Abstract
Hyperactive amygdala functioning may underlie emotional dysregulation during smoking abstinence and represents one neurobiological target for pharmacological cessation aids. Available pharmacotherapies (e.g., nicotine replacement and varenicline) aid only a subset of individuals with smoking cessation and therefore elucidating the neurobiological impact of these medications is critical to expedite improved interventions. In a fMRI study employing a within-subject, double-blind, placebo-controlled design, we assessed task performance and amygdala functioning during an emotional face matching paradigm following administration of nicotine and varenicline to 24 abstinent smokers and 20 nonsmokers. All participants underwent ~17 days of varenicline and placebo pill administration and were scanned, on different days under each condition, wearing a transdermal nicotine or placebo patch. During the amygdala reactivity paradigm, nicotinic acetylcholine receptor (nAChR) stimulation by nicotine and varenicline decreased reaction time (RT) in abstinent smokers but not in nonsmokers. When considering all smokers as a single homogenous group, no drug-induced effects on amygdala reactivity were detected. However, in an exploratory analysis we parsed participants into subgroups according to individual differences in the propensity to demonstrate stable performance augmentation following nAChR stimulation (stable RT-improvers [SI] vs. variable RT-improvers [VI]). Using this exploratory approach, drugs appeared to modulate amygdala reactivity in only one smoker subgroup but not in either nonsmoker subgroup. Specifically, in the SI-smoker cohort abstinence-induced elevated amygdala reactivity was down-regulated by nAChR stimulation. In contrast, varenicline and nicotine did not modulate amygdala functioning in the VI-smoker cohort who displayed moderate levels of amygdala reactivity in the absence of drug administration. These results suggest that pharmacotherapies most robustly dampened amygdala functioning in smokers appearing susceptible to abstinence-induced effects. Such findings provide a step towards fractionating the smoker phenotype by discrete neurobiological characteristics.
Keywords: varenicline, nicotine, withdrawal, amygdala, emotion, functional magnetic resonance imaging (fMRI)
1. INTRODUCTION
A major impediment for cigarette smokers attempting to quit is the tobacco abstinence syndrome characterized by anxiety, irritability, and difficulty concentrating (Hughes, 2007; Piasecki, 2006). Nicotine reverses abstinence-induced emotional dysregulation (Kassel et al., 2003) and performance deficits (Heishman et al., 1994) suggesting that early relapse occurs, in part, to relieve such symptoms (Baker et al., 2004). Indeed, those smokers presenting with higher degrees of affective disturbances and/or poorer task performance shortly after cessation are those most liable for recidivism (Patterson et al., 2010; Piper et al., 2011). Thus, early abstinence represents a critical period for interventions to assist smokers with cessation. Currently available pharmacological cessation aids (e.g., varenicline and nicotine replacement) are efficacious in only a subset of individuals and therefore elucidation of the neurobiological impact of these medications is important to expedite the development of improved interventions.
Amygdala’s role in affect-related processes is well established (Phelps and LeDoux, 2005) and the neural substrates mediating negative emotional states during acute drug abstinence are critically centered on amygdala and its interconnected circuitry (Koob, 2009; Koob and Le Moal, 2005). In chronic smokers acutely deprived of nicotine, elevated amygdala activity co-varies with increased smoking urges (Wang et al., 2007) and cigarette smoking dampens this regional hyperactivity (Rose et al., 2003; Zubieta et al., 2005). Additionally, elevated amygdalar responses to smoking-related cues have been associated with increased smoking urges (Chase et al., 2011; Kuhn and Gallinat, 2011; Smolka et al., 2006) and greater susceptibility to relapse (Janes et al., 2010). Such findings suggest that amelioration of hyperactive amygdala functioning represents one neurobiological target upon which cessation pharmacotherapies may exert an efficacious response, particularly in the subset of smokers with greater abstinence-induced hyperactivity. Recent neuroimaging investigations indicate that varenicline, the first non-nicotine medication specifically developed for smoking cessation, down-regulates amygdala functioning in chronic smokers (Franklin et al., 2011a; Loughead et al., in press; Loughead et al., 2010).
