Abstract
We evaluated the association of body mass index (BMI), waist (WC) and hip circumferences (HC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) with diabetes in Caucasians, Native Hawaiians, and Japanese Americans aged 45–75 years in the Multiethnic Cohort. Diabetes cases were obtained from self-reports and by linkages with health insurance plans. We estimated adjusted prevalence odds ratios (POR) and compared the area under the receiver operating characteristic curves (AUC). All measures were positively associated with diabetes prevalence; the PORs were 1.24–1.64 in men and 1.52–1.83 in women. In all 3 ethnic groups, the AUCs in men were greater for BMI than for the other measures, while in women the AUCs were greater for combined models than for BMI alone, but the differences were small and not clinically significant. It does not appear that one anthropometric measure best reflects diabetes prevalence or performs better in one ethnic group than in another.
Keywords: type 2 diabetes, ethnicity, anthropometry, waist circumference, BMI, adiposity
Introduction
Obesity is a major risk factor for type 2 diabetes,1 but different methods to assess excess body fat often yield diverse results, in particular across populations. A common measure to define obesity is the body mass index (BMI), a combination of weight and height. According to WHO recommendations, cutpoints that classify overweight and obesity were set at 25 kg/m2 and 30 kg/m2, since these margins confer higher risks of diabetes and cardiovascular diseases.2 Scientific evidence points at differential associations between BMI, percentage of body fat, and health risk in Asian populations as opposed to Europeans, with a substantial proportion of Asians having a high metabolic disease risk at BMIs lower than the existing WHO cutpoints.3–5 Besides total amount, the distribution of body fat seems critical, since abdominal fat and more specifically visceral fat has a strong association with insulin resistance and type 2 diabetes mellitus.6 The relatively higher proportion of abdominal visceral relative to subcutaneous adipose tissue among Japanese Americans than Caucasians7 appears to be responsible for the adverse effects of excess body weight.8 Thus, additional anthropometric measures, such as waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), or waist-to-height ratio (WHtR), may provide a better indicator for abdominal obesity.9 Although all measures are associated with diabetes, it is controversial which one has the best predictive power.5;10–12 Evidence from 18 studies conducted in the Asia-Pacific region suggests a stronger association of diabetes with WC or WHR than BMI in some populations.5 Therefore, we evaluated the association of different anthropometric measures of obesity with diabetes in a cross-sectional design among Caucasians, Native Hawaiians and Japanese Americans in the Hawaii component of the Multiethnic Cohort Study (MEC).
Methods
Study population
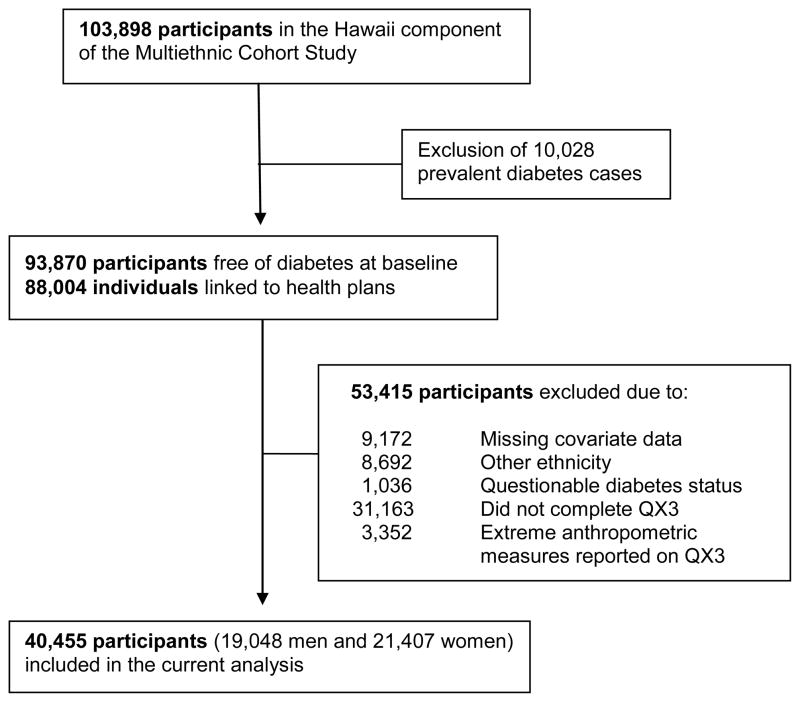
The MEC was established in 1993–1996 to evaluate associations between diet and cancer among five different ethnic groups living in Hawaii and California.13 More than 215,000 men and women, aged 45–75 years at baseline were recruited into the cohort by returning a mailed self-administered survey consisting of a food frequency questionnaire and additional questions on demographics, medical conditions, anthropometric measures, and lifestyle factors. Response rates ranged from 28% to 51% in the different ethnic-sex groups and comparisons with US census data indicated that the MEC participants represent all levels of education, although cohort members were somewhat better educated than the general population.13 Annual linkages with state and national death certificate files have been performed to obtain information on vital status. A linkage with health plans to identify incident diabetes cases was only possible in Hawaii; thus, the present study is limited to the Hawaii component of the MEC, consisting of 103,898 participants of primarily Caucasian, Japanese American, and Native Hawaiian ancestry.4 For all analyses related to diabetes risk, subjects with prevalent diabetes at study entry (n=10,028), questionable incident diabetes status (n=1,036), other ethnicity (n=8,692), and missing covariate data at baseline (n=9,172) were excluded (Figure 1). Of the remaining 74,970 cohort members, 31,163 did not complete a follow-up questionnaire (QX3) assessing waist and hip measures, and 3,352 participants were excluded because their WC or HC values were outside the range of the sex-ethnic mean ±3 standard deviations (SD), their weight was ≤31.8 kg or ≥204.5 kg, or their BMI was <10 kg/m2, leaving 40,455 participants (19,048 men and 21,407 women). Study protocols were approved by the Committee on Human Studies at the University of Hawaii and by the Institutional Review Board of Kaiser Permanente.
Figure 1.
Cohort participants included in the current analysis, Hawaii component of the MEC
Exposure assessment
The baseline questionnaire (QX1) collected information on age, ethnicity, education, smoking, physical activity, and a diet history using a validated food frequency questionnaire during the previous year.13;14 Participants were also asked if they had ever been diagnosed with high blood pressure or had taken blood pressure medication. Weight and height at baseline were self-reported and BMI as weight divided by height squared was calculated. As part of follow-up, QX3 in 2003–2007 reassessed weight and asked about WC and HC. Participants were provided with a tape measure and instructions to measure (in standing position) WC at the navel and HC at the widest area between waist and thighs. Since height was not reassessed at QX3, but is unlikely to differ from baseline, BMI at QX3 was calculated as weight at QX3 divided by baseline height squared, WHR as waist divided by HC, and WHtR as waist divided by height. BMI was categorized as normal weight (BMI <25.0 kg/m2), overweight (25.0–29.9 kg/m2), and obese (BMI ≥30.0 kg/m2).
Case ascertainment
As described in detail elsewhere,4 diabetes cases were identified by the following three methods: through a short follow-up questionnaire (QX2) asking about medical conditions in 1999–2003 (response rate 84%), a medication questionnaire including diabetes drugs administered in 2003–2006 (response rate 38%), and linkage with health insurance plans in 2007.4 All participants of the cohort, known to be alive and not refusing to participate, were linked with the diabetes care registries of the two major health insurers, which capture 90% of the population of Hawaii: Kaiser Permanente Hawaii and Blue Cross/Blue Shield. Diabetes cases that were self-reported in the follow-up questionnaire but not confirmed by the health plans were deemed questionable, and thus, not included in this analysis.
Statistical analysis
We compared characteristics of the study population to those excluded and tested for difference with χ2- or t-test. Anthropometric measures of the study population are given as percentages or mean +/− SD by sex and ethnicity. Logistic regression models, stratified by sex, were used to estimate prevalence odds ratios (POR) and 95% confidence intervals (CI) for prevalent diabetes associated with one sex-specific SD increase in anthropometric values to allow for comparisons across measures. We adjusted the analyses for BMI (normal weight, overweight, obese), ethnicity (Caucasian, Native Hawaiian, Japanese American), education (≤12 years, 13–15 years, ≥16 years), physical activity (never, ½–1, 2–3, ≥4 h/week), smoking status (never smoker, former smoker, current smoker), hypertension (yes, no), and alcohol intake (<1 drink/month as non-drinkers, ≥1 drink/month as drinkers). Red meat and dietary fiber intake were also included as covariates since they were significantly associated with diabetes risk in previous analyses.15;16 After calculating energy densities (g/4184 kJ*d), intakes were grouped into quintiles. To assess the ability of each anthropometric variable to discriminate between those with and without diabetes, areas under the receiver operating characteristic curves (AUC), also called c-statistic, were compared by using a contrast matrix with a method described by DeLong et al.17 To determine performance of the anthropometric measures by ethnicity, separate analyses for each ethnic group were performed. We also stratified by normal vs. overweight/obese status at QX3 and repeated analysis after excluding subjects whose weight had changed by more than +/− 5 kg between QX1 and QX3. Finally, we estimated diabetes risk for cross-tabulations of 3 BMI categories and 2 WC categories using 102 cm for men and 88 cm for women as cutpoints.18
Results
The proportion of diabetes cases was similar among the 40,455 participants with information on waist and hip measurements as in the 34,515 individuals who did not respond to QX3 (Table 1). However, the study population for the current analysis was slightly younger and leaner, had a higher proportion of well educated subjects and non-smokers, and reported a lower prevalence of hypertension. All adiposity measures (Table 2) differed significantly by ethnicity (p<0.0001). Native Hawaiian men and women had a higher mean BMI (28.7 and 28.6 kg/m2) than Caucasians (26.5 and 25.4 kg/m2) and Japanese Americans (25.2 and 23.6 kg/m2). Similarly, WC and HC were greatest in Native Hawaiians, followed by Caucasian, while Japanese Americans had the lowest anthropometric values. The relative measures of WHR and WHtR showed small, but statistically significant differences, across ethnic groups.
Table 1.
Characteristics of the study population and of subjects without waist measures
| Study population | Subjects not part of QX3 | p-value* | |||
|---|---|---|---|---|---|
|
| |||||
| N | % | N | % | ||
| Number of subjects | 40,455 | 100 | 34,515 | 100 | |
| Age (mean±SD) | 57 ± 8.6 | 60.2 ± 9.5 | <0.0001 | ||
| BMI (mean±SD) | 24.7 ± 4.1 | 25.2 ± 4.8 | <0.0001 | ||
| Ethnicity | |||||
| Caucasian | 16,314 | 40.3 | 12,994 | 37.6 | <0.0001 |
| Native Hawaiian | 4,970 | 12.3 | 5,545 | 16.1 | |
| Japanese American | 19,171 | 47.4 | 15,976 | 46.3 | |
| Education | |||||
| ≤12 years | 11,108 | 27.5 | 14,730 | 42.7 | <0.0001 |
| 13–15 years | 12,259 | 30.3 | 10,170 | 29.5 | |
| ≥16 years | 17,088 | 42.2 | 9,615 | 27.9 | |
| Smoking Status | |||||
| Never smoker | 19,137 | 47.3 | 14,069 | 40.8 | <0.0001 |
| Former smoker | 16,062 | 39.7 | 14,100 | 40.9 | |
| Current smoker | 5,256 | 13.0 | 6,346 | 18.4 | |
| Diabetes Status | |||||
| No | 35,804 | 88.5 | 30,607 | 88.7 | 0.46 |
| Yes | 4,651 | 11.5 | 3,908 | 11.3 | |
| Hypertension History | |||||
| No | 28,122 | 69.5 | 21,294 | 61.7 | <0.0001 |
| Yes | 12,333 | 30.5 | 13,221 | 38.3 | |
From χ2- and t-tests
Table 2.
Anthropometric measures by sex and ethnicity at QX3*
| All | Caucasian | Native Hawaiian | Japanese American | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Men (n) | 19,048 | 8,027 | 2,121 | 8,900 | ||||
| Body mass index (kg/m2) | 26.2 | 4.1 | 26.5 | 4.0 | 28.7 | 5.0 | 25.2 | 3.5 |
| Weight change since QX1 (kg) | 0.62 | 6.99 | 1.36 | 7.62 | 0.53 | 9.17 | −0.03 | 5.58 |
| Waist circumference (cm) | 95.7 | 10.8 | 98.8 | 11.0 | 100.5 | 12.4 | 91.7 | 8.7 |
| Hip circumference (cm) | 100.7 | 8.9 | 103.4 | 8.8 | 105.3 | 10.5 | 97.3 | 7.0 |
| Waist-hip ratio | 0.95 | 0.06 | 0.96 | 0.06 | 0.95 | 0.06 | 0.94 | 0.06 |
| Waist-to-height ratio | 0.55 | 0.06 | 0.55 | 0.06 | 0.57 | 0.07 | 0.55 | 0.05 |
|
| ||||||||
| Women (n) | 21,407 | 8,287 | 2,849 | 10,271 | ||||
| Body mass index (kg/m2) | 25.0 | 4.9 | 25.4 | 4.9 | 28.6 | 6.1 | 23.6 | 3.9 |
| Weight change since QX1 (kg) | 1.09 | 6.66 | 1.70 | 7.36 | 1.65 | 9.09 | 0.44 | 4.99 |
| Waist circumference (cm) | 86.5 | 13.0 | 88.1 | 13.3 | 94.3 | 14.7 | 83.0 | 10.9 |
| Hip circumference (cm) | 101.2 | 11.1 | 104.0 | 11.0 | 108.8 | 13.5 | 96.9 | 8.2 |
| Waist-hip ratio | 0.85 | 0.08 | 0.85 | 0.08 | 0.87 | 0.08 | 0.86 | 0.08 |
| Waist-to-height ratio | 0.54 | 0.08 | 0.54 | 0.08 | 0.58 | 0.09 | 0.53 | 0.07 |
All differences by ethnicity as assessed by t-tests are significant (p<0.0001)
All anthropometric measures were significantly associated with diabetes prevalence in men (Table 3). The adjusted POR for a one SD increase ranged from 1.25 (95% CI: 1.20–1.31) for WHR to 1.64 (95% CI: 1.56–1.72) for BMI. With 0.731, the AUC for BMI was significantly greater than for WHR, WHtR, WC, and HC (all p<0.05), but the differences were small (<0.03). When we added BMI to the other models, the PORs for the respective measures were attenuated and remained significant except for the combination of BMI and HC. However, the AUCs did not improve. After stratification by ethnicity, the AUCs tended to be lower for Native Hawaiians and Japanese Americans than for Caucasians, irrespective of the anthropometric measure or combination of measures. For BMI, the AUC was 0.750 for Caucasians, 0.696 for Native Hawaiians, and 0.676 for Japanese Americans. As for the overall population, the AUCs were greater for BMI than for the other measures in all ethnic groups although not always significant and did not increase by including another anthropometric measure into the model.
Table 3.
Prevalence odds ratios (POR) for diabetes associated with a one standard deviation increment in each anthropometric measure and corresponding areas under the receiver operating characteristics (AUC) in men, Hawaii component of the MECa
| Measuresb | All men | Caucasian | Native Hawaiian | Japanese American | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| POR | 95% CI | AUC | POR | 95% CI | AUC | POR | 95% CI | AUC | POR | 95% CI | AUC | |
| BMI | 1.64 | (1.56, 1.72) | 0.731 | 1.77 | (1.63, 1.92) | 0.750 | 1.40 | (1.27, 1.54) | 0.696 | 1.68 | (1.57, 1.80) | 0.676 |
| WC | 1.53 | (1.46, 1.60) | 0.721* | 1.63 | (1.50, 1.77) | 0.741* | 1.36 | (1.23, 1.50) | 0.687 | 1.54 | (1.44, 1.65) | 0.664* |
| HC | 1.43 | (1.37, 1.50) | 0.713* | 1.48 | (1.36, 1.61) | 0.730* | 1.28 | (1.17, 1.41) | 0.678* | 1.48 | (1.38, 1.59) | 0.657* |
| WHR | 1.25 | (1.20, 1.31) | 0.702* | 1.32 | (1.22, 1.43) | 0.711* | 1.22 | (1.09, 1.36) | 0.673* | 1.22 | (1.14, 1.29) | 0.642* |
| WHtR | 1.52 | (1.46, 1.60) | 0.723* | 1.69 | (1.56, 1.84) | 0.745 | 1.37 | (1.24, 1.51) | 0.689 | 1.48 | (1.39, 1.59) | 0.662* |
|
| ||||||||||||
| WC | 1.10 | (1.02, 1.18) | 0.731 | 1.14 | (0.99, 1.30) | 0.751 | 1.08 | (0.92, 1.26) | 0.696 | 1.08 | (0.97, 1.20) | 0.676 |
| + BMI | 1.53 | (1.42, 1.65) | 1.60 | (1.40, 1.83) | 1.32 | (1.14, 1.54) | 1.59 | (1.42, 1.77) | ||||
| HC | 0.97 | (0.91, 1.04) | 0.731 | 0.95 | (0.84, 1.07) | 0.750 | 0.96 | (0.83, 1.12) | 0.696 | 1.00 | (0.90, 1.12) | 0.676 |
| + BMI | 1.67 | (1.56, 1.80) | 1.84 | (1.62, 2.09) | 1.44 | (1.24, 1.68) | 1.68 | (1.51, 1.86) | ||||
| WHR | 1.09 | (1.04, 1.14) | 0.732 | 1.14 | (1.05, 1.23) | 0.751 | 1.10 | (0.99, 1.23) | 0.697 | 1.05 | (0.98, 1.12) | 0.677 |
| + BMI | 1.60 | (1.52, 1.68) | 1.70 | (1.55, 1.85) | 1.37 | (1.24, 1.51) | 1.65 | (1.53, 1.78) | ||||
| WHtR | 1.11 | (1.03, 1.19) | 0.731 | 1.25 | (1.09, 1.44) | 0.752 | 1.10 | (0.94, 1.29) | 0.696 | 1.01 | (0.91, 1.13) | 0.676 |
| + BMI | 1.51 | (1.40, 1.63) | 1.47 | (1.28, 1.69) | 1.30 | (1.12, 1.52) | 1.66 | (1.48, 1.87) | ||||
Obtained by logistic regression using sex-specific standard deviations as units; covariates include age, ethnicity, education, physical activity, hypertension, processed red meat intake, dietary fiber intake, smoking and alcohol intake.
Abbreviations: BMI-body mass index, WC-waist circumference, HC=hip circumference, WHR=waist-hip ratio, WHtR=waist-to-height ratio
P<0.05 for DeLong test of difference in AUC between models as compared to the BMI only model
In women (Table 4), all anthropometric measures were also positively associated with diabetes prevalence; the PORs for one SD ranged from 1.52 (95% CI: 1.44–1.59) for WHR to 1.83 (95% CI: 1.74–1.92) for WC. The AUC for BMI (0.762) did not differ from the ones for WC (0.762) and WHtR (0.763), but was significantly higher than for HC (0.744) and WHR (0.741) (p<0.05). Adding any of the other anthropometric measures to the BMI model significantly increased the AUC as compared to the BMI model, but the differences were very small (<0.01). As in men, the AUCs were smaller for Native Hawaiian and Japanese American women (0.723 and 0.718 respectively) than for Caucasians (0.776). In all three ethnic groups, the AUCs were greater for combined models than for BMI alone, but again the differences were small (<0.04).
Table 4.
Prevalence odds ratios (POR) for diabetes associated with a one standard deviation increment in each anthropometric measure and corresponding areas under the receiver operating characteristics (AUC) in women, Hawaii component of the MECa
| Measuresb | All women | Caucasian | Native Hawaiian | Japanese American | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| POR | 95% CI | AUC | POR | 95% CI | AUC | POR | 95% CI | AUC | POR | 95% CI | AUC | |
| BMI | 1.77 | (1.68, 1.86) | 0.762 | 1.85 | (1.68, 2.03) | 0.776 | 1.51 | (1.39, 1.64) | 0.723 | 1.96 | (1.82, 2.12) | 0.718 |
| WC | 1.83 | (1.74, 1.92) | 0.762 | 1.99 | (1.79, 2.20) | 0.782 | 1.62 | (1.47, 1.78) | 0.723 | 1.87 | (1.74, 2.02) | 0.714 |
| HC | 1.59 | (1.51, 1.67) | 0.744* | 1.67 | (1.51, 1.84) | 0.750* | 1.42 | (1.31, 1.54) | 0.706* | 1.69 | (1.56, 1.83) | 0.691* |
| WHR | 1.52 | (1.44, 1.59) | 0.741* | 1.73 | (1.55, 1.93) | 0.746* | 1.40 | (1.26, 1.56) | 0.688* | 1.48 | (1.39, 1.58) | 0.692* |
| WHtR | 1.80 | (1.71, 1.89) | 0.763 | 2.04 | (1.84, 2.26) | 0.788 | 1.60 | (1.45, 1.76) | 0.722 | 1.80 | (1.68, 1.93) | 0.713 |
|
| ||||||||||||
| WC | 1.44 | (1.34, 1.56) | 0.767* | 1.60 | (1.37, 1.88) | 0.787* | 1.35 | (1.16, 1.56) | 0.728 | 1.40 | (1.25, 1.55) | 0.723* |
| + BMI | 1.36 | (1.26, 1.46) | 1.31 | (1.12, 1.53) | 1.23 | (1.08, 1.40) | 1.53 | (1.37, 1.71) | ||||
| HC | 0.92 | (0.84, 1.01) | 0.763* | 0.92 | (0.76, 1.11) | 0.777 | 1.00 | (0.86, 1.16) | 0.723 | 0.88 | (0.77, 1.01) | 0.719* |
| + BMI | 1.89 | (1.73, 2.06) | 1.99 | (1.66, 2.38) | 1.51 | (1.30, 1.76) | 2.16 | (1.89, 2.45) | ||||
| WHR | 1.35 | (1.29, 1.43) | 0.771* | 1.52 | (1.35, 1.71) | 0.792* | 1.30 | (1.16, 1.45) | 0.731* | 1.30 | (1.22, 1.39) | 0.726* |
| + BMI | 1.66 | (1.58, 1.75) | 1.72 | (1.55, 1.90) | 1.47 | (1.35, 1.59) | 1.81 | (1.67, 1.96) | ||||
| WHtR | 1.43 | (1.33, 1.54) | 0.767* | 1.74 | (1.47, 2.06) | 0.790* | 1.30 | (1.11, 1.51) | 0.728 | 1.35 | (1.21, 1.50) | 0.722* |
| + BMI | 1.35 | (1.25, 1.45) | 1.21 | (1.03, 1.43) | 1.26 | (1.10, 1.44) | 1.54 | (1.37, 1.73) | ||||
Obtained by logistic regression using sex-specific standard deviations as units; covariates include age, ethnicity, education, physical activity, hypertension, processed red meat intake, dietary fiber intake, smoking and alcohol intake.
Abbreviations: BMI-body mass index, WC-waist circumference, HC=hip circumference, WHR=waist-hip ratio, WHtR=waist-to-height ratio
P<0.05 for DeLong test of difference in AUC between models as compared to the BMI only model
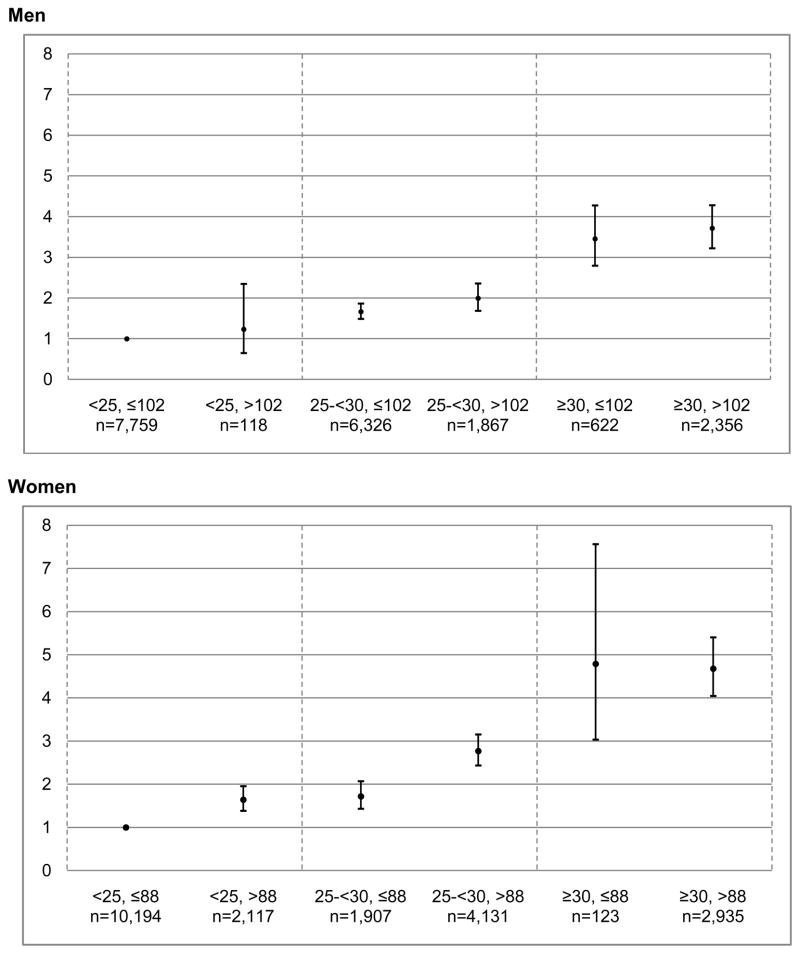
Combined stratification by BMI and WC indicated a higher prevalence of diabetes for individuals with elevated WC in most BMI categories (Figure 2), but the 95% CIs overlapped for men in all categories and also for obese women. Excluding the 6,444 men and 7,015 women whose weight changed by more than +/− 5 kg between QX1 and QX3 increased the AUCs by approximately 0.01 (data not shown). Also, stratification by normal vs. overweight/obese status only improved the AUC values by 0.04–0.07 among men and women with normal BMI.
Figure 2.
Prevalence odds ratios (and 95% confidence intervals) for diabetes by BMI and Waist Circumference for Men and Women, Hawaii component of the MECa
aObtained by logistic regression using stratification of waist and BMI as units; reference is category with BMI<25 kg/m2 and WC≤102 cm in men and 88 cm in women; covariates include age, ethnicity, education, physical activity, hypertension, processed red meat intake, dietary fiber intake, smoking and alcohol intake.
Discussion
This comparison of Caucasians, Native Hawaiians, and Japanese Americans found little evidence across ethnic groups that measures of central adiposity had greater discriminatory power than BMI to distinguish diabetes cases from non-cases. In men, BMI was the best predictor and the combination of BMI with one of the other measures did not improve models. On the other hand, BMI, WC, and WHtR performed equally well among women and combining BMI with any of the other measures improved the predictive power significantly, but the gains in AUCs were small. The relative importance of the different anthropometric measures was similar across ethnic groups, but the AUCs were generally higher in Caucasians than in the other two ethnic groups. The differences in AUC between different anthropometric measures were, although sometimes statistical significant, small and, therefore, unlikely to have clinical importance not even when working with different ethnic groups.
The results of our analysis are in agreement with a meta-analysis of 32 prospective studies10 that detected no significant difference in the magnitude of the association for BMI, WC, and WHR with incident diabetes. Another review confirmed that in prospective studies all anthropometric measures performed equally well but indicated that cross-sectional studies show greater discriminatory power for WC and WHR than for BMI without reaching statistical significance.11 Looking at ethnic-specific reports,11 a Taiwanese study described a non-significant stronger association of WHR than BMI with diabetes.19 A greater predictive power for waist measures than BMI was also shown in a cross-sectional evaluation of health surveys from the USA and England; the AUCs for WC and WHR were greater by 0.03–0.08 than BMI.20 Data from 18 study populations in the Asia-Pacific region suggested a stronger association of diabetes with WC or WHR than for BMI in Asians5 and in a cross-sectional analysis of 16 Asian cohorts, measures of central adiposity were better predictors than BMI overall but not in Chinese or Japanese.21 Given the small differences in these reports (<0.01) and the results of our own study, there is no consistent evidence that any measure of central adiposity currently available performs better than the others. Our findings of higher PORs and AUCs for Caucasians than Japanese Americans agree with the observation by Huxley et al.5 who also found a stronger association between diabetes and BMI in Whites than in Asians using a standardized BMI measure. At the same time, Asians experience a higher diabetes risk at each BMI category as illustrated by the higher prevalence22 and incidence4 of diabetes in Japanese Americans as compared to Caucasians at equal BMI in the MEC. Our results (Figure 2) are comparable to a German report23 that found a higher diabetes risk within BMI categories for persons with higher WC.
Limitations of the present study include the reliance on self-reported anthropometric data and the cross-sectional design, i.e., for most participants WC and HC were measured after the onset of diabetes, but exclusion of participants whose weight had changed between QX1 and QX3 did not materially modify the results. Underreporting of weight is common and likely to be more prevalent among overweight and obese individuals.24 Self-measurements of WC and HC may also be erroneous, but we excluded extreme values to control this problem. We had no information on type of diabetes but given the mean age at diagnosis of 57 years, more than 90% of cases are likely type 2. The ascertainment of diabetes has obvious weaknesses, foremost that the diagnoses were obtained through different methods with their own limitations. Although health plan information is superior to self-reports, cases may have been missed due to lack of health insurance, contact with the health care system, and accurate identification of cases from insurance records.4 Given that approximately 10% of the population of Hawaii is not part of the two insurance plans used for linkage, we might have excluded some cases that could not be confirmed by a registry. Although data on the quality of the Hawaii registries are not available, validation studies of similar registries have shown high specificity and adequate sensitivity.25 Since this population was not screened for diabetes, some non-cases might have undiagnosed diabetes. Detection bias is possible given that obese subjects or those with high-risk ethnic backgrounds may be more likely to undergo testing for diabetes. In comparison to those excluded, the present study population showed more favorable sociodemographic and health related characteristics; thus, generalization of results should be made with caution. Strengths of this study include the large sample size and the high proportion of Native Hawaiian and Japanese American participants. Next to weight and height, several measures of central obesity were assessed and their performances were compared. Furthermore, information on many diabetes risk factors was included in the analysis.
Simple anthropometric measures of central adiposity are attractive as proxies for body fat distribution, since direct measurements with computed tomography (CT) or magnetic resonance imaging (MRI) are laborious, expensive, and not feasible in large-scale studies.9 The biological mechanisms to explain the link between body fat accumulation and diabetes risk point at a central role of visceral adipose tissue in lipid and glucose metabolism due to the production of various hormones and cytokines.8;26 Although associations between visceral abdominal fat mass assessed by CT and diabetes risk have been demonstrated,6;8;27 WC and other central adiposity measures do not have a consistently stronger association with diabetes, possibly because WC does not discriminate between visceral fatness and subcutaneous fatness.10 Our data do not support the hypothesis that WC and other measures of central adiposity are better predictors of diabetes in Asians than Caucasians despite the higher susceptibility of Asians to accumulate abdominal fat.28 Nevertheless, identifying and monitoring persons with high WC is important when screening for diabetes risk in susceptible Asian ethnic groups.29;30
Conclusion
All measures of adiposity evaluated in this study were significantly associated with a higher diabetes risk, irrespective of sex or ethnicity. Observed differences in the discriminatory power of the anthropometric measures as compared to BMI were too small to be clinically relevant suggesting that there is not one optimal adiposity measure that best reflects diabetes risk.11 However, as observed in other studies,20;23 the significant number of normal BMI individuals with high WC found in all ethnic groups who are at risk for type 2 diabetes need to receive appropriate screening tests.
Acknowledgments
The Multiethnic Cohort is supported by NCI grant R37CA54281 (PI: Dr. L.N. Kolonel). The recruitment of Native Hawaiians was funded by grant DAMD 17-94-T-4184 (PI: Dr. A. Nomura). The diabetes project is funded by R21 DK073816 (PI: Dr. G. Maskarinec). We thank Mark M. Schmidt and Aileen Uchida at Kaiser Permanente Center for Health Research, Honolulu, HI and Deborah Taira Juarez and Krista Hodges at HMSA (Blue Cross/Blue Shield of Hawaii) for their assistance in linking the cohort with the health plans.
References
- 1.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr. 2004;80:257–263. doi: 10.1093/ajcn/80.2.257. [DOI] [PubMed] [Google Scholar]
- 2.WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 3.Stommel M, Schoenborn CA. Variations in BMI and prevalence of health risks in diverse racial and ethnic populations. Obesity (Silver Spring) 2010;18:1821–1826. doi: 10.1038/oby.2009.472. [DOI] [PubMed] [Google Scholar]
- 4.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58:1732–1738. doi: 10.2337/db08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9 (Suppl 1):53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 (Suppl 1):S302–S304. doi: 10.1007/s00592-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 9.Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness--a critical review. Int J Obes Relat Metab Disord. 1998;22:719–727. doi: 10.1038/sj.ijo.0800660. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 11.Qiao Q, Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr. 2010;64:30–34. doi: 10.1038/ejcn.2009.93. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Choi SJ, Lee KS, Park HY. Waist circumference and waist-to-height ratio as predictors of cardiovascular disease risk in Korean adults. Circ J. 2009;73:1643–1650. doi: 10.1253/circj.cj-09-0161. [DOI] [PubMed] [Google Scholar]
- 13.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stram DO, Hankin JH, Wilkens LR, Henderson B, Kolonel LN. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in hawaii. J Nutr. 2010;140:68–74. doi: 10.3945/jn.109.112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbrecher A, Erber E, Grandinetti A, Kolonel L, Maskarinec G. Meat consumption and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr. 2010:1–7. doi: 10.1017/S1368980010002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CH, Ho CC, Yang CF, Huang YC, Lai CH, Liaw YP. Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr Res. 2010;30:585–593. doi: 10.1016/j.nutres.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Diaz VA, Mainous AG, III, Baker R, Carnemolla M, Majeed A. How does ethnicity affect the association between obesity and diabetes? Diabet Med. 2007;24:1199–1204. doi: 10.1111/j.1464-5491.2007.02244.x. [DOI] [PubMed] [Google Scholar]
- 21.Nyamdorj R, Qiao Q, Lam TH, et al. BMI compared with central obesity indicators in relation to diabetes and hypertension in Asians. Obesity (Silver Spring) 2008;16:1622–1635. doi: 10.1038/oby.2008.73. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Grandinetti A, Matsuura G, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis. 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Feller S, Boeing H, Pischon T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: implications for routine clinical practice. Dtsch Arztebl Int. 2010;107:470–476. doi: 10.3238/arztebl.2010.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 25.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol. 2004;14:507–516. doi: 10.1016/j.annepidem.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 27.Rendell M, Hulthen UL, Tornquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–749. doi: 10.1210/jcem.86.2.7260. [DOI] [PubMed] [Google Scholar]
- 28.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 29.Moy FM, Atiya AS. Waist circumference as a screening tool for weight management: evaluation using receiver operating characteristic curves for Malay subjects. Asia Pac J Public Health. 2003;15:99–104. doi: 10.1177/101053950301500205. [DOI] [PubMed] [Google Scholar]
- 30.Narksawat K, Podang J, Punyarathabundu P, Podhipak A. Waist circumference, body mass index and health risk factors among middle aged Thais. Asia Pac J Public Health. 2007;19:10–15. doi: 10.1177/101053950701900303. [DOI] [PubMed] [Google Scholar]