc
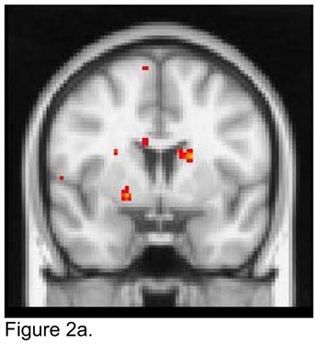
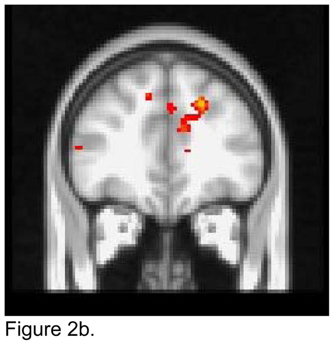
For the right NAcc seed, apathetic patients showed lower FC than non-apathetic patients with the right amygdala, putamen and globus pallidus, and with the left caudate (Figure 2a). Apathetic patients exhibited higher FC than non-apathetic patients with the dmPFC and the left dACC (Figure 2b).
Figure 2.
T maps of the right nucleus accumbens resting state connectivity: 2a. Apathetic Patients <NonApathetic Patients; 2b. Apathetic Patients > NonApathetic Patients. Images were thresholded using clusters determined by z>2.3 and a corrected cluster significance threshold of p<0.05.
Figure 2a. Seed in the Right Nucleus Accumbens:
Apathetic Patients < NonApathetic Patients
Figure 2b. Seed in the Right Nucleus Accumbens:
Apathetic Patients > NonApathetic Patients
When apathetic patients and controls were compared, apathetic patients showed lower FC with the putamen and the globus pallidus bilaterally. Non-apathetic patients had lower FC than controls with the OFC bilaterally.
dACC Seeds
For both the left and the right dACC seeds, both depressed and non-depressed subjects demonstrated significant FC within a network that included the dACC, left PFC, supramarginal, superior parietal, inferior parietal regions, and thalamus. Apathetic patients had lower FC of the dACC with frontal gyri and higher FC with the insula, middle frontal gyrus, and the OFC than non-apathetic patients.
Left dACC
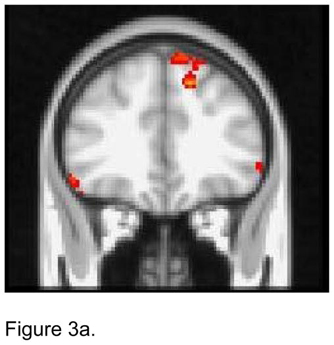
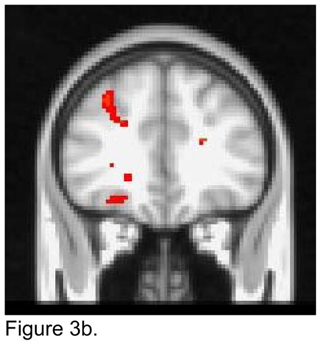
For the left dACC seed, apathetic patients had lower FC than non-apathetic patients with both the superior and the inferior frontal gyruses bilaterally and the left middle frontal gyrus and the right superior parietal region (Figure 3a). Apathetic patients demonstrated higher FC than non-apathetic patients with the right insula, OFC, and the middle frontal gyrus (Figure 3b).
Figure 3.
T maps of the left dorsal anterior cingulate cortex (ACC) resting state connectivity: 3a. Apathetic Patients <NonApathetic Patients; 3b. Apathetic Patients > NonApathetic Patients. Images were thresholded using clusters determined by z>2.3 and a corrected cluster significance threshold of p<0.05.
Figure 3a. Seed in the Left Dorsal ACC:
Apathetic Patients < NonApathetic Patients
Figure 3b. Seed in the Left Dorsal ACC:
Apathetic Patients > NonApathetic Patients
When compared with healthy controls, apathetic depressed patients had lower FC with the superior and the middle frontal gyrus bilaterally and with the left subgenual ACC. Relative to controls, apathetic depressed patients had higher FC with the dmPFC and the right insula. Non-apathetic patients had lower FC than healthy controls with the right OFC and left rostral ACC and higher FC with left superior frontal gyrus and right inferior frontal gyrus.
Right dACC
When the seed was placed in the right dACC, apathetic depressed patients demonstrated lower FC than non-apathetic depressed patients with the ventrolateral PFC bilaterally and the left superior frontal gyrus (Figure 4a). Apathetic patients exhibited higher FC than non-apathetic patients with right structures, i.e. middle frontal gyrus, OFC, and insula (Figure 4b).
