Abstract
The cellular basis of the Frank-Starling “Law of the Heart” is the length-dependence of activation, but the mechanisms by which the sarcomere detects length changes and converts this information to altered calcium sensitivity has remained elusive. Here the effect of titin-based passive tension on the length-dependence of activation (LDA) was studied by measuring the tension-pCa relation in skinned mouse LV muscle at two sarcomere lengths (SLs). N2B KO myocardium, where the N2B spring element in titin is deleted and passive tension is elevated, was compared to WT myocardium. Myofilament lattice structure was studied with low-angle X-ray diffraction; the myofilament lattice spacing (d10) was measured as well as the ratio of the intensities of the 1,1 and 1,0 diffraction peaks (I11/I10) as an estimate of the degree of association of myosin heads with the thin filaments. Experiments were carried out in skinned muscle in which the lattice spacing was reduced with Dextran-T500. Experiments with and without lattice compression were also carried out following PKA phosphorylation of the skinned muscle. Under all conditions that were tested, LDA was significantly larger in N2B KO myocardium compared to WT myocardium, with the largest differences following PKA phosphorylation. A positive correlation between passive tension and LDA was found that persisted when the myofilament lattice was compressed with Dextran and that was enhanced following PKA phosphorylation. Low-angle X-ray diffraction revealed a shift in mass from thin filaments to thick filaments as sarcomere length was increased. Furthermore, a positive correlation was obtained between myofilament lattice spacing and passive tension and the change in I11/I10 and passive tension and these provide possible explanations for how titin-based passive tension might regulate calcium sensitivity.
Introduction
An important aspect of cardiac adaptation is the immediate systolic pressure increase in response to increased venous return, an effect known as the Frank-Starling mechanism (FSM) of the heart [1-3]. It had been suggested that increased calcium release in response to increased sarcomere length might be an important contributor to the FSM but this now seems unlikely [2, 4] and instead increased calcium sensitivity of force development appears to be the dominant effect [2, 5]. Thus, when sarcomeres are stretched, the myofilaments produce more force for the same level of calcium, i.e., the myofilaments display length-dependent activation (LDA). Various myofilament components are likely to contribute to LDA: the thin-filament based troponin (Tn) complex[6, 7], the thick-filament based proteins cMyBP-C and MLC2 [8, 9], and titin, the third myofilament of the cardiac sarcomere[10-15]. The titin filament directly senses stretch and interacts with both actin and myosin; titin is, therefore, a good candidate for a major role in the FSM. This study is focused on the role of titin in LDA, using the N2B KO model in which one of the spring elements of titin (the N2B element) is deleted[16]. Titin is a giant filamentous protein that spans the half-sarcomeric distance from Z-disk to M-band[17]. Titin’s I-band region functions as a molecular spring that develops passive tension when sarcomeres are stretched[18] and it is now well accepted that titin is important for the diastolic health of the heart[16, 17, 19, 20]. A role for titin in LDA has been suggested by experiments in which titin was degraded by trypsin and LDA was reduced[10, 21, 22] and also by experiments in which changes in titin-based passive tension due to variable passive stress relaxation[11] or to the variable expression of titin isoforms [13, 23] correlated with changes in LDA. A recent study on a rat model that expresses highly compliant titin isoforms and in which LDA was reduced[15] supports the notion that titin plays an important role in determining calcium sensitivity.
In the aforementioned titin studies, LDA was measured in skinned muscle, which has the advantage that the calcium concentration can be well controlled. However, skinning also results in myofilament lattice spacing expansion [24]. We therefore studied LDA in skinned muscle from WT and N2B KO mice, using both tension-pCa measurements and low angle X-ray diffraction, in which the lattice spacing was reduced with Dextran-T500. We measured the myofilament lattice spacing (d10) and the ratio of the intensities of the 1,1 and 1,0 diffraction peaks (I11/I10) as an estimate of the proximity of myosin heads to the thin filaments. Because the beta-adrenergic tone of cardiac muscle affects LDA[25] and to rule out subtle differences in the PKA phosphorylation status between WT and KO muscle, experiments with and without lattice compression were carried out following PKA phosphorylation of the skinned muscle (see Methods), normalizing thereby the phosphorylation status between WT and N2B KO myocardium. It was found that PKA phosphorylation increases LDA, mainly by lowering calcium sensitivity at the short sarcomere length (1.95 μm) and, under all experimental conditions, calcium sensitivity at the long sarcomere length (2.3 μm) is greatest in N2B KO myocardium. X-ray diffraction revealed that both myofilament lattice spacing and I1,1/I1,0 became smaller as sarcomere length was increased and that the reduction in myofilament lattice spacing and in I1,1/I1,0 scaled with passive tension.
Methods
Animal model
Wild type (WT) mice and N2B element-deficient mice (N2B KO) were used in which exon 49 of the titin gene had been deleted[16]. Mice were genotyped as described previously[16], and the results were confirmed by 1% agarose protein gels[26]. Three months old male N2B KO and WT mice were used, and all surgical procedures and skinning protocols were as described previously[27]. Briefly, mice were sacrificed by cervical dislocation, the hearts were removed, and papillary muscles from the left ventricle (LV) were dissected. The papillary muscles were skinned and the remaining LV was quick-frozen for protein analysis. Experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
Muscle preparations
Skinned papillary muscles were dissected into small strips (cross-sectional area (CSA) ~0.016 mm2; length ~1.0 mm) and small aluminum clips were glued at each end of the muscle[28]. The muscles were attached to a force transducer (model 403 Aurora Scientific) and a length controller (model 322C, Aurora Scientific) that were part of a custom-designed experimental setup (model 802D, Aurora Scientific). The stage was mounted on top of an inverted microscope and contained 8 isolated wells. The muscle was visualized via a CCD camera attached to the microscope, and through this image, the sarcomere length was measured on-line using a spatial autocorrelation function (model 901, Aurora scientific). The experimental stage was temperature controlled at 15 °C (except during PKA incubation, see below). The thickness and width of the preparation were measured and the CSA was calculated by assuming an elliptical cross-section. The CSA was used to convert measured forces into tension (in mN/mm2).