Varenicline is thought to aid cessation by ameliorating aversive withdrawal symptoms during abstinence while also attenuating nicotine’s reinforcing effects upon re-exposure. In nicotine’s absence, varenicline acts primarily as a partial agonist at α4β2 nicotinic acetylcholine receptors (nAChRs) producing ~50–60% the relative action of nicotine; whereas in nicotine’s presence, the drug acts as an antagonist, binding with higher affinity and preventing full activation by nicotine (Rollema et al., 2007). Varenicline-induced effects in animal models of mood and cognition appear mediated by α4β2 and/or α7 nAChR interactions (Rollema et al., 2009). In the clinic, varenicline reduces abstinence-induced affective and cognitive disturbances as well as the subjective rewarding aspects of a smoked cigarette (Patterson et al., 2009). This partial agonist/antagonist profile, targeting both negative and positive reinforcement mechanisms perpetuating tobacco use, may account for varenicline’s greater relative efficacy over other pharmacotherapies (Gonzales et al., 2006; Jorenby et al., 2006). We modeled this putative “dual action” profile by administering varenicline alone and in combination with transdermal nicotine to a group of overnight abstinent smokers, as well as to a nonsmoker (negative control) group.
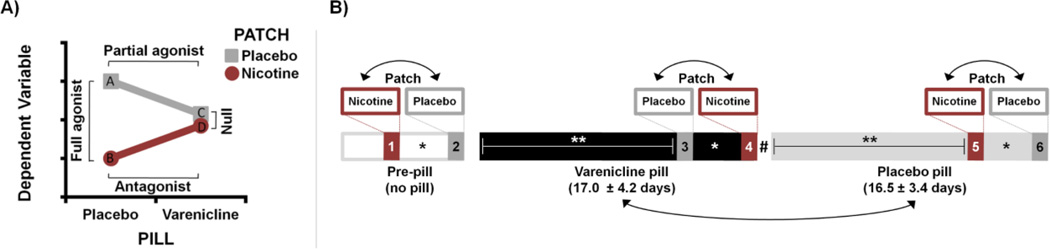
The primary aim of this study was to use the partial agonist/antagonist varenicline and the full agonist nicotine as pharmacological probes to interrogate abstinence-induced effects on amygdala functioning and to assess potential differential pharmacological responses in subsets of smokers. To examine amygdala functioning, we employed functional magnetic resonance imaging (fMRI) coupled with an emotional face matching paradigm known to yield a reliable measure of amygdala reactivity (Hariri et al., 2002b; Sergerie et al., 2008) and previously utilized to assess the impact of pharmacological manipulations (Bigos et al., 2008; Labuschagne et al., 2010; Paulus et al., 2005). In a placebo-controlled, within-subject design, participants underwent ~17 days each of varenicline and placebo pill administration (PILL factor) and were scanned near the end of both medication periods wearing, on different days, a transdermal nicotine or placebo patch (PATCH factor). Based on preclinical data regarding the pharmacological properties of varenicline (Rollema et al., 2009) and operationalized in the current study, we anticipated varenicline (PILL) × nicotine (PATCH) interactions across dependent variables (Fig. 1A). We hypothesized: 1) varenicline and nicotine would speed reaction times (RT) in abstinent smokers but not in nonsmokers; and 2) these pharmacological probes would dampen abstinence-induced elevated amygdala reactivity in smokers. We further examined differential amygdalar responses following drug administration in an exploratory analysis by parsing individuals into “stable” versus “variable/non RT-improvers” according to the degree to which nAChR stimulation augmented task performance. Using a decrease in RT following drug administration (relative to a corresponding placebo) as a proxy for a drug response, we operationally defined stable RT-improvers (SI) as those participants showing RT decreases following all drug administrations, whereas variable/non RT-improvers were defined as those who did not show a RT decrease following all drug administrations. We reasoned that those smokers demonstrating stable RT-improvements following nAChR stimulation would also be those most likely to show drug-induced changes in regional brain functioning.
Figure 1.
Study overview schematics. (A) Illustration of anticipated varenicline (PILL) × nicotine (PATCH) pharmacological interaction and the nature of effects on a dependent variable (DV) during smoking abstinence. Abstinence-induced effects on the DV (e.g., reaction time, amygdala reactivity) are expected to be greatest following smoking deprivation and in the absence of drug administration (i.e., under placebo-pill/placebo-patch conditions: data point “A”). Administration of nicotine is expected to reduce this abstinence-induced elevation (data point “B”) yielding a full agonist response (A vs. B). Similarly, administration of varenicline alone is expected to reduce the DV (data point “C”) yielding a partial agonist effect (A vs. C). Administration of varenicline in combination with nicotine (data point “D”) is then expected to attenuate the nicotine-induced response, as varenicline binds to nAChRs with higher affinity than nicotine and blocks the full agonist response, yielding an antagonist effect (B vs. D). These partial agonist and antagonist effects combine to produce a null effect of nicotine versus placebo patch (C vs. D) in the presence of varenicline. (B) Illustration of study design. All participants completed six fMRI assessments. Before beginning a pill regimen (pre-pill), participants completed assessments wearing transdermal nicotine and placebo patches on separate days. Subsequently, participants underwent varenicline (mean ± SD: 17.0 ± 4.2 days) and placebo pill administration (16.5 ± 3.4 days) and again completed nicotine and placebo patch scans towards the end of both medication periods. Double-headed arrows indicate the randomization of drug order across participants. * Nicotine and placebo patch scans were separated by an average of 2.9 ± 1.7 days. ** Neuroimaging assessments occurred 13.9 ± 2.3 days after the onset of each PILL period. # A washout interval did not separate varenicline and placebo pill epochs.
