Abstract
Background
Whereas some studies have shown clear evidence for an augmentation effect of d-cycloserine (DCS) on exposure therapy for anxiety disorders, other studies have shown weak effects or no effect at all. Some preclinical data suggest that the DCS augmentation effect is moderated by the success of the extinction trials. Therefore, we conducted a re-analysis of existing data to examine whether the effects of DCS on clinical outcome would vary as a function of response to the exposure session (i.e. exposure success).
Methods
In a clinical trial, patients with height phobia received two sessions involving 30 minutes of virtual reality exposure therapy and were randomly assigned to a pill placebo (N=14) or 50 mg of DCS (N=15) immediately after each session.
Results
Mixed-effects regression analysis showed that the effects of DCS administration on clinical improvement was moderated by the level of fear experienced just prior to concluding exposure sessions. Patients receiving DCS exhibited significantly greater improvement in symptoms relative to patients who received placebo when subjective fear was low at the end of the exposure. In contrast, when end fear was still elevated, patients receiving DCS improved less compared to those receiving placebo.
Conclusions
DCS appears to enhance the benefits of exposure treatment when applied after a successful session, but it seems to have detrimental effects when administered after inadequate/unsuccessful exposures.
Trial Registry
The Trial is registered at ClinicalTrials.gov (NCT01102803).
Keywords: CBT, Cognitive Behavioral Therapy, Exposure therapy, Fear extinction, d-cycloserine, Moderators, Acrophobia
Introduction
Despite the overall strength of d-cycloserine (DCS) augmentation effects for extinction learning (1, 2), there is evidence of failures to find an augmentation effect in both animal (3, 4) and human (5-7) paradigms. A number of animal studies have investigated the limits of DCS augmentation effects and indicate that augmentation effects are achieved only with animals that have demonstrated extinction at the time the DCS is administered. For example, Weber and colleagues (4) separated animals into those that had and those that had not demonstrated extinction learning; only the former group showed DCS augmentation effects. Likewise, in a re-analysis of a null-finding study (3) of DCS augmentation, Bouton and colleagues (8) subsequently performed a median split of their sample based on the extinction effects during the drug session; again, a significant DCS augmentation effect was seen only for the animals that had demonstrated stronger extinction learning.
Animal studies have also shown that this learning-specific DCS augmentation effect can be manipulated by restricting the number of extinction trials. Specifically, both Lee and colleagues (9) and Bouton and colleagues (8) found that DCS augmentation was evident only when a sufficient number of trials was offered for extinction learning. Importantly, Lee and colleagues (9) also found evidence for DCS augmentation of fear reconsolidation when extinction trials were limited; subjects that had received only limited cue exposure showed an increase in fear during a subsequent test. Hence, whether DCS has beneficial or detrimental effects appears to be linked with the sufficiency of fear learning achieved during (extinction) training.
These findings have direct application to the understanding of the differential effects of DCS augmentation observed in clinical studies with humans. Against a backdrop of significant DCS augmentation of exposure therapy (10-13), there are studies that have shown little benefit (6, 7, 14), and, most recently, evidence of detrimental effects relative to placebo augmentation (5). The adequacy of extinction learning is a prominent potential explanation for these variable results, with the hypothesis that inadequate exposure therapy led to reconsolidation effects in the study by Litz and colleagues (5). These findings encourage examination of the relation between successful extinction learning and the direction and strength of DCS effects in humans.
We used studies reporting the re-analyses of null results with animals (4, 8) as a model for a re-analysis of a null trial of DCS augmentation in humans (15). In our target study, we tested the efficacy of post-session, instead of pre-session, dosing of 50 mg of DCS. Pre-session dosing has been the strategy employed in all studies to date (1). We used post-session administration because, if effective, it will provide the clinician with the opportunity to apply DCS more judiciously (i.e., only after sessions deemed successful). We found that 50 mg of DCS administered following each of two sessions of 30 minutes of graded virtual reality exposure did not result in better overall clinical outcomes than identical exposure combined with placebo administration (15).