Skinned muscle solutions
We used relaxing solution (RS), pre-activating solution (Pre-A), and maximal activating solution (AS, pCa 4.5). All solutions contained the following: BES, 40mM; DTT, 1 mM; creatine phosphate (PCr), 33 mM; creatine phosphokinase (CPK), 200 U/ml; the ionic strength was adjusted to 180mM with K-proprionate; pH 7.0 at 15°C.
| Solution | MgCl2(mM) | Na-ATP(mM) | EGTA(mM) | Ca-EGTA(mM) | K-Prop. (mM) |
|---|---|---|---|---|---|
| Relaxing | 6.86 | 5.96 | 10 | - | 3.28 |
| Pre-activating | 6.66 | 5.98 | 1 | - | 30.44 |
| Activating | 6.64 | 6.23 | - | 10 | 2.09 |
The solutions contained protease inhibitors (phenylmethylsulfonyl fluoride (PMSF), 0.5 mM; Leupeptin, 0.04 mM; E64, 0.01 mM). Sub-maximal activating solutions were obtained by mixing RS and AS with the free [Ca2+] calculated according to Fabiato and Fabiato[29] with modifications according to Stienen and colleagues [30].
Mechanical experiments and data analysis
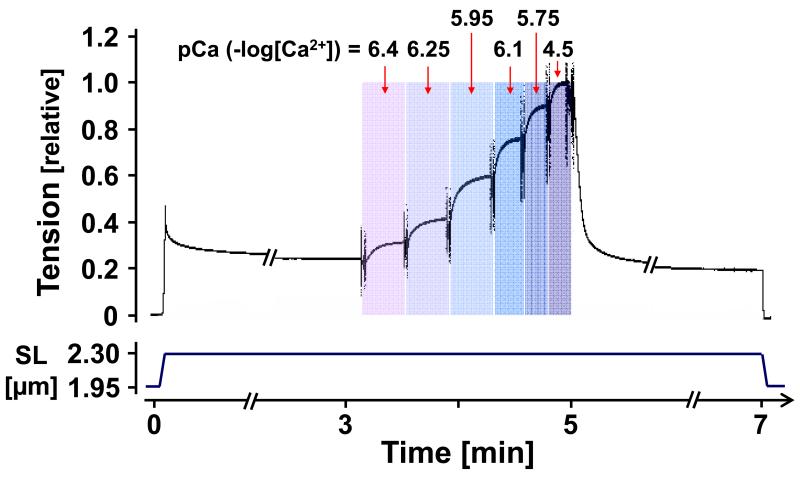
The relaxed muscle was set at a sarcomere length (SL) of 1.95 μm, activated maximally (pCa 4.5), and when a plateau in force was reached, the muscle was relaxed. The muscle was then activated in the following sequence in order to obtain a force-pCa curve at a SL of 1.95 μm: pre-activating solution, pCa 6.4, 6.25, 6.1, 5.95, 5.75 and 4.5 activating solutions, relaxing solution. The same sequence of treatments was repeated at SL 2.3 μm where the fibers were stretched (20% L0/s) in the pre-activating solution and held for 3 min to allow for stress relaxation, see Fig.1. (The maximal activations that preceded these measurements settled the preparation well in their clips that attached them to the hooks. Furthermore a series of test stretches (on passive muscle) using different SL amplitudes allowed us to predictably reach the desired SL of 2.3 μm. This approach is superior to simply setting the final SL by manually operating a micromanipulator that is attached to one of the ends of the muscle). After the activation sequence, the muscle was relaxed and released back to SL 1.95 μm. In order to test the effect of PKA, the fibers were incubated for 1 hour at 22°C in relaxing solution containing 1U/μl of PKA catalytic subunit from bovine heart (#P2645, Sigma) and washed in fresh relaxing solution. The sequence of activations used to obtain a force-pCa curve described above was repeated at SL of 1.95 and 2.3 μm in order to compare pre- and post-PKA treatment. A maximal activation (pCa 4.5) at SL of 1.95 μm was carried out once more to assess the rundown during the experiment, including four force-pCa curves (rundown was always less than 5%). At the end of the experiment, in order to determine the contribution of titin and collagen to passive tension, thick and thin filaments were extracted to remove titin’s anchors in the sarcomere, by incubating the skinned muscle in relaxing solution containing 0.6 M KCl and then in relaxing solution containing 1.0 M KI for 30 min each[31]. The remaining tension, assumed to be collagen based, was subtracted from the pre-extraction tension to determine titin-based passive tension.
Figure 1. Experimental protocol.
Skinned muscle was stretched, held, and then released. During the hold phase and after stress relaxation had ceased, the muscle was exposed to various pCa activating solutions.
Experiments were also carried out in relaxing solution containing 3% Dextran T-500 (Dextran) with muscles incubated with Dextran for 30 min before an experiment. Skinning causes swelling of fibers (i.e. increasing myofilament lattice spacing), the presence of Dextran reduces lattice spacing towards physiological levels [24]. Dextran was washed out with a fresh relaxing solution for 50 min before the PKA-treatment. After PKA-incubation for an hour, fibers were incubated again in Dextran and the tension-pCa curves measured.
Passive tensions were measured just prior to activation, and active tensions were measured at the tension plateau for each activation. Each sub-maximal active tension was normalized by the maximal active tension. The tension-pCa curves were fit to the Hill equation: T/Tmax(relative tension) = [Ca2+]nH/(K+[Ca2+]nH), where nH is the Hill coefficient, and pCa50=(−logK)/nH. We used the pCa that results in half-maximal tension, or pCa50 as an indicator of calcium sensitivity. We then determined the differences between pCa50 of the tension-pCa curves measured at SL 1.95 and 2.3 μm (ΔpCa50) and used this as an index of length-dependent activation (LDA).