2. MATERIAL AND METHODS
2.1 Participants
A total of 24 cigarette smokers (12 females) and 20 nonsmokers (10 females), all right-handed and 18–55 years of age completed the study. Participants reported no history of drug dependence (other than nicotine in smokers), neurological or psychiatric disorders, cardiovascular or renal impairment, diabetes, or contra-indications for MRI scanning. We recruited non-treatment seeking daily smokers who reported smoking 10 or more cigarettes per day for a minimum of two years. Nonsmokers reported no history of daily cigarette use and no smoking within two years preceding the study. Participant characteristics are reported in Table 1. Notably, the smoker sample was older (p = 0.04) and less educated than the nonsmoker sample (p = 0.004). As such, age and years of education were included as covariates when comparing smokers versus nonsmokers. Data from two male nonsmokers were excluded from behavioral and imaging analyses due to excessive head motion during scanning. Before beginning the study, participants gave written informed consent in accordance with the Institutional Review Board for the National Institute on Drug Abuse Intramural Research Program. Volunteers were remunerated for participation.
Table 1.
Demographic and smoking characteristics.
| Smokers (n = 24) |
Nonsmokers (n = 20) |
|
|---|---|---|
| Agea | 35.7 ± 9.9 (22–52) | 30.1 ± 7.1 (20–45) |
| Education (years)a | 13.7 ± 1.9 (8–16) | 15.2 ± 1.3 (12–16) |
| Estimated IQb | 106.6 ± 11.7 (87–127) | 112.7 ± 11.6 (87–130) |
| Gender (female/male) | 12/12 | 10/10 |
| Cigarettes per day | 17.7 ± 7.9 (10–40) | --- |
| Years daily smoking | 18.0 ± 10.6 (3–39) | --- |
| Fagerström scorec | 5.0 ± 1.9 (2–9) | --- |
| Age of first cigarette | 15.8 ± 3.6 (10–25) | --- |
Data are expressed as mean ± SD (range).
Smokers were older (t[42] = 2.1, p = 0.04) and less educated (t[42] = 3.0, p = 0.004) relative to nonsmokers.
Groups did not differ in terms of Wechsler Adult Intelligence Scale-Revised estimated IQ scores (t[42] = 1.7, p = 0.1).
Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
2.2 Design and drugs
This was a two-drug, placebo-controlled, double-blind crossover study involving six fMRI assessments per participant on separate days. At three points during a varenicline administration regimen (PILL factor: pre-pill vs. varenicline vs. placebo), all participants underwent imaging on two occasions, once each with a transdermal nicotine or placebo patch (PATCH factor). Following two initial pre-pill neuroimaging visits, each participant underwent ~17 days of varenicline and placebo pill administration and completed nicotine and placebo patch sessions towards the end of both medication periods (Fig. 1B). The study physician maintained and randomized drug order while those conducting data collection remained blinded. For clarity of presentation, data from the pre-pill sessions are omitted from the main text, but can be found in Supplemental Information.
Varenicline (Chantix®, Pfizer, New York, NY) and placebo pills were distributed in identical blister packs. Varenicline was administered according to standard guidelines (http://labeling.pfizer.com) at a dosage of 0.5 mg once daily for days 1–3 of the active medication interval, 0.5 mg twice daily for days 4–7, and 1 mg twice daily beginning on day 8. Active medication was encapsulated and resembled placebo sucrose capsules. A washout interval did not separate medication periods. Given a ~24-h elimination half-life (Faessel et al., 2006), we assumed varenicline carryover effects were negligible in those participants first receiving active medication as subsequent scanning under placebo pills occurred approximately two weeks after the last active dose. Medication adherence (Supplemental Information section ‘1.1 Medication adherence’) and side effects were monitored through regular telephone assessments and at in-person visits. Participants confirmed taking a medication dose the morning of neuroimaging assessments. Other than a 12 h abstinence period before each neuroimaging day, smokers were not asked to alter their smoking behaviors during participation.
Transdermal nicotine (NicoDerm CQ®, GlaxoSmithKline, Research Triangle Park, NC) or placebo patches were applied to the upper back at the beginning of neuroimaging visits. All nonsmokers were administered 7 mg nicotine patches. For smokers, we employed a multiple dosing strategy to match daily nicotine intake: 21 mg (10–15 cigs/day; n = 11), 28 mg (16–20 cigs/day; n = 9), 35 mg (21–25 cigs/day; n = 1), and 42 mg (> 25 cigs/day; n = 3). Patches were worn for the duration of imaging visits, which lasted ~9 h and involved two MRI sessions. Pharmacokinetic data indicate plasma nicotine concentrations reach a peak within 2–4 h after patch application, remain relatively stable for the next 4–6 h, and then gradually decrease beginning ~8–10 h post-patch (Palmer et al., 1992). As such, data collection occurred within a 2–9 h post-patch window associated with steady plasma nicotine levels.
2.3 Procedures
Participation involved a total of 9 visits (1 orientation, 2 neurocognitive, and 6 neuroimaging days). During the orientation, participants gave consent and received training on multiple neuroimaging tasks (one reported herein). Instructions were explained at a bench computer and tasks were practiced in a mock scanner.
Smoking abstinence was not required of smokers before the orientation or neurocognitive visits. However, overnight abstinence was required on neuroimaging days. We instructed smokers to have their last cigarette 12 h before their scheduled arrival. Upon arrival, all participants were tested for recent drug and alcohol use, and for expired carbon monoxide (CO) levels. Given that the half-life of CO during sleep can be up to 4–8 h (SRNT-Subcommittee, 2002), we used a guideline of less than or equal to 15 parts per million (ppm) to verify abstinence. Indicative of compliance, smokers’ CO levels were lower on neuroimaging days (mean ± SD: 7.1 ± 2.6 ppm) in comparison to visits not requiring abstinence (18.9 ± 8.9 ppm; t[23] = −8.2, p < 0.001); nonsmokers’ CO levels did not differ (abstinence required: 1.9 ± 0.3 ppm; not required: 1.8 ± 0.4 ppm; p = 0.2). Smokers did not smoke during visits.
During each neuroimaging assessment, participants completed two MRI sessions lasting ~2 h each, one in the morning and one in the afternoon. The morning session began ~2–2.5 h after patch application during which participants completed multiple neuroimaging tasks (not discussed further). The afternoon session began ~6–7 h after patch application. During this session, participants completed an amygdala reactivity paradigm after an anatomical scan and other neuroimaging tasks. The reactivity paradigm was completed ~1.75 h after scanner entry and 8–9 h post-patch.
2.4 Heart rate
To confirm that pharmacological manipulations produced an observable response at the physiological level, we measured heart rate (HR) each visit before transdermal patch application and then at multiple post-patch time points (30, 60, and 120 min). Data were normalized to percent increase from pre-patch levels and subsequently averaged across post-patch values.
2.5 Task and performance measure
We adopted a variant of the amygdala reactivity paradigm (Hariri et al., 2002b) previously used in a similar repeated-measures design (Bigos et al., 2008). On each trial of the task, participants viewed three simultaneously presented stimuli (a trio) and selected one of the two choice items (bottom) matching the target (top). Four blocks of face presentation were interleaved with five blocks of geometric shape presentation. “Face blocks” consisted of six 4 s trials (2, 4, or 6 s inter-trial interval). On any given trial, individual stimuli constituting each trio were of similar gender and emotional expression (angry, fearful, surprised, or neutral) and participants indicated via a button press which face was identical to the target. Trios were displayed on the screen for a constant 4 s duration regardless of the speed with which participants responded. During each session, a total of 24 pseudo-randomly ordered trios were displayed, 12 of each gender and 6 of each emotional expression. Given the sparse number of stimuli per expression-type and their intermingling within blocks, this paradigm was not optimized to probe affect-specific responses per se. Rather we were interested in eliciting a reliable amygdala response across sessions that could be probed for drug effects. Additionally, neutral facial expressions were used for two reasons: 1) to provide a large enough pool of images to draw from allowing for presentation of novel stimuli each session thereby mitigating habituation effects, and 2) novel neutral face presentation also engages the amygdala (Kukolja et al., 2008). Facial stimuli were obtained from a standard set of images (www.macbrain.org). “Shape blocks” consisted of six 4 s trials (2 s inter-trial interval) and involved presentation of ovals and circles. Task-performance measures were reaction times (RT) from correct-response trials and error rates (including missed-response trials) collapsed across face-and shape-trials.
RT from correct-response trials were collapsed across face and shape trial-types and assessed as a measure of task performance. We collapsed RT across trial-types because pharmacological manipulations augmented performance in abstinent smokers similarly on both face and shape trials (Supplemental Information: section “1.3 Task performance”). While there is abundant evidence that smokers encounter abstinence-induced performance deficits and that nAChR stimulation speeds RT during monotonous tasks (e.g., Heishman et al., 1994; Kleykamp et al., 2011; Myers et al., 2008), there is also growing appreciation of individual differences in the susceptibility to such performance deficits (Patterson et al., 2010; Rukstalis et al., 2005). This individual variability appears clinically relevant as it: 1) predicts relapse propensity (Patterson et al., 2010), 2) may be mediated by genetic variation (Greenwood et al., in press; Winterer et al., 2010), and 3) could be leveraged to indicate which smokers would most benefit from specific pharmacological interventions (Loughead et al., 2009; Newhouse et al., 2004). We reasoned that smokers most susceptible to abstinence-induced deficits would receive the greatest benefit from nAChR stimulation by nicotine and varenicline in terms of RT-improvement and decreased amygdala reactivity.
We parsed participants (both smokers and nonsmokers) into subgroups/cohorts based on their propensity to show consistent behavioral improvements following nAChR stimulation. This (sub)grouping scheme was characterized by the question: Did nicotine (both times administered) and varenicline consistently improve a participant’s task performance? We operationalized performance improvement (a proxy for an observable drug response) as a decrease in RT following drug administration relative to a corresponding placebo condition. We calculated three difference values: two associated with nicotine administration (Δ-NIC1: pre-pill/placebo-patch minus pre-pill/nicotine-patch; and, Δ-NIC2: placebo-pill/placebo-patch minus placebo-pill/nicotine-patch) and one with varenicline administration (Δ-VAR: [pre-pill/placebo-patch + placebo-pill/placebo-patch / 2] minus varenicline-pill/placebo-patch). Positive values indicated drug administration improved a participant’s performance whereas negative values indicated drugs did not improve performance. If all three difference values were positive, a participant was categorized as a stable RT-improver (SI). If any one of the difference values was not positive, the participant was categorized as a variable/non RT-improver (VI). Presumably, individual differences in the propensity to demonstrate augmented performance following nAChR stimulation are a stable trait best captured by a composite metric derived from multiple observations. Such a strategy is arguably less susceptible to “misclassification” due to session-specific variance and thus provides a more reliable estimate of behavioral improvement in comparison to categorization using a single difference value.
2.6 MR image collection
Data were acquired with a Siemens 3T Magnetom Allegra scanner (Erlangen, Germany). During the task, thirty-nine 4-mm thick slices were obtained 30° oblique to the axial plane using a T2*-weighted, single-shot gradient-echo, echo-planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) effects (202 volumes; repetition time [TR] = 2,000 ms; echo time [TE] = 27 ms; flip angle [FA] = 80°; field of view = 220 × 220 mm; image matrix = 64 × 64). High-resolution oblique-axial structural images were also acquired using a 3D magnetization prepared rapid gradient-echo (MPRAGE) T1-weighted sequence (TR = 2,500 ms; TE = 4.38 ms; FA = 8°; voxel size = 1 mm3).
2.7 MR image processing and analyses
We processed and analyzed data with AFNI (Cox, 1996). Functional data were slice-time and motion corrected, and aligned with anatomical images. Time series were normalized to percent signal change and submitted to voxel-wise multiple regression. For each participant and session, regressors for the two task conditions (i.e., face and shape blocks) were modeled as box-car functions convolved with a canonical hemodynamic response. Six motion-correction nuisance regressors were also included. We calculated individual contrast images comparing face versus shape blocks, normalized the images into Talairach space with re-sampled 3 mm isotropic voxels, and spatially blurred them with a 3 mm Gaussian kernel.
To identify regions showing differential activity during face versus shape blocks, individual participant’s contrast images were averaged across the six sessions and then submitted to a group-level, one-sample t-test. We applied a voxel-wise threshold of p < 10−10 to the resulting statistical map with a minimum cluster size of 20 voxels. We used this stringent threshold because the blocked-design and session-averaged contrast images led to a large effect size and high statistical power. These procedures yielded group-level functionally defined clusters encompassing right (3780 mm3; center of mass [Talairach, mm]: x = 24, y = −6, z = −16) and left amygdala regions (2727 mm3; x = −22, y = −4, z = −16).
To characterize drug effects, we examined percent signal change from group-level, task-defined regions of interest (ROIs). We extracted signal change by averaging across all voxels within a given ROI. As there were no significant effects of hemisphere/laterality detected on amygdala reactivity, left and right amygdala values were averaged before further statistical analyses. Regional percent signal change was assessed in separate mixed-effects ANOVAs. Task-defined ROI assessment may potentially bias subsequent inferences if the comparison generating the ROI is not statistically independent from the contrast of interest (Kriegeskorte et al., 2009). By defining ROIs based on a group-level analysis collapsed across all participants and visits, this six-session average was independent of the main drug comparisons of interest, and hence did not bias results.
2.8 Statistical analyses
HR and RT data were analyzed in GROUP (smoker vs. nonsmoker) × PILL (varenicline vs. placebo) × PATCH (nicotine vs. placebo) mixed-effects ANOVAs controlling for age and education. Given individual differences in the propensity to show behavioral improvements following drug administration, both smokers and nonsmokers were parsed into SI and VI cohorts (see results below). Therefore, brain activity extracted from task-defined ROIs was assessed in exploratory COHORT (SI vs. VI) × GROUP × PILL × PATCH mixed-effects ANOVAs controlling for age, education, and error rates. As differing error rates may contribute to variability in amygdala activity (Pourtois et al., 2010), we included a composite error rate measure1 as a covariate of no interest to account for additional variance. Data are reported as estimated marginal mean ± standard error controlling for covariates. Unless otherwise stated, statistical corrections (i.e., Bonferroni correction) were applied and corrected p values are reported.
3. RESULTS
3.1 Heart rate
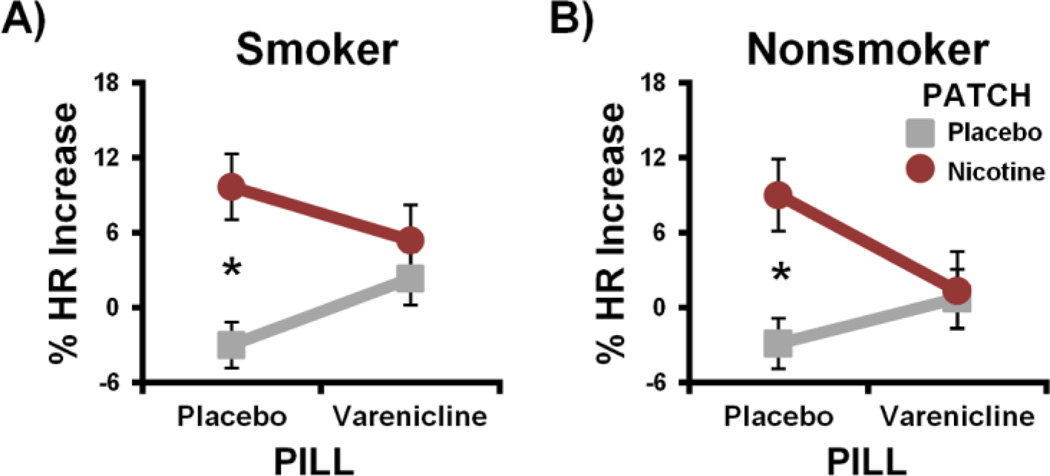
HR was modulated by pharmacological manipulations similarly in both smokers and nonsmokers as indicated by the absence of a significant GROUP × PILL × PATCH interaction (F[1,40]2 = 0.2, p > 0.7; Fig. 2 and Supplemental Fig. S1). Within the smoker group, both nicotine and varenicline produced a physiological response in accord with the hypothesized pharmacological interaction pattern (PILL × PATCH: F[1,23] = 6.4, p = 0.04; Fig. 2A). Specifically, nicotine (vs. placebo) patch administration increased HR under placebo pill sessions (t[23] = 4.4, p < 0.001), but not under varenicline sessions (p > 0.6). A similar pattern was observed within the nonsmoker group (Fig. 2B), such that nicotine-induced HR increases during placebo pill sessions (t[19] = 4.0, p = 0.002) were absent during varenicline sessions (p > 0.8). These results confirm that nicotine and varenicline produced a physiological response in both smokers and nonsmokers and, despite differences in terms of nicotine dose and previous history with nicotine, indicate that the two groups reacted similar (in terms of HR) to the drug manipulations.
Figure 2.
Varenicline and nicotine modulated heart rate (HR) similarly in smokers and nonsmokers. Nicotine-induced HR increases observed under placebo pill conditions were attenuated under varenicline conditions in both the smoker (A) and nonsmoker groups (B). * pcorrected < 0.05.
3.2 Task performance
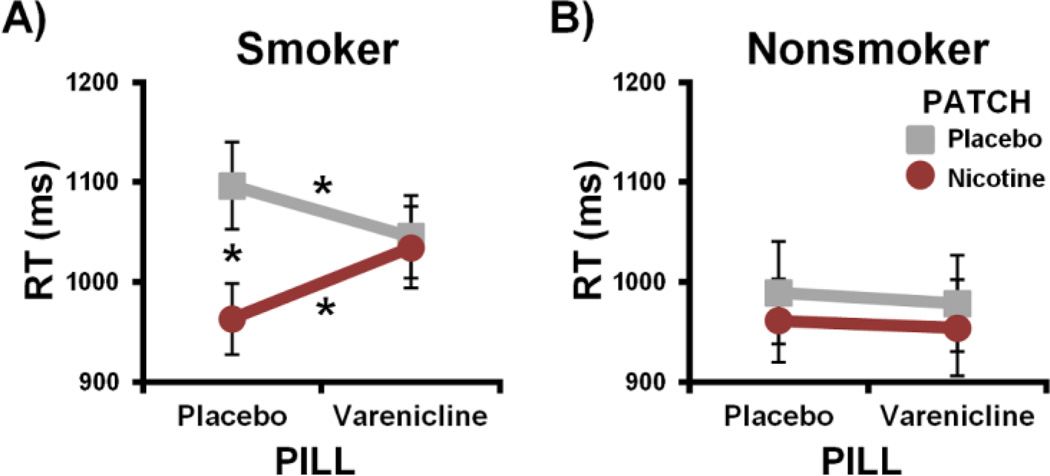
In contrast to the HR measure, pharmacological manipulations differentially impacted RT in the smoker and nonsmoker groups (GROUP × PILL × PATCH: F[1,38]3 = 4.2, p = 0.047; Fig. 3 and Supplemental Fig. S2). Within the smoker group, nicotine and varenicline modulated RT in accord with the hypothesized pharmacological interaction pattern (PILL × PATCH: F[1,23] = 9.5, p = 0.01; Fig. 3A). Specifically, nicotine (vs. placebo) patch sessions were associated with faster responding under placebo pill (t[23] = −3.9, p = 0.003; Cohen’s d = −0.69), but not under varenicline conditions (p = 0.7). No nicotine-induced RT reduction was observed under active medication as varenicline: 1) decreased RT in the absence of nicotine as predicted (t[23] = −2.1, pone-tailed = 0.046; Cohen’s d = −0.24); and 2) attenuated nicotine’s impact thereon (t[23] = 2.5, pone-tailed = 0.02; Cohen’s d = 0.32). Within the nonsmoker group, performance was not affected by administration of either drug (p values > 0.3; Fig. 3B). Thus, nicotine and varenicline augmented task performance in abstinent smokers while having no detectable impact on nonsmokers’ RT. When assessing error rates (Supplemental Fig S2), no significant GROUP, PILL, or PATCH effects were detected (p values > 0.1), arguing against a speed-accuracy tradeoff explanation of RT differences.
Figure 3.
The impact of nAChR stimulation on reaction time (RT) in smokers and nonsmokers. (A) Across all smokers, nicotine-induced effects observed under placebo pill sessions were absent under active medication as varenicline decreased RT (grey line) and attenuated nicotine’s impact thereon (red line). (B) Although nonsmokers displayed a physiological (HR) response to drugs, pharmacological manipulations did not significantly impact their behavioral performance. * pcorrected < 0.05.
Not all abstinent smokers displayed consistent RT reductions following nAChR stimulation. As such, we parsed participants into subgroups/cohorts according to whether the individual showed a RT decrease (i.e., improvement) following each drug administration. If drugs decreased RT each administration (i.e., Δ-NIC1 Δ-NIC2 and Δ-VAR were all positive) a participant was categorized as a stable RT-improver (SI), otherwise the participant was categorized as a variable/non RT-improver (VI). Using this subject-level criterion, 11 smokers (7 nonsmokers) were categorized as SIs and 13 smokers (11 nonsmokers) as VIs. One interpretation of why some abstinent smokers showed stable RT-improvements following drug administration is that these are the smokers who are particularly susceptible to abstinence-induced RT slowing (Supplemental Figure S3). We hypothesized that those smokers showing robust behavior augmentation would also be those most likely to show drug-induced decreases in amygdala reactivity. While the two smoker subgroups were distinguished by objective pharmacologically-induced effects on RT, the two smoker cohorts did not significantly differ in terms of demographic or smoking characteristics (Supplemental Table S1).
3.3 Imaging results
Across all sessions and participants, emotional face presentation produced increased activation in bilateral amygdala, dorsolateral prefrontal cortex (dlPFC), visual cortex, and fusiform gyri (Supplemental Table S2). Activity from these task-defined ROIs was extracted and assessed for drug effects. When considering all smokers as a single homogeneous group and all nonsmokers, no significant GROUP (smoker vs. nonsmoker), PILL (varenicline vs. placebo), or PATCH effects (nicotine vs. placebo) were detected with respect to amygdala reactivity (p values > 0.1). However, given the above indication that subsets of participants reacted differently to nAChR stimulation at the behavioral level, we subsequently assessed amygdala reactivity in exploratory COHORT (SI vs. VI) × GROUP × PILL × PATCH mixed-effects ANOVAs.
Pharmacological manipulations differentially impacted amygdala reactivity across subgroups of participants as indicated by a significant four-way interaction (COHORT × GROUP × PILL × PATCH: F[1,35]4 = 4.5, p = 0.04; Fig. 4A and Supplemental Fig. S4). Focusing on the smokers, the two cohorts differed in their response to drug manipulations (COHORT × PILL × PATCH: F[1,19]5 = 5.4 puncorrected = 0.04). The SI-smoker cohort showed elevated amygdala reactivity during the placebo-pill/placebo-patch session which was reduced by nAChR stimulation in accord with the hypothesized pharmacological interaction pattern (PILL × PATCH: F[1,7]5 = 6.0, puncorrected = 0.04). In contrast, the VI-smoker cohort displayed moderate levels of amygdala reactivity during the placebo-pill/placebo-patch session and drug administration did not modulate regional activity as indicated by the absence of a PILL × PATCH effect (F[1,9]5 = 0.7; p = 0.4). With respect to nonsmokers, the two subgroups reacted similarly to pharmacological manipulations (COHORT × PILL × PATCH: F[1,13]4 = 0.01; p = 0.9) such that drug-induced changes in amygdala reactivity were not detected in either cohort (p values > 0.2). These outcomes in nonsmokers provide a “negative control” for the analytical strategy used to identify differential responses within smokers. In sum, the four-way COHORT × GROUP × PILL × PATCH interaction was driven by pharmacological effects observed only in SI-smokers but not in VI-smokers or either nonsmoker cohort. Using a less stringent threshold to define ROIs, we observed a similar four-way interaction within ventromedial PFC (Supplemental Fig. S5).
Figure 4.
Drug effects on task-induced neural responses in subgroups of smokers and nonsmokers. (A) Amygdala reactivity was differentially modulated by drugs across subgroups. Pharmacologically induced decreases in amygdala reactivity (i.e., PILL × PATCH interaction) were observed only in the SI-smoker cohort (shaded), but not in any of the other three cohorts. Distinct drug-effect patterns were observed in alternative ROIs, suggesting the regional specificity and/or mechanistic selectivity of pharmacological effects within amygdala: right dlPFC (B) and visual cortex (C). * puncorrected < 0.05.
To determine the regional specificity and/or mechanistic selectivity of pharmacological effects within amygdala, we assessed responses from additional task-defined ROIs in a REGION (amygdala vs. dlPFC vs. visual cortex) × COHORT × GROUP × PILL × PATCH mixed-effects ANOVA. Distinct drug-effects patterns were observed across these ROIs as indicated by a significant five-way interaction (REGION × COHORT × GROUP × PILL × PATCH: F[2,34]6 = 3.4, p = 0.04). More specifically, in the right dlPFC we detected a significant COHORT × GROUP × PATCH interaction (F[1,35] = 7.3, p = 0.01; Fig. 4B). This distinct drug-effects pattern indicated that nicotine, but not varenicline, reduced dlPFC reactivity only in SI-smokers (PATCH: F[1,10] = 13.6, p = 0.004) but not in VI-smokers (PATCH: F[1,12] = 0.01, p = 0.9; COHORT × PATCH: F[1,19] = 7.3, p = 0.01) or either nonsmoker cohort (p values > 0.14). In the right dlPFC ROI no indications of a varenicline effect were observed within SI-smokers suggesting that nicotine’s influence in this region was modulated by nAChRs other than those upon which varenicline acts. Using a less stringent threshold to define ROIs, we observed a similar interaction effect within left insula/lateral PFC (Supplemental Fig. S5).
With respect to the visual cortex ROI, we did not detect a significant COHORT × GROUP × PILL × PATCH interaction (c.f., amygdala ROI; F[1,35] = 0.1, p = 0.8) nor a significant COHORT × GROUP × PATCH interaction (c.f., dlPFC ROI; F[1,35] = 2.6, p = 0.1). In other words, there was no evidence that subgroups of smokers (p values > 0.3) or nonsmokers (p values > 0.2) showed differential responses to drug administration in visual cortex