Figure 4.
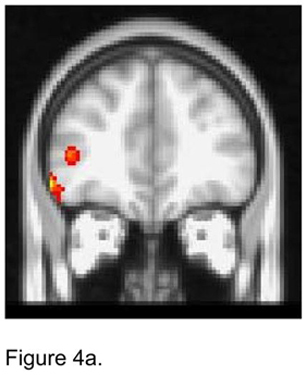
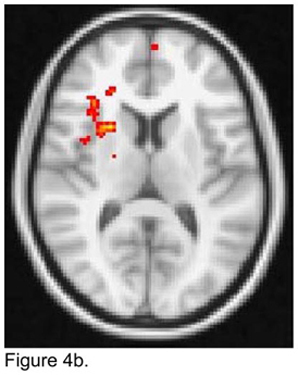
T maps of the right dorsal anterior cingulate cortex (ACC) resting state connectivity: 4a. Apathetic Patients <NonApathetic Patients; 4b. Apathetic Patients > NonApathetic Patients. Images were thresholded using clusters determined by z>2.3 and a corrected cluster significance threshold of p<0.05.
Figure 4a. Seed in the Right Dorsal ACC Seed:
Apathetic Patients < NonApathetic Patients
Figure 4b. Seed in the Right Dorsal ACC Seed:
Apathetic Patients > NonApathetic Patients
Apathetic depressed patients had lower FC than healthy controls with left structures, i.e. subgenual ACC, caudate, and middle frontal gyrus and increased FC with the left dACC and the dorsomedial PFC. Non-apathetic patients had lower FC than healthy controls with the right OFC and vmPFC and higher FC with the left superior frontal gyrus and the right inferior frontal gyrus.
DISCUSSION
The principal finding of this study is that resting FC of the NAcc and the dACC distinguished older patients with apathy and major depression from depressed older patients without apathy and from normal older subjects. Apathetic depressed patients had lower FC of the NAcc with the amygdala, caudate, putamen, globus pallidus, and thalamus and increased FC with the dmPFC, the superior frontal cortex, and the insula than non-apathetic patients. Further, apathetic patients had lower FC of the dACC with dorsolateral and ventrolateral prefrontal cortices and higher FC with the insula and the OFC than non-apathetic patients.
To our knowledge this is the first study to identify abnormalities in resting FC distinguishing apathetic depressed older patients from non-apathetic depressed patients and normal subjects. A strength of this study is that its subjects were free of psychotropic drugs for at least two weeks. Its limitations include a small number of subjects, the lack of random sampling, and the use of a 1.5 T scanner; a more powerful scanner may have yielded additional findings because of its higher signal-to-noise ratio.
The structures on which this study focused are anatomically connected (Bonelli and Cummings, 2007). Specifically, the dACC (BA24) provides input to the ventral striatum, which includes the NAcc, olfactory tubercle, ventromedial caudate, and ventral putamen. Projections from the ventral striatum innervate the rostromedial globus pallidus interna and ventral pallidum and the rostrodorsal substantia nigra. The ventral pallidum provides input to the magnocellular mediodorsal thalamus, which in turn projects to the dACC thus closing the dACC-subcortical loop. An indirect loop has also been identified projecting from the ventral striatum to the rostral pole of globus pallidus externa, which connects to the medial subthalamic nucleus, and from there projections are sent to the ventral pallidum.
Along with anatomical connections, the functions of NAcc and dACC are directly related to motivational, cognitive, and behavioral functions of the kind found impaired in apathetic states. The NAcc is a central node in networks processing diverse reward functions and serves as an interface between limbic and motor processing. The NAcc is selectively activated during the perception of pleasant pictures and during imagery of pleasant, emotional scenes (Costa et al., 2010; Sabatinelli et al., 2007). The dACC is a processing station of top-down and bottom-up stimuli assigning appropriate control to other structures. It may play a special role in reward circuitry, particularly in reward-based decision making, learning, and the performance of novel, nonautomatic tasks (Bush et al., 2002).
Apathetic depressed patients had lower FC than non-apathetic patients of the NAcc with the amygdala, putamen, caudate and globus pallidus and higher FC with dACC and dmPFC than non-apathetic patients. The NAcc is part of the cortico-striato-thalamo-cortical loop. Significant sources of input to the NAcc come from the amygdala, and from dopaminergic fibers of the ventral tegmental area (VTA) via the mesolimbic pathway. The amygdala processes motivationally salient stimuli (Lindquist et al., 2012), while the VTA serves functions related to reward, motivation, and cognition. Thus the NAcc, amygdala, VTA is a complex for processing various aspects of reward related perception and behavior. The NAcc gives input to the ventral part of globus pallidus, which in turn projects to the thalamus and from there to the prefrontal cortex and the striatum. FC abnormalities in these networks may contribute to the perception and experience of rewards and contribute to apathy in late-life depression.
Apathetic depressed patients had lower FC of the dACC with the superior and inferior frontal gyri and higher FC with the insula, the middle frontal gyrus, and the OFC than non-apathetic patients. These prefrontal cortices are responsible for high level processing of working memory including monitoring and manipulation of information and maintenance of distinct stimuli including response mapping rules and adaptive decision making. The insula is connected with the ACC and is a node critical for the experience of basic emotions (happiness, sadness, aversion, fear, anger) and with emotional salience of body representation and the environment including environmental monitoring and response selection. The OFC plays a central role in integrating the affective value of reinforcers in overall perception, and in decision making and expectation (Kringelbach, 2005). Thus, the FC differences between apathetic and non-apathetic depressed patients are between the dACC, a central distribution node, and structures related to processing of both various emotions as well as complex behavioral responses.
Identifying the causes of apathy in late life depression is beyond the scope of this study. However, vascular compromise may contribute to apathy and may account in part for the poor antidepressant response of apathetic depressed patients. Consistent with our finding of low FC among medial frontal structures and the ventral striatum are findings suggesting that lesions in the basal ganglia and in frontal subcortical regions have been associated with apathy (Levy and Dubois, 2006; van Reekum et al., 2005). Healthy elders with apathy have more subcortical white matter hyperintensities (WMH) than those without apathy (Yao et al., 2009). Apathy was associated with history of stroke/TIA as well as cardiovascular risk factors in a large probability sample of community residing elders (Ligthart et al., 2012). There was a dose-effect relationship between apathy and hypertension, type 2 diabetes mellitus, obesity, and C-reactive protein. Similarly, lower ankle-brachial blood pressure, an index of peripheral vascular disease, may be an independent risk factor for apathy (Sugawara et al., 2011). Among individuals older than 85 years, those with apathy had more vascular diseases at baseline and during a 5 year follow-up (van der Mast et al., 2008). Both apathy (Chaturvedi and Sarmukaddam, 1986; Lavretsky et al., 1999; Levkovitz et al., 2011) as well as increased WMH volume of (Sheline et al., 2008) (Gunning-Dixon et al., 2010) and low fractional anisotropy in areas, including frontal and frontal-subcortical white matter regions has been associated with low remission rate of late-life major depression (Alexopoulos et al., 2010; Alexopoulos et al., 2009; Alexopoulos et al., 2008). These abnormalities are principally located in areas likely to interrupt connections among frontal and ventral striatum structures and may be contributed to by cerebrovascular disease among other factors (Hoptman et al., 2009).
In conclusion, resting FC between the NAcc and the dACC on the one hand and structures related to processing of rewards and of related behavioral responses distinguished older apathetic depressed patients from non-apathetic depressed patients and from normal older subjects. In addition to replication, these findings point to the need for studies of the structural integrity and functional responses of NAcc and dACC networks in depressed older apathetic patients, who are often unresponsive to classical antidepressants and remain disabled and suffering.
Acknowledgments
Role of the funding source: Personnel and imaging cost of this work was supported by NIMH grants R01 MH65653, R01 MH079414, P030 MH085943, T32 MH019132 (GSA), K23 MH74818 (FGD) and the Sanchez Foundation. Escitalopram and placebo were provided free of cost by Forest Pharmaceuticals, Inc.
We thank Raj Sangoi (RT) (R) (MR) for his assistance in scanning the research participants and Eric Woods for participating in the clinical evaluation.
Appendix
Table 2.
Regions showing group functional connectivity differences between apathetic and non-apathetic depressed patients.
| Seed | Region | Voxels | Z | MNI Coordinates | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left Nucleus Accumbens | Apathy<No Apathy | R Inferior Frontal | 407 | 4.13 | 56 | 30 | −2 |
| R Ventral Striatum | 221 | 4.97 | 22 | 18 | −16 | ||
| R Putamen | 174 | 4.07 | 26 | 14 | −4 | ||
| L Caudate | 94 | 4.21 | −14 | 4 | 18 | ||
| Apathy>No Apathy | L Dorsal ACC | 274 | 3.76 | −8 | 20 | 32 | |
| R Sup Frontal | 108 | 3.85 | 20 | 42 | 28 | ||
| Right Nucleus Accumbens | Apathy<No Apathy | R Amygdala | 82 | 3.08 | 26 | 6 | −18 |
| L Caudate | 66 | 3.53 | 12 | −4 | 18 | ||
| R Globus Pallidus | 56 | 3.18 | 20 | 2 | −6 | ||
| Apathy>No Apathy | L Dorsal ACC | 106 | 2.97 | −8 | 0 | 40 | |
| L Dorsomedial PFC | 97 | 3.01 | −8 | 30 | 34 | ||
| Left Dorsal Anterior Cingulate Cortex | Apathy<No Apathy | L Sup Frontal | 532 | 3.60 | −12 | 22 | 54 |
| R Mid Frontal | 315 | 4.19 | 46 | −2 | 48 | ||
| L Sup Parietal | 134 | 4.09 | −24 | −66 | 58 | ||
| L Sup Frontal | 78 | 3.08 | −40 | 12 | 52 | ||
| L Inf Frontal | 71 | 3.43 | −58 | 26 | 12 | ||
| Apathy>No Apathy | R Mid Frontal | 211 | 3.42 | 26 | 30 | 28 | |
| R Insula | 69 | 2.89 | 34 | 24 | 8 | ||
| R Orbital Frontal | 65 | 3.56 | 30 | 30 | −16 | ||
| Right Dorsal Anterior Cingulate Cortex | Apathy<No Apathy | R Mid Frontal | 315 | 4.03 | 46 | 4 | 48 |
| R Inf Frontal | 148 | 3.88 | 38 | −14 | 32 | ||
| L Sup Frontal | 115 | 3.74 | −4 | 20 | 60 | ||
| R Sup Frontal | 113 | 3.74 | 10 | 18 | 52 | ||
| R Inf Frontal | 77 | 4.05 | 18 | 83 | 32 | ||
| Apathy>No Apathy | R Mid Frontal | 226 | 3.79 | 34 | 36 | 38 | |
| R Orbital Frontal | 134 | 3.38 | 31 | 83 | 30 | ||
| R Insula | 71 | 3.34 | 38 | 4 | 14 | ||
Footnotes
Conflicts: Dr. Alexopoulos has received grant support from Forest Pharmaceuticals; has consulted to Hoffman-LaRoche, Lilly, Pfizer, and Otsuka; and has served at the speakers’ bureaus of Astra Zeneca, Avanir, Forest, Merck, Novartis, and Sunovion. No other authors report conflicts of interest.
Contributors: Dr. Alexopoulos conceptualized and designed and oversaw the conduct of the study, obtained funding and wrote the first draft of the manuscript. Dr. Hoptman oversaw acquisition of MRI data. Drs. Lim and Hoptman guided image analysis and provided consultation on data interpretation. Drs. Gunning and Yuen performed processing and analysis of MR images and contributed to revisions of the manuscript for important technical and intellectual content. Ms. Kanellopoulos and Ms. Seirup developed and managed the research database and performed statistical analyses in SPSS and SAS and contributed to the interpretation of data. All authors contributed to the manuscript and have approved the final version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Glatt CE, Hoptman MJ, Kanellopoulos D, Murphy CF, Kelly RE, Jr, Morimoto SS, Lim KO, Gunning FM. BDNF val66met polymorphism, white matter abnormalities and remission of geriatric depression. J Affect Disord. 2010;125:262–268. doi: 10.1016/j.jad.2010.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE, Jr, Kanellopoulos D, Klimstra S, Lim KO, Young RC, Hoptman MJ. Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord. 2009;119:132–141. doi: 10.1016/j.jad.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Andersson S, Krogstad JM, Finset A. Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychological medicine. 1999;29:447–456. doi: 10.1017/s0033291798008046. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM. Structural correlates of apathy in Alzheimer’s disease. Dementia and geriatric cognitive disorders. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues in clinical neuroscience. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. The Hopkins Verbal Leaning Test-Revised. Psychological Assessment Resources, Inc; Lutz, FL: 2001. [Google Scholar]
- Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain : a journal of neurology. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase TN. Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotoxicity research. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta psychiatrica Scandinavica. 1986;74:183–186. doi: 10.1111/j.1600-0447.1986.tb10603.x. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR American journal of neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain’s reward circuitry. Human brain mapping. 2010;31:1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan J, Ustun T. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer Robert L, Miriam Gibbon, Williams Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fisch RZ. Masked depression: its interrelations with somatization, hypochondriasis and conversion. International journal of psychiatry in medicine. 1987;17:367–379. doi: 10.2190/cr7j-wu5n-hc5x-2jq5. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsell Y, Jorm AF, Fratiglioni L, Grut M, Winblad B. Application of DSM-III-R criteria for major depressive episode to elderly subjects with and without dementia. Am J Psychiatry. 1993;150:1199–1202. doi: 10.1176/ajp.150.8.1199. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human brain mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RH, Leopold DA, Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic resonance imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. Depression without sadness: functional outcomes of nondysphoric depression in later life. Journal of the American Geriatrics Society. 1997;45:570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Golden CJ. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Co; Chicago, IL: 1978. [Google Scholar]
- Grace J, Malloy P Psychological Assessment Resources, I. FrSBe, frontal systems behavior scale : professional manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, Brickman AM, Hoptman MJ, Young RC, Alexopoulos GS. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. 2010;126:395–401. doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurological Neurosurgery in Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, Etwaroo GR, Kanellopoulos D, Alexopoulos GS. Blood pressure and white matter integrity in geriatric depression. J Affect Disord. 2009;115:171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophrenia research. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Human brain mapping. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- Lampe IK, Heeren TJ. Is apathy in late-life depressive illness related to age-at-onset, cognitive function or vascular risk? International psychogeriatrics / IPA. 2004;16:481–486. doi: 10.1017/s1041610204000766. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- Levkovitz Y, Sheer A, Harel EV, Katz LN, Most D, Zangen A, Isserles M. Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain stimulation. 2011;4:266–274. doi: 10.1016/j.brs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, Paulsen JS, Litvan I. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Ligthart SA, Richard E, Fransen NL, Eurelings LS, Beem L, Eikelenboom P, van Gool WA, Moll van Charante EP. Association of vascular factors with apathy in community-dwelling elderly individuals. Archives of general psychiatry. 2012;69:636–642. doi: 10.1001/archgenpsychiatry.2011.1858. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: a meta-analytic review. The Behavioral and brain sciences. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. J Affect Disord. 1993;28:7–14. doi: 10.1016/0165-0327(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis. 1994;182:235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer’s disease. Dementia and geriatric cognitive disorders. 2006;21:144–147. doi: 10.1159/000090674. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, Huang W, Studenski S. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. Int J Geriatr Psychiatry. 2008;23:238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annual review of neuroscience. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. Therapy and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1985. The Halstead-Reitan neuropsychological test battery. [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain : a journal of neurology. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of neurophysiology. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Sapir A, d’Avossa G, McAvoy M, Shulman GL, Corbetta M. Brain signals for spatial attention predict performance in a motion discrimination task. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17810–17815. doi: 10.1073/pnas.0504678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, McKinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. Journal of neurology, neurosurgery, and psychiatry. 2008;79:1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- Sugawara N, Yasui-Furukori N, Umeda T, Kaneda A, Sato Y, Takahashi I, Matsuzaka M, Danjo K, Nakaji S, Kaneko S. Ankle brachial pressure index as a marker of apathy in a community-dwelling population. Int J Geriatr Psychiatry. 2011;26:409–414. doi: 10.1002/gps.2541. [DOI] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, D’Esposito M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cerebral cortex. 2007;17:1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- van der Mast RC, Vinkers DJ, Stek ML, Bek MC, Westendorp RGJ, Gussekloo J, de Craen AJM. Vascular disease and apathy in old age. The Leiden 85-plus Study. Int J Geriat Psychiatry. 2008;23:266–271. doi: 10.1002/gps.1872. [DOI] [PubMed] [Google Scholar]
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? Journal of Neuropsychiatry & Clinical Neurosciences. 2005;17:7–19. doi: 10.1176/jnp.17.1.7. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Human brain mapping. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yao H, Takashima Y, Mori T, Uchino A, Hashimoto M, Yuzuriha T, Miwa Y, Sasaguri T. Hypertension and white matter lesions are independently associated with apathetic behavior in healthy elderly subjects: the Sefuri brain MRI study. Hypertension research : official journal of the Japanese Society of Hypertension. 2009;32:586–590. doi: 10.1038/hr.2009.65. [DOI] [PubMed] [Google Scholar]