X-ray diffraction experiments and data analysis
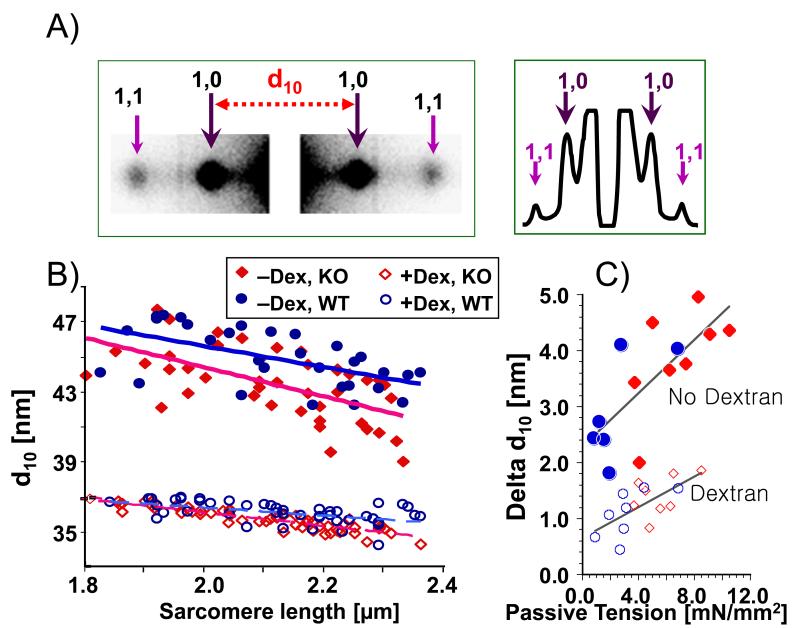
To measure the structural changes in the myofilament lattice, small-angle X-ray diffraction experiments were performed at the BioCAT beamline 18-D at the Advanced Photon Source, Argonne National Laboratory (Argonne, IL). A separate group of skinned papillary muscles from N2B KO and WT mice LV was prepared for X-ray diffraction experiments (the procedure of fiber preparation was as described above). Experiments were carried out in relaxing solutions or relaxing solution containing 3% Dextran. The fibers were mounted between a force transducer and a servomotor in a custom-designed experimental chamber. The chamber has Kapton windows allowing passage of the X-ray beam, and is designed to be able to measure tension and X-ray diffraction patterns simultaneously. This setup also allows simultaneous sarcomere length measurement using laser diffraction with a linear CCD based detector and a commercial peak analyzer. The X-ray exposure times were ~0.5s and the X-ray diffraction patterns were collected on a CCD-based X-ray detector (PCCD 168080, Aviex L.L.C., Napierville, IL, USA). A representative X-ray diffraction pattern and the intensity distribution profile is shown in Fig. 5A. From the images of X-ray diffraction pattern, spacings between the 1,0 and 1,1 equatorial reflections were converted to d10 lattice spacings using Bragg’s law (the thick to thick filament spacing can be estimated by d10 × 2/√3[32]). Integrated intensities of the 1,0 and 1,1 equatorial reflections (I1,0 and I1,1, respectively) were determined from the areas under the reflection peak on the one-dimensional projections[33]. The intensity ratio (I1,1/I1,0) can be used to estimate mass (cross-bridge) transfer away from the thin filament region toward the thick filament backbone.
Figure 5. X-ray diffraction studies of N2B WT and KO skinned myocardium.
(A) (left) typical example of equatorial X-ray diffraction patterns; spacings between the 1,0 and 1,1 equatorial reflections in the diffraction pattern were converted to d10 lattice spacings. (right) example of intensity distribution of X-ray diffraction pattern. (B) Lattice spacing (d10) was significantly decreased with SL regardless of genotype and the presence of Dextran. The slope of this negative correlation was greater in KO compared to WT, a difference that was statistically significant in the presence of Dextran (p<0.01). (C) Δd10 between SL 1.95 and 2.3 μm was significantly correlated with passive tension at SL of 2.3 μm both in the absence (p<0.01) and in the presence (p<0.05) of Dextran.
Gel-electrophoresis
Muscles were solubilized [34] and proteins were electrophoresed using 1% agarose gels[26]. The gels were stained with Coomassie blue and scanned with an Epson Expression 1680 scanner. The images were analyzed with One-D scan EX software (Scanalytics Inc.).
Statistical analysis
Data are expressed as mean ± SEM. A t-test was used with statistical significance set to p<0.05 to identify differences in genotype or in the presence of Dextran. A paired t-test was used with statistical significance set to p<0.05 in order to test the effect of PKA-treatment. Symbols on Figures and in Tables (*, #, †) indicate the following levels of statistical significance: one symbol, p<0.05; two symbols, p<0.01; three symbols, p<0.001. Mechanical experiments used 7 WT and 7 KO mice and X-ray diffraction experiments 8 WT and 9 KO mice.
Results
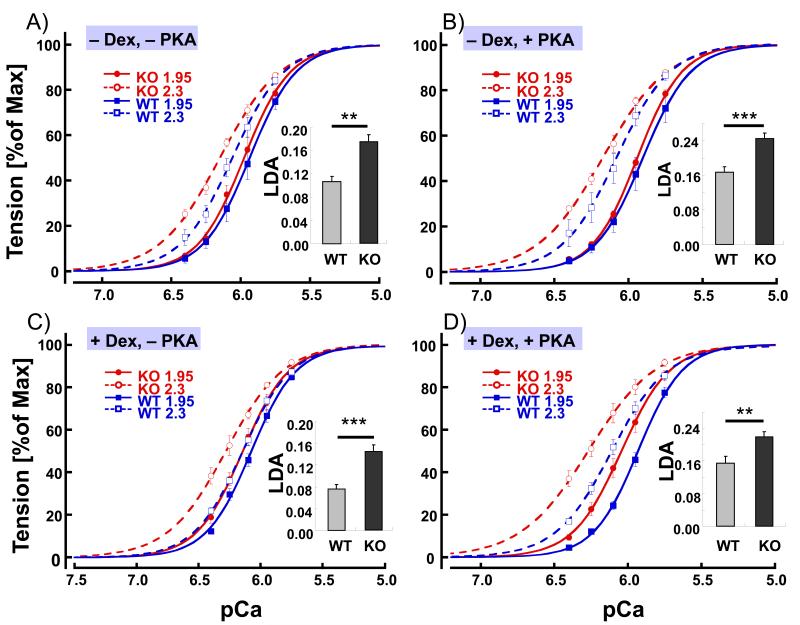
Skinned muscle strips, stretched to a sarcomere length (SL) of either 1.95 μm or 2.3 μm, were activated at various calcium concentrations (expressed as pCa or −log[Ca2+]), and active tension at each pCa was determined as a fraction of the maximal tension measured at pCa 4.5. Calcium sensitivity was determined from the pCa50 (pCa where active tension is half of the value at pCa 4.5). An example of an experiment is shown in Fig. 1, and average tension-pCa curves are shown in Fig. 2. WT mice were compared to KO mice that are deficient in the N2B spring element of titin and that develop, therefore, increased levels of passive tension. The SLs at the slack length of skinned LV muscle strips were not significantly different between KO and WT (1.93 (±0.004) for KO, 1.94 (±0.005) for WT) and passive tension at SL 1.95 μm was near zero in both genotypes. However, passive tension at SL 2.3 μm was significantly higher in N2B KO mice (Table 1). Our goal was to study whether or not the level of passive tension affects length dependence of calcium sensitivity (LDA). We found that pCa50 at SL1.95 μm was the same in the two genotypes (6.08, see Table 1, 1st and 5th row). However, pCa50 at SL 2.3μm in KO was greater than in WT muscle (6.26, vs. 6.19, Table 1, 5th and 1st row). As a result, LDA (ΔpCa50) was significantly larger in KO mice (ΔpCa50 0.18 in KO and 0.11 in WT, Fig. 2A). Thus the muscle type with the highest passive tension (KO) has the greatest LDA.