The current report represents a re-analysis of these data, examining whether the effects of post-session DCS administration on clinical outcome would vary as a function of response to the exposure session (i.e., exposure success). Because exposure therapy during each of the two sessions was delivered in a graded fashion (i.e., patients moved up a hierarchy of increasingly more challenging situations [virtual heights]), we used the fear rating patients provided just prior to concluding the exposure exercises as an index of exposure success, with lower end fear ratings indexing evidence of fear extinction and higher end fear ratings indexing relatively unsuccessful fear extinction. We used the Clinical Global Impressions Improvement scale, an established clinician-rated measure for measuring improvement in clinical trials (16), to index improvement in height phobia severity. We predicted that the adequacy of exposure training during the session would moderate the effects of DCS on clinical outcome, such that the advantage of DCS over placebo would be greater for cases evidencing lower end fear ratings than for cases evidencing higher end fear ratings during the previous session. To test this hypothesis, we used a mixed-effects regression approach (see data analytic section below) relating the interaction between treatment condition (DCS vs. Placebo) and end fear ratings during one session (i.e., session 1 or session 2, respectively) to CGI-I scores obtained at the beginning of the following session (i.e., session 2 and posttreatment, respectively).
Methods and Materials
Participants
A description of the study design, procedures and main outcome findings have been reported elsewhere (15). Participants were 29 medication-free adults (Mage= 33.38; 76% female; 59% Non-Hispanic White, 24% African-American, 17% other) diagnosed with DSM-IV-TR acrophobia, as determined by the Structured Clinical Interview for DSM’IV Diagnosis (SCID-I), who reported elevated levels of fear (> 50 on a 0-100 scale) on the highest floor of a virtual elevator environment. Patients were excluded if they endorsed a lifetime history of bipolar disorder, schizophrenia, psychosis, delusional disorders, obsessive-compulsive disorder, as well as current or recent diagnosis of substance use disorders, PTSD, panic disorder, eating disorders, or current or recent suicidality or suicidal behavior. For safety reasons, participants were also excluded if they had a history of head trauma causing loss of consciousness, or seizure, cognitive impairment, or were pregnant.
Study Design and Treatments
Eligible participants received two sessions involving 30 minutes of virtual reality exposure therapy and were randomly assigned to one of two blinded arms: (1) administration of pill placebo immediately after each session (N=14) or (2) administration of 50 mg of DCS after each session (N=15). Acrophobia symptom severity was assessed at the beginning of each therapy session (week 1 and week 2), at posttreatment (at week 3), and at 1-month follow-up. Clinical improvement was assessed at the beginning of session 2 (week 2), at posttreatment (at week 3), and at 1-month follow-up. Every 5 minutes throughout the exposure session participants reported their subjective fear levels (i.e., subjective units of distress [SUDS]) (17) on a 0 to 100 scale (0 indicates no fear, 100 indicates extreme fear or panic). The present study only reports on data collected at baseline, during the sessions, and at posttreatment. The Southern Methodist University Institutional Review Board approved the study protocol and participants provided written informed consent.
Virtual Reality Exposure Therapy (VRE)
VRE was manualized, administered by advanced doctoral students, and supervised by the first and last authors (JAJS and CDT). In the first session (60 minutes), which occurred a week after the baseline visit, therapists first provided participants with a rationale for VRE. Wearing a virtual reality helmet and goggles, participants were then guided through their first graded exposure exercises, which involved moving up a simulated glass elevator, and stepping out on a series of catwalks, balconies, and rooftop in a hotel setting. The first exposure exercise began at a floor which had yielded SUDS > 50 during the baseline assessment. Based on feedback from the patient, therapists gradually moved the patients up to higher floors, for a total of 30 minutes of VRE exposures. At the second session, which occurred one week following the first session, participants received an additional 30 minutes of VRE exposures, which were completed in a similar fashion. Immediately after each session, participants ingested the study pill (DCS or Placebo).
Medication
Study capsules were compounded by Abrams Royal Pharmacy in Dallas, TX containing: (a) 50 mg DCS (derived from Seromycin 250 mg capsules) and polyethylene glycol 3350 powder or (b) polyethylene glycol 3350 powder. All capsules were identical in appearance to maintain the blind. The rationale for selecting 50 mg as the dose was that this dose showed efficacy in previous work examining exposure enhancement with pre-session administration of DCS (10, 11).
Measures
Fear
Participants reported their subjective fear levels (i.e., subjective units of distress [SUDS]) (17) on a 0 to 100 scale (0 indicates no fear, 50 indicates moderate fear, and 100 indicates extreme fear or panic) every 5 minutes throughout the exposure session. The fear rating provided just prior to concluding the exposure exercises was used as an index for exposure success (i.e., fear extinction), with lower ratings indicating higher success, and higher ratings indicating lower success. Because beginning fear was assessed at the first floor and end fear was assessed at the last floor attempted during the exposure session, we did not use a change score (i.e., difference between end fear and beginning fear). Instead, we used end fear and corrected for beginning fear and number of virtual floors successfully “passed” (see data analysis below).
Clinical Global Impressions Severity and Improvement scales (CGI-S and CGI-I)
The CGI-S and CGI-I are widely used measures of global psychopathology severity and improvement initially developed for the study of psychotropic drugs (16). The CGI-S and CGI-I allow clinicians to determine whether a particular condition has improved, worsened, or remained the same. The CGI-S asks clinicians to evaluate the participant’s severity on a scale of 1 to 7 (1 = normal, not at all ill; 2 = borderline mentally ill; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill; 7 = extremely ill). The CGI-I asks clinicians to rate the level of improvement (starting at the beginning of session 2) using a 7-point scale (1 = very much improved; 2 = much improved; 3 = minimally improved; 4 = no change; 5 = minimally worse; 6 = much worse; 7 = very much worse). In making their CGI ratings, the therapists used the SCID-I (including the simple phobia module) as well as additional measures of acrophobia symptoms (Acrophobia Questionnaire (18), and Attitudes Toward Heights Questionnaire (19)).
Data Analysis
Because patients participated in two exposure/pill administration sessions, each participant contributed up to two data points in the analyses; 2 patients only had one data point because they dropped out of the study before they could be assessed on CGI-I after the second exposure session, 26 patients had two data points, and one patient dropped out between the first exposure and the follow-up session, thereby contributing no data to the analysis. All dropouts were in the placebo condition.
Because patients contributed more than one data point to the analyses, we used mixed-effects regression models (MRM), which allows for data points to be correlated and participants to contribute differing numbers of data points to the analysis. The MRM model consisted of a patient’s CGI-I at a given session being predicted by drug condition (DCS vs. Placebo), fear level at the end of the previous session, and the interaction of the drug condition and fear level at the end of the previous session. We also included the following control variables: baseline severity (CGI-S, assessed the week before the first exposure session), fear level at the beginning of the previous exposure session, and number of virtual floors successfully “passed” during the previous session. The first two control variables were included to ensure that our results would not be merely a function of severity, and the last variable was included to take into account the fact that end fear at a session could in part be related to the number of floors to which a patient was exposed. Finally, the MRM model used full information maximum likelihood estimation and the Satterthwaite correction for calculating degrees of freedom (resulting in different degrees of freedom for the tests of the significance of each regression coefficient). We used an “unstructured” matrix for the covariance matrix of the errors of the repeated measures, thereby allowing the error of the repeated measures to be correlated and their variances to differ across time.
Results
There were no between-group (DCS vs. Placebo) differences on any of the demographic variables or any of the indices of clinical severity assessed at baseline (see Table 1). We observed considerable variability in end fear ratings (M=21.84; SD=14.54; range: 0-62) and CGI-I scores (M=2.57; SD=0.98; range: 1-4), but the mean scores indicated that, on average, patients showed reasonable fear extinction and improvement in clinical status, with no evidence of worsening in clinical status (i.e., no participant was rated higher than “4” on the CGI-I).
Table 1.
Baseline data
| Variable | DCS (n = 15) |
PLA (n = 14) |
P Value |
|---|---|---|---|
| Acrophobia Avoidance Questionnaire (AAVQ) , M (SD) | 20.73 (7.74) | 23.06 (10.60) | .50 |
| Acrophobia Anxiety Questionnaire (AAQ), M (SD) | 48.27 (20.98) | 58.36 (25.71) | .26 |
| Attitude Towards Heights Questionnaire (ATHQ), M (SD) | 45.67 (8.53) | 47.71 (12.98) | .62 |
| Clinical Global Impression of Severity (CGI-S), M (SD) | 4.13 (.52) | 4.14 (.36) | .96 |
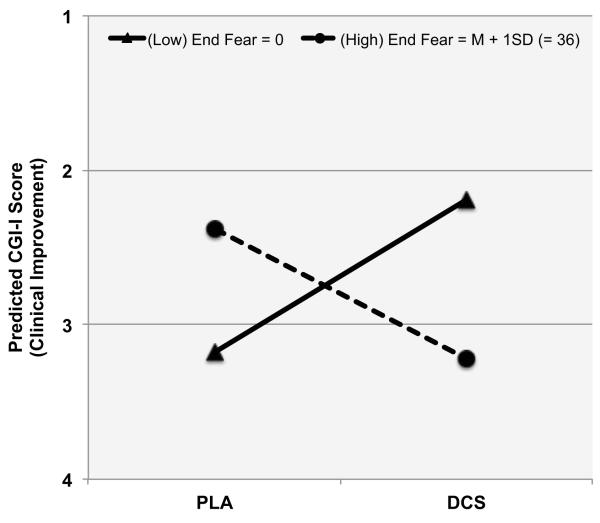
As hypothesized, the effect of pill administration (DCS vs. Placebo) on CGI-I at the beginning of the next session was moderated by the session end fear level [β= −.05, t (50) = 2.84, p = .007 for the interaction between condition and end fear level]. To examine the nature of this interaction, we followed the procedure recommended by Aiken and West (20), which computes the model-based predicted effect of the intervention (DCS vs. Placebo) at different levels of the moderator (end fear level). This approach has the advantage of using all the data from all participants to calculate the effect of the intervention, as opposed to examining the intervention effects separately within different subgroups. As illustrated in Figure 1, we evaluated the model-based predicted effects of DCS (vs. Placebo) administration both for patients who exhibited strong extinction learning before the pill administration (those who reported end fear = 0 [“no fear”]) and for patients who exhibited less extinction learning and thus higher end fear (end fear = M + 1SD = 36). For patients reporting an end fear of 0 [“no fear”], those receiving DCS were rated almost 1 full point more improved on the CGI-I than those receiving placebo [β = .99, t (44) = 2.10, p = .04]. For participants who reported end fear at 1 SD above the mean of the sample (end fear = 36), those receiving DCS were rated .84 points less improved on the CGI-I than those receiving placebo [β = −.84, t(48) = 2.75, p = .025].
Figure 1.
CGI-I outcome effects of the interaction between drug condition and exposure adequacy as assessed by end fear scores.
Note. CGI-I is the Clinical Global Impressions Improvement scale that uses a 7-point scale, with lower scores indicating greater improvement 7-point scale (1 = very much improved; 2 = much improved; 3 = minimally improved; 4 = no change; 5 = minimally worse; 6 = much worse; 7 = very much worse). End Fear is the fear rating provided just prior to concluding the exposure exercises. Fear was rated 0-100 scale (0 = no fear; 50 = moderate fear; 100 = extreme fear or panic), and thus 0 indicates exposure success and higher ratings indicate less exposure success.
Examining the interaction from an additional perspective can add further insight into our understanding of the effects of DCS. Again using the Aiken and West approach (20) to calculate model based predictions of simple slopes using all the data, we found that the relation between end fear at the previous session and CGI-I at the current session was not significant for participants in the Placebo condition [p=.12]. However, for those receiving DCS, the relation between prior session end fear and current session CGI-I was significant [β = .03, t(48)= 2.43, p = .019], with higher end fear being associated with higher (worse) CGI-I at the next session. This result is consistent with the idea that DCS enhances retention of whatever emotional learning occurred during a session.
Discussion
This manuscript reports on a re-analysis of a null finding yielded by a study evaluating the effects of post-session administration of DCS for augmenting exposure therapy (15). Taking into consideration pre-clinical data suggesting that the effects of DCS on extinction retention may depend on the degree of fear extinction learning, we tested the hypothesis that the effects of post-session DCS administration on clinical improvement would be moderated by the level of fear reported by patients just prior to concluding their exposure sessions (i.e., end fear). Consistent with prediction, our results indicated that, when end fear was low (i.e., high extinction learning), patients who received DCS exhibited significantly greater improvement in symptoms relative to patients who received placebo. Conversely, when end fear was higher (i.e., lower extinction learning), patients receiving DCS showed significantly less subsequent improvement in symptom severity relative to those receiving placebo. The observed effects were clinically meaningful, with CGI-I scale score differences between DCS and placebo estimated to be 1 point for those reporting very low fear (i.e., “0”) at the end of the previous session, and .84 points in the opposite direction for patients reporting fear at 1 SD above the sample mean (i.e., “36”) at the end of the previous session.
Our findings may provide a context for understanding the mixed results with respect to the efficacy of pre-session administration of DCS for enhancing exposure therapy outcomes for the anxiety disorders. Specifically, following a series of studies showing strong effects (10-13), and other studies have shown weak effects (14) or no effect at all (5-7). The findings of the present study suggest that the weak or null effects observed in these previous studies may be accounted for by large individual variability in fear extinction observed in therapy sessions. Indeed, it was around the desire for judicious dosing of DCS that we originally investigated post-session dosing of DCS (15), as encouraged by the success of this strategy in animal studies (1, 21). Our current results suggest that the decision to administer DCS post-session should be made in relation to the degree of exposure success achieved during each session by each patient. As such, future investigations could explicate the type and degree of clinical response to an exposure session that best predicts beneficial augmentation effects from DCS.
These findings add to a growing body of literature indicating that the effective application of DCS for augmenting exposure therapy may require clinicians to take into consideration individual-difference variables (22), including degree of response achieved in each exposure session. Importantly, the findings of the current study show that DCS can indeed exert its desired effects (i.e., enhancing outcome of exposure therapy) when applied after a successful exposure session. However, this study also shows that when administered in combination with a session characterized by inadequate fear attenuation DCS can interfere with exposure therapy. Preclinical research indicates that the effect of DCS is to enhance whatever emotional learning has occurred; without sufficient extinction learning on drug, there is no or little beneficial outcome to enhance (8). Moreover, insufficient exposure may also set the stage for reconsolidation of active fear memories (23), and there is evidence that DCS may be able to enhance these adverse reconsolidation effects as well, leading to poorer outcome relative to placebo administration, as potentially observed in this and other studies (9, 24).
A number of limitations deserve mention. First, the sample size was relatively small. The fact that we found significant interactive effects with a small sample speaks to the magnitude of the observed effects. Nonetheless, our findings require replication with larger and more diverse samples with other anxiety disorders. Second, we only examined post-administration of 50 mg of DCS, and therefore cannot make inferences with respect to dose-response or timing of administration effects. Third, our analyses indicated that the fear level reported just prior to concluding exposure interacted with DCS to predict clinical improvement, while controlling for fear at the beginning of the session, number of floors successfully passed during exposure and baseline clinical severity. Although statistically these analyses capture the moderating effects of within-session attenuation of fear, future studies should examine whether end fear or within-session reduction in fear is the critical dimension for moderating the effects of DCS. Standardizing the exposure therapy sessions would facilitate this aim. Finally, our study represents a post-hoc analysis that, at best, provides proof-of-concept evidence for post-session dosing of DCS as combined with judicious selection of which sessions should be followed by DCS administration. Pre-session dosing may still offer more powerful DCS augmentation effects than post-session dosing, and as such, other procedures to maximize exposure success (e.g., adding DCS to later, but not initial, exposure trials to try to ensure greater success during exposures(12)) may provide effective clinical strategies for enhancing DCS augmentation effects.
If replicated and extended using data from studies validating the efficacy of pre-session DCS dosing, the results of the present study provide the basis for developing an algorithm for the application of DCS in conjunction with exposure therapy for the anxiety disorders. Rather than using a blanket approach (i.e., pre-session administration), which has been promoted by research so far, select and targeted dosing of DCS (e.g., at specific stages of treatment or following sessions that are successful) may ultimately be more likely to yield the clinical outcomes that clinicians and patients desire.
Supplementary Material
Disclosures and Acknowledgements
This report was supported by Diversity Supplement to R01MH075889 from the National Institute of Mental Health. We appreciate the support of the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health (NIMH) or the National Institutes of Health (NIH).
Dr. Smits receives royalties from various book publishers for work unrelated to this study. Dr. Rosenfield. Dr. Powers, Dr. Telch, and Dr. Tart report no biomedical financial interests or potential conflicts of interest. Dr. Hofmann has served as a consultant for Merck and Schering-Plough and receives book royalties from various book publishers for work unrelated to this study. Dr. Otto serves as consultant for MicroTransponder, Inc. and receives royalties from various book publishers for work unrelated to this study. Dr. Pollack’s disclosures over the last three years include: Advisory Boards and Consultation: Eli Lilly, Medavante, Otsuka, Targia Pharmaceuticals Research Grants: Bristol Myers Squibb, Euthymics, Forest Laboratories, GlaxoSmithKline, NCCAM, NIDA, NIMH Equity: Medavante, Mensante Corporation, Mindsite, Targia Pharmaceutical Royalty/patent: SIGH-A, SAFER interviews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann SG, Smits JA, Asnaani A, Gutner CA, Otto MW. Cognitive enhancers for anxiety disorders. Pharmacol Biochem Behav. 2011;99:275–284. doi: 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral neuroscience. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 4.Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012 doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- 7.Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of the effect of D-cycloserine on enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- 15.Tart CD, Handelsman PR, Deboer LB, Rosenfield D, Pollack MH, Hofmann SG, et al. Augmentation of exposure therapy with post-session administration of d-cycloserine. J Psychiatr Res. doi: 10.1016/j.jpsychires.2012.09.024. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy W. Assessment Manual for Pharmacology. US Giovernment Printing Office; Washington, DC: 1976. [Google Scholar]
- 17.Wolpe . Psychotherapy by Reciprocal Inhibition. Stanford University Press; Palo Alto, CA: 1958. [Google Scholar]
- 18.Cohen D. Comparison of self-report and overt-behavioral procedures for assessing acrophobia. Behav Ther. 1977;8:17–23. [Google Scholar]
- 19.Abelson JL, Curtis GC. Cardiac and neuroendocrine responses to exposure therapy in height phobics: desynchrony within the ‘physiological response system’. Behav Res Ther. 1989 doi: 10.1016/0005-7967(89)90091-0. [DOI] [PubMed] [Google Scholar]
- 20.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage Publications; Newbury Park, CA: 1991. [Google Scholar]
- 21.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 22.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 24.Lee JL, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.