Figure 2. Tension–pCa relations in WT and N2B KO skinned myocardium.
Tension-pCa curves and LDA (inset) of WT and KO myocardium in absence of Dextran and before PKA treatment (A; −Dex, −PKA), absence of Dextran and after PKA treatment (B; −Dex, +PKA), presence of Dextran and before PKA treatment (C; +Dex, −PKA), and presence of Dextran and after PKA treatment (D; +Dex, +PKA) at SL of 1.95 and 2.3 μm. Under all conditions LDA of KO myocardium was significantly greater than LDA of WT (inset). Results from 7 WT and 7 KO mice.
Table 1.
Passive and active tension (T), pCa50 and Hill coefficients.
| Dex | PKA | PT [mN/mm2] | Max AT [mN/mm2] | ΔAT % | nH | pCa50 | ΔpCa50 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Steady | 1.95 μm | 2.3 μm | 1.95 μm | 2.3 μm | 1.95 μm | 2.3 μm | |||||
| WT | − | − | 17±1.4 | 7.7±.6 | 35±3.2 | 45±4.9 | 130±1 | 2.6±.09 | 2.5±.12 | 6.08±.07 | 6.19±.06 | 0.11±.01 |
| − | + | 15±1.1 ## | 7.0±.5 ## | 45±4.1## | 57±4.6## | 127±2 | 2.7±.09 | 2.4±.12 | 6.01±.05# | 6.18±.05 | 0.17±.01## | |
| + | − | 20±1.5 | 8.8±.8 | 39±3.88 | 49±4.3 | 128±2 | 2.4±.08† | 2.3±.05 | 6.19±.06 | 6.28±.07 | 0.08±.01 | |
| + | + | 17±1.0 ## | 7.7±.7 ## | 45±6.2# | 52±5.9 | 119±3## | 2.6±.18# | 2.3±.07 | 6.09±.08### | 6.25±.07# | 0.16±.01# | |
| KO | − | − | 55±3.9*** | 21±1.9*** | 35±2.3 | 37±2.2 | 107±2** | 2.8±.12 | 2.0±.07** | 6.08±.05 | 6.26±.05 | 0.18±.01** |
| − | + | 48±3.9***### | 19±2.1 ***## | 45±4.3# | 47±4.4# | 103±2** | 2.9±.07 | 2.0±.08* | 6.05±.05 | 6.29±.05 | 0.25±.01***## | |
| + | − | 56±4.9*** | 20±1.9*** | 42±3.2 | 45±3.6 | 110±3 | 2.5±.16 | 2.0±.08** | 6.24±.05f | 6.38±.05 | 0.14±.01** | |
| + | + | 49±4.3***### | 18±1.6***## | 49±3.3# | 47±2.4 | 97±1* | 2.8±.1 | 2.0±.06** | 6.12±.04### | 6.34±.04 | 0.22±.01**# | |
Asterisks (*): comparison between KO and corresponding WT data (t-test); ΔAT: maximal active tension difference from value at SL 1.95 and 2.3 μm; ΔpCa50 values difference from value at SL 1.95 and 2.3 μm, Number (#) sign: comparison between PKA and no PKA results using a paired t-test; Dagger (†) sign: comparison between Dex and no Dex results using a t-test. Single mark, p<.05; double, p<.01; triple, p<.001; PT, titin-based passive tension
Because it is well known that the beta-adrenergic tone of cardiac muscle affects LDA, subtle differences in the PKA phosphorylation status between WT and KO might be expected to affect the results. To rule out this potential complication, the experiments described above were repeated following PKA phosphorylation of the skinned muscle (see Methods), there by normalizing the phosphorylation status. Following PKA phosphorylation LDA (ΔpCa50) was increased in both WT and KO muscle (in WT from 0.11 (no PKA) to 0.17 (PKA) and in KO from 0.18 (no PKA) to 0.25 (PKA)), an effect that is mainly due to a reduction in pCa50 at SL 1.95 μm (Table 1, 2nd and 6th rows). Importantly, following PKA phosphorylation, LDA remained significantly larger in KO than in WT muscle (Fig. 2B).
Additionally, we tested the effect of reducing the myofilament lattice spacing back toward physiological levels (the lattice expands as a result of skinning[24]) using Dextran (Methods). Direct measurements of lattice spacing with low-angle X-ray diffraction showed a ~18% decrease in d1,0 in both genotypes (Table 2). The presence of Dextran (reduced lattice spacing) did not affect titin-based tension (Table 1, 3rd and 7th rows). However, Dextran increased pCa50 by 0.1 – 0.2 units at both SL 1.95 μm and 2.3 μm (Table 1, 3rd and 7th rows). Dextran showed a trend to decrease LDA, but the difference was not statistically significant (Table 1, 3rd and 7th rows). The KO muscles continued to have an increased LDA (Fig.2C). Finally we studied skinned muscle in Dextran following PKA treatment and found that PKA phosphorylation increased LDA in both WT and KO muscle at reduced myofilament lattice spacing (in WT ΔpCa50 increased from 0.08 (no PKA) to 0.16 (PKA) and in KO from 0.14 (no PKA) to 0.22 (PKA)). This affect was mainly due to a reduction in pCa50 at SL 1.95 μm (Table 1, 4th and 8th rows, Fig. 2D) in the presence of Dextran.
Table 2.
Summary data from X-ray diffraction experiments.
| Dex | Geno | SL | PT | LS | Intensity ratio | ||
|---|---|---|---|---|---|---|---|
| Short SL | Long SL | short | long | ||||
| no | WT (6) | 2.3±.01 | 2.5±.9 | 45.7±.6 | 43.6±.5§§§ | 0.30±.01 | 0.27±0.2 |
| KO (8) | 2.3±.01 | 6.9±.8** | 45.0±.5 | 41.8±.6*§§§ | 0.37±.01** | 0.32±0.2 | |
| yes | WT (9) | 2.3±.01 | 3.2±.6 | 36.6±.1††† | 35.7±.2†††§§ | 0.35±.01† | 0.29±0.1§§ |
| KO (9) | 2.3±.01 | 6.5±1.1* | 36.5±.1 ††† | 35.1 ±.1*†††§§§ | 0.34±.01† | 0.29±0.1§ | |
Asterisks (*): comparison between KO and corresponding WT data (t-test); SL, sarcomere length; PT, passive tension; LS, lattice spacing; Section (§) sign: comparison between short and long length results using a paired t-test; Dagger (†) sign: comparison between Dex and no Dex results using a t-test. Single mark, p<0.05; double, p<0.01; triple, p<0.001
Reducing myofilament lattice spacing did not affect maximal active tension whereas PKA treatment increased the maximal active tension in both genotypes (Table 1). This increase was most pronounced at SL 1.95 μm. Except for the effect of increasing SL in N2B KO muscle, no effects of experimental conditions on the Hill coefficient (nH) were observed (Table 1).
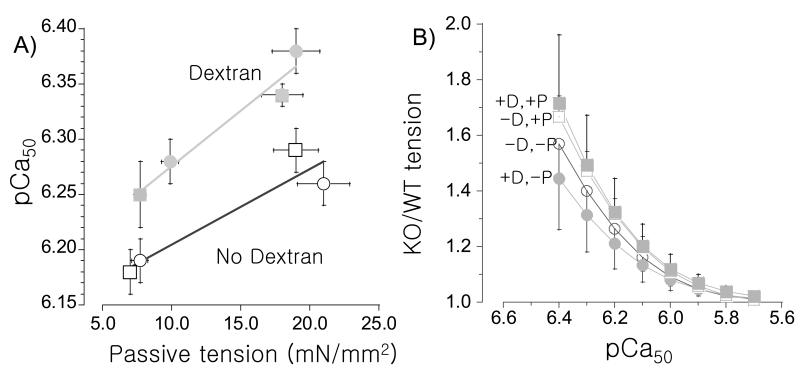
An important finding of our work is that at SL 2.3 μm, calcium sensitivity is significantly greater (increased pCa50) at high passive tension; compressing the myofilament lattice with Dextran also increases calcium sensitivity, but this occurs to a similar degree at all passive tension levels (Fig. 3A). The effect of this increase in calcium sensitivity on active tension is well captured by expressing the active tension level at SL 2.3 μm in KO relative to WT myocardium. This shows that at pCa> ~6.0, tensions are significantly higher in the KO and that PKA-phosphorylation enhances this effect (Fig.3B). We also plotted for each muscle the relationship between titin-based passive tension (SL 2.3 μm) and LDA (ΔpCa50). Regardless of PKA-phosphorylation state and the presence or absence of Dextran, LDA showed a significant correlation with titin-based passive tension (Fig.4). Dextran did not significantly affect the relation between titin-based passive tension and LDA (Fig. 4A) whereas PKA treatment increased the effect (Fig. 4B). Thus, titin-based passive tension is significantly correlated to LDA with the largest effect following PKA phosphorylation.
Figure 3. pCa50 at SL 2.3 μm in absence (open symbol) and presence (closed symbol) of Dextran (A) and tension of KO relative to WT at SL 2.3 μm as a function of pCa (B).
(A) pCa50 is significantly higher at higher passive tension both before PKA treatment (circles) and after treatment (squares). (B) Active tensions of KO muscle are expressed relative to those of WT muscle. At pCa >6.0 tension is higher in the KO with the largest increase following PKA treatment. Results from 7 WT and 7 KO mice. (D: dextran; P: PKA treated; circles: no PKA treatment, squares: PKA treated.)
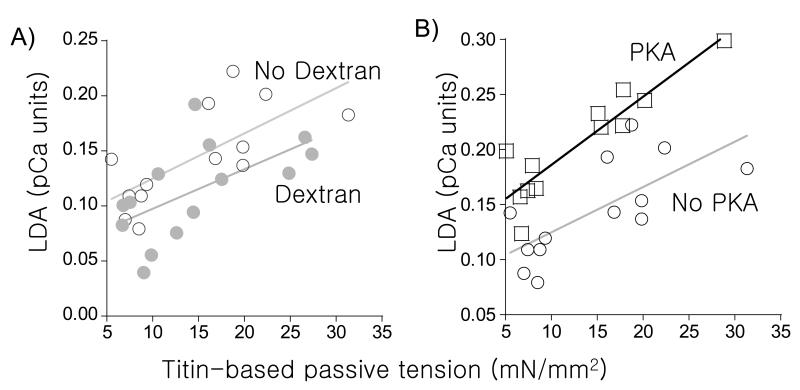
Figure 4. Length dependence of activation (LDA) vs. titin-based passive tension in N2B WT and KO skinned myocardium.
(A) LDA (ΔpCa50 from SL 1.95 μm to 2.3 μm) is significantly correlated with ΔTitin-based passive tension (tension at SL 2.3 μm minus tension at 1.95 μm) in absence (open circles) and presence of Dextran (closed circles). Lines are the linear regression fit (p<0.01 for no Dex, p<0.05 for Dex). (Data from WT and N2B muscle grouped; no PKA treatment). (B) Dependence of LDA on ΔTitin-based passive tension is enhanced following PKA treatment. (Data from WT and N2B muscle grouped; no Dextran). Results from 7 WT and 7 KO mice
We used small-angle X-ray diffraction to assess changes in myofilament lattice structure with increasing sarcomere length and passive tension in N2B KO and WT myocardium, to see if structural changes correlate with LDA. Separate groups of N2B KO and WT myocardium were prepared using the same procedures as for the mechanical experiments described above. An example X-ray diffraction pattern is shown in Fig.5A. The spacing between 1.0 equatorial reflections was converted to d1,0 lattice spacing (LS). Myofilament lattice spacing (LS) in both WT and KO myocardium decreased with increasing SL (Fig. 5B). The Dextran-treated fibers showed a similar reduction in LS in both genotypes (see Table 2). Linear regression curves were fitted to the data set from each group (KO or WT), in the presence or absence of Dextran. KO myocardium showed a greater slope than that of WT myocardium, and this difference in slope was significant in the presence of Dextran (Fig.5B). Changes in LS from SL of 1.95 μm to 2.3 μm (Delta LS) were positively correlated to the passive tension, both with or without Dextran (Fig. 5C).
The ratio of the intensities of the 1,1 and 1,0 diffraction peaks (I11/I10) was used as an estimate of the proximity of myosin heads to the thin filaments (Fig 5A-right). Figure 6A and Table 2 show that the intensity ratio was negatively correlated with elongation of the sarcomere (Fig.6A). To study whether passive tension per se has an effect on the intensity ratio we determined the change in intensity ratio from SL 1.95 to 2.3 μm (delta I11/I10) for muscle in Dextran (both WT and N2B KO) and regardless of genotype segregated the data into low passive tension and high passive tension groups (see also caption of Fig. 6B). Delta I11/I10 was significantly reduced at high passive tension (Fig.6B) i.e. the decrease in I11/I10 with increasing sarcomere length was greater at low passive tension than at high.
Figure 6. Effect of SL and passive tension on I11/I10.
(A) Intensity ratio I11/I10 was decreased proportionally to SL regardless of genotype and presence of Dextran, however, no significant difference in the slope of correlation was found between genotypes, (B) Delta I11/I10 ratio when sarcomeres were stretched from 1.95 μm to 2.3 μm for low passive tension, PT (1-4 mN/mm2) and high passive tension data (5-14 mN/mm2). High and Low PT muscle have significantly different delta I11/I10 ratios. (WT and N2B KO muscles in presence of Dextran)
Discussion
It is well-established that the cellular basis underlying the Frank-Starling relationship is the length-dependence of activation, but the mechanisms by which the sarcomere detects length changes and converts this information in altered calcium sensitivity has remained elusive. Here we studied the effect of titin-based passive tension on the length-dependence of activation by measuring, at two SLs, the tension-pCa relation in skinned mouse LV muscle. We compared WT mice with N2B KO mice that develop high passive tension levels. Under all conditions that were tested, LDA was significantly larger in the N2B KO myocardium compared to WT myocardium, with a significant correlation between LDA and the level of titin-based passive tension. Low-angle X-ray diffraction revealed a shift in mass from thin filaments to thick filaments as sarcomere length was increased and a correlation between myofilament lattice structure and passive tension. Below we discuss these results in detail.
An important ‘tool’ that we used was the N2B KO mouse model in which titin’s N2B element has been deleted. The N2B element is one of three spring elements found in cardiac titin (the PEVK and tandem Ig segments being the two other elements) [35-37]. The N2B KO myocardium develops higher titin-based passive tension than WT myocardium (Table 1), most likely because the absence of the N2B element in the N2B KO results in a higher extension of the remaining spring element [16], increasing thereby the fractional extension of these segments and increasing force accordingly[38]. No changes in isoform expression are known to occur in sarcomeric proteins in the N2B KO mouse [27] and thus the model is well suited to study the effect of titin-based passive tension on calcium sensitivity of force development.
Considering the importance of the FSM, many studies of calcium sensitivity have been carried out using cells and muscles that were skinned, because of the ease of controlling calcium levels in this type of preparation. A drawback of skinning is that the myofilament lattice spacing (d10) is greatly expanded due to the removal of the osmotic constraint imposed by the sarcolemma. The myofilament spacing (d10) has been well-characterized for intact rat myocardium and within the physiological sarcomere length range d10 varies from ~37 nm (short SL) to-~35 nm (long SL) [39, 40]. Following skinning, the values are ~44-42 nm[40], which are in line with the values of our study in skinned mouse myocardium (Fig. 5, Table 2). To test the effect of passive tension on LDA in skinned muscle that has been compressed back towards physiological lattice spacings we used the osmotic agent Dextran, which reduced the lattice spacing to ~37 nm (short SL)-~35 nm (long SL). Lattice compression did not affect maximal active tension nor the Hill coefficient, nH (Table 1), but it significantly sensitized the myofilaments at both short and long SL, as reflected by the increased pCa50 at each SL, but without affecting LDA (Table 1). A similar relation between passive tension and LDA exists in the absence and in the presence of Dextran (Fig. 4A) indicating that passive tension modulates LDA at physiological lattice spacings.
Because beta-adrenergic stimulation is known to affect the FSM[25], we normalized possible differences between WT and N2B KO in baseline phosphorylation level of PKA sites on sarcomeric proteins by PKA treating skinned muscle, and studied LDA before and after PKA treatment. PKA phosphorylation increased LDA, largely due to a reduction in calcium sensitivity at the short SL (1.95 μm). A PKA-induced desensitization has been reported in other studies on cardiac muscle from a range of species [41-46]. One of these was on mouse skinned myocardium and examined the effect of PKA phosphorylation at two SLs and, consistent with our study, found an increase in LDA[41]. These studies were carried out on skinned muscle without the addition of osmotic agents that reduce the myofilament lattice back towards physiological values and our study is the first that shows that when physiological lattice spacing is restored with Dextran, PKA phosphorylation reduces calcium sensitivity (increased pCa50) and increases LDA (Table 1). The underlying mechanism probably involves known PKA targets in the sarcomere, cTnI[47], cMyBP-C[48] and titin[49]. Considering that the PKA sites on titin are located in the N2B element[49] and that the N2B element is absent in the N2B KO where PKA has a large effect on LDA (Table 1) it seems unlikely that phosphorylation of titin is involved in the PKA effects. Of the other targets it has been shown that cTnI is likely to be very important[41], and that this cTnI effect requires the presence of cMyBP-C [8]. Recently it has been reported that it is phosphorylation of cMyBP-C by PKA that is primarily responsible for displacing heads away from the thick filament backbones towards the thin filament leading to increased I11/I10[50].
The present study can be compared with previous work on mouse skinned cardiac myocytes in which the degree of passive stress relaxation prior to activation was varied and the relationship between the level of passive tension and LDA was measured [11]. Both studies found a similar positive correlation between passive tension and LDA. This relationship between passive tension and LDA in the mouse is also similar to that obtained in studies on skinned rat cardiac myocytes and rat trabeculae, in which titin-based passive tension was varied by trypsin-based degradation of titin [14, 22, 51]. A positive correlation between titin-based passive tension and LDA was also obtained by comparing left ventricular and left atrial bovine myocardium, which develops high and low passive tension, respectively [13]. Furthermore, a recent study on a mutant rat that expresses a giant titin isoform reported that the lower passive tension of the mutant rat was accompanied by much reduced LDA[15]. Finally, a study on trout cardiac myocytes also found a positive correlation between titin-based passive tension and Ca2+ sensitivity of active tension[52]. Thus, the length-dependence of activation that underlies the FSM of the heart is a function of titin-based passive tension and this has now been found in many mammalian species as well as in a non-mammalian species.
The mechanism by which titin-based passive tension affects LDA is unknown. It has been suggested that the reason for increased calcium sensitivity at longer sarcomere lengths is decreased lattice spacing (“the lattice spacing hypothesis”) since in many experiments there is a good correlation between LS and calcium sensitivity[14, 53-55]. Thus, one possible mechanism that has previously been suggested, is that titin reduces the myofilament lattice spacing by generating a radial force that compresses the lattice and that increases the number of cycling crossbridges by bringing myosin heads closer to binding sites on actin[11]. In the present study we found a dependence of the lattice spacing on titin-based passive tension in both the absence and presence of Dextran (Fig. 5C), consistent with the lattice spacing hypothesis. High passive tension reduces the myofilament spacing by several nm and this might greatly impact the interaction between actin and myosin. However, extensive previous work by de Tombe and colleagues has shown that changes in myofilament lattice spacing are not sufficient to explain changes in LDA under all experimental conditions[32, 40] and it seems prudent to explore alternative mechanisms by which titin-based passive tension might affect LDA. One possibility is given by our recent finding that titin-based passive tension strains the thick filament[56] and we have speculated that increased thick filament backbone strain is communicated to the crossbridges, priming them for binding to actin. Indeed it was recently found that the proximity of the myosin heads to the thin filament, as reflected by the I11/I10 intensity ratio, is reduced as SL is increased in membrane intact muscle[39] a finding that is consistent with the present study (Fig. 6A) using skinned muscle. Thus the myosin head position is not constant but appears to respond to SL. Additionally we found that the change in I11/I10 is less when passive tension is high (Fig. 6B) suggesting that titin-based tension might affect the degree of association of myosin heads with the thin filaments. More work is required to extend this finding and establish whether it relates to LDA.
In summary we studied LDA in LV myocardium from WT and N2B KO mice and found a correlation between passive tension and calcium sensitivity that persists when the myofilament lattice is compressed with Dextran and that is enhanced following PKA phosphorylation. A positive correlation was found between myofilament lattice spacing and passive tension as well as between the degree of association of myosin heads with the thin filament and the amount of passive tension, either of which might explain how titin-based passive tension regulates calcium sensitivity. More precise measurements of structural changes in the thick filaments, the thin filaments, and the myofilament lattice are required to more fully understand the mechanisms by which titin-based passive tension enhances LDA.
We studied passive tension, length-dependent activation(LDA), and muscle structure.
A positive correlation between titin-based passive tension and LDA was found
Passive tension and myofilament lattice spacing were positively correlated A shift in mass from thin filaments to thick filaments occurred.
Acknowledgements
We would like to acknowledge the contributions of Chen-Ching Yuan, Sami Somo, Cynthia Yang and Hsiao Man Hsu who analyzed the X-ray intensity data as well as funding by NIH grant HL62881 (HG), DFG (MG) and an AHA postdoctoral fellowship (EJL). “Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. “This project was supported by grants from the National Center for Research Resources (2P41RR008630-17) and the National Institute of General Medical Sciences (9 P41 GM103622-17) from the National Institutes of Health.” The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Research Resources, National Institute of General Medical Sciences or the National Institutes of Health”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. Journal of molecular and cellular cardiology. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. Journal of molecular and cellular cardiology. 1985;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 3.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiological reviews. 2000;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 4.Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. The Journal of experimental biology. 2008;211(Pt 13):2005–2013. doi: 10.1242/jeb.003145. [DOI] [PubMed] [Google Scholar]
- 5.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circulation research. 1986;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 6.Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circulation research. 1998;83(5):471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 7.Terui T, Sodnomtseren M, Matsuba D, Udaka J, Ishiwata S, Ohtsuki I, Kurihara S, Fukuda N. Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle. The Journal of general physiology. 2008;131(3):275–283. doi: 10.1085/jgp.200709895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazorla O, Szilagyi S, Vignier N, Salazar G, Kramer E, Vassort G, Carrier L, Lacampagne A. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovascular research. 2006;69(2):370–380. doi: 10.1016/j.cardiores.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Ait Mou Y, le Guennec JY, Mosca E, de Tombe PP, Cazorla O. Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Archiv: European journal of physiology. 2008;457(1):25–36. doi: 10.1007/s00424-008-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Guennec JY, Cazorla O, Lacampagne A, Vassort G. Is titin the length sensor in cardiac muscle? Physiological and physiopathological perspectives. Advances in experimental medicine and biology. 2000;481:337–348. doi: 10.1007/978-1-4615-4267-4_20. discussion 348-351. [DOI] [PubMed] [Google Scholar]
- 11.Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circulation research. 2001;88(10):1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda N, Terui T, Ohtsuki I, Ishiwata S, Kurihara S. Titin and troponin: central players in the frank-starling mechanism of the heart. Current cardiology reviews. 2009;5(2):119–124. doi: 10.2174/157340309788166714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. The Journal of physiology. 2003;553(Pt 1):147–154. doi: 10.1113/jphysiol.2003.049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Archiv: European journal of physiology. 2005;449(5):449–457. doi: 10.1007/s00424-004-1354-6. [DOI] [PubMed] [Google Scholar]
- 15.Patel JR, Pleitner JM, Moss RL, Greaser ML. Magnitude of length-dependent changes in contractile properties varies with titin isoform in rat ventricles. American journal of physiology Heart and circulatory physiology. 2012;302(3):H697–708. doi: 10.1152/ajpheart.00800.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radke MH, Peng J, Wu Y, McNabb M, Nelson OL, Granzier H, Gotthardt M. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circulation research. 2004;94(3):284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 18.Trombitas K, Jin JP, Granzier H. The mechanically active domain of titin in cardiac muscle. Circulation research. 1995;77(4):856–861. doi: 10.1161/01.res.77.4.856. [DOI] [PubMed] [Google Scholar]
- 19.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circulation research. 2009;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 20.Borbely A, van Heerebeek L, Paulus WJ. Transcriptional and posttranslational modifications of titin: implications for diastole. Circulation research. 2009;104(1):12–14. doi: 10.1161/CIRCRESAHA.108.191130. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda N, Granzier H. Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart. Current vascular pharmacology. 2004;2(2):135–139. doi: 10.2174/1570161043476357. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda N, Granzier HL. Titin/connectin-based modulation of the Frank-Starling mechanism of the heart. Journal of muscle research and cell motility. 2005;26(6-8):319–323. doi: 10.1007/s10974-005-9038-1. [DOI] [PubMed] [Google Scholar]
- 23.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophysical journal. 2000;79(6):3226–3234. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millman BM. The filament lattice of striated muscle. Physiological reviews. 1998;78(2):359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- 25.Sarnoff SJ. Myocardial contractility as described by ventricular function curves; observations on Starling’s law of the heart. Physiological reviews. 1955;35(1):107–122. doi: 10.1152/physrev.1955.35.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24(11):1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 27.Lee EJ, Peng J, Radke M, Gotthardt M, Granzier HL. Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice. Journal of molecular and cellular cardiology. 2010;49(3):449–458. doi: 10.1016/j.yjmcc.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clinica chimica acta; international journal of clinical chemistry. 2007;375(1-2):1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de physiologie. 1979;75(5):463–505. [PubMed] [Google Scholar]
- 30.Ebus JP, Stienen GJ, Elzinga G. Influence of phosphate and pH on myofibrillar ATPase activity and force in skinned cardiac trabeculae from rat. The Journal of physiology. 1994;476(3):501–516. doi: 10.1113/jphysiol.1994.sp020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Cazorla O, Labeit D, Labeit S, Granzier H. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. Journal of molecular and cellular cardiology. 2000;32(12):2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 32.Farman GP, Walker JS, de Tombe PP, Irving TC. Impact of osmotic compression on sarcomere structure and myofilament calcium sensitivity of isolated rat myocardium. American journal of physiology Heart and circulatory physiology. 2006;291(4):H1847–1855. doi: 10.1152/ajpheart.01237.2005. [DOI] [PubMed] [Google Scholar]
- 33.Irving TC, Millman BM. Changes in thick filament structure during compression of the filament lattice in relaxed frog sartorius muscle. Journal of muscle research and cell motility. 1989;10(5):385–394. doi: 10.1007/BF01758435. [DOI] [PubMed] [Google Scholar]
- 34.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circulation research. 2004;94(4):505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 35.Helmes M, Trombitas K, Centner T, Kellermayer M, Labeit S, Linke WA, Granzier H. Mechanically driven contour-length adjustment in rat cardiac titin’s unique N2B sequence: titin is an adjustable spring. Circulation research. 1999;84(11):1339–1352. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 36.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin’s extensibility. Biophysical journal. 1999;77(6):3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linke WA, Stockmeier MR, Ivemeyer M, Hosser H, Mundel P. Characterizing titin’s I-band Ig domain region as an entropic spring. Journal of cell science. 1998;111(Pt 11):1567–1574. doi: 10.1242/jcs.111.11.1567. [DOI] [PubMed] [Google Scholar]
- 38.Kellermayer MS, Smith SB, Bustamante C, Granzier HL. Complete unfolding of the titin molecule under external force. Journal of structural biology. 1998;122(1-2):197–205. doi: 10.1006/jsbi.1998.3988. [DOI] [PubMed] [Google Scholar]
- 39.Farman GP, Gore D, Allen E, Schoenfelt K, Irving TC, de Tombe PP. Myosin head orientation: a structural determinant for the Frank-Starling relationship. American journal of physiology Heart and circulatory physiology. 2011;300(6):H2155–2160. doi: 10.1152/ajpheart.01221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. American journal of physiology Heart and circulatory physiology. 2000;279(5):H2568–2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- 41.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. The Journal of physiology. 2003;547(Pt 3):951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanft LM, McDonald KS. Sarcomere length dependence of power output is increased after PKA treatment in rat cardiac myocytes. American journal of physiology Heart and circulatory physiology. 2009;296(5):H1524–1531. doi: 10.1152/ajpheart.00864.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald KS, Hanft LM, Domeier TL, Emter CA. Length and PKA Dependence of Force Generation and Loaded Shortening in Porcine Cardiac Myocytes. Biochemistry research international. 2012;2012:371415. doi: 10.1155/2012/371415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker JS, Walker LA, Margulies K, Buttrick P, de Tombe P. Protein kinase A changes calcium sensitivity but not crossbridge kinetics in human cardiac myofibrils. American journal of physiology Heart and circulatory physiology. 2011;301(1):H138–146. doi: 10.1152/ajpheart.00838.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovascular research. 2000;46(3):487–495. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 46.van der Velden J, Boontje NM, Papp Z, Klein LJ, Visser FC, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Calcium sensitivity of force in human ventricular cardiomyocytes from donor and failing hearts. Basic research in cardiology. 2002;97(Suppl 1):I118–126. doi: 10.1007/s003950200040. [DOI] [PubMed] [Google Scholar]
- 47.Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 48.Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. The Biochemical journal. 1988;249(3):709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circulation research. 2002;90(11):1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 50.Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, Fitzsimons DP, Irving TC, Moss RL. Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium. Journal of molecular and cellular cardiology. 2012 doi: 10.1016/j.yjmcc.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cazorla O, Vassort G, Garnier D, Le Guennec JY. Length modulation of active force in rat cardiac myocytes: is titin the sensor? Journal of molecular and cellular cardiology. 1999;31(6):1215–1227. doi: 10.1006/jmcc.1999.0954. [DOI] [PubMed] [Google Scholar]
- 52.Patrick SM, Hoskins AC, Kentish JC, White E, Shiels HA, Cazorla O. Enhanced length-dependent Ca2+ activation in fish cardiomyocytes permits a large operating range of sarcomere lengths. Journal of molecular and cellular cardiology. 2010;48(5):917–924. doi: 10.1016/j.yjmcc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs F, Smith SH. Calcium, cross-bridges, and the Frank-Starling relationship. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2001;16:5–10. doi: 10.1152/physiologyonline.2001.16.1.5. [DOI] [PubMed] [Google Scholar]
- 54.McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circulation research. 1995;77(1):199–205. doi: 10.1161/01.res.77.1.199. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda N, Terui T, Ishiwata S, Kurihara S. Titin-based regulations of diastolic and systolic functions of mammalian cardiac muscle. Journal of molecular and cellular cardiology. 2010;48(5):876–881. doi: 10.1016/j.yjmcc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Irving T, Wu Y, Bekyarova T, Farman GP, Fukuda N, Granzier H. Thick-filament strain and interfilament spacing in passive muscle: effect of titin-based passive tension. Biophysical journal. 2011;100(6):1499–1508. doi: 10.1016/j.bpj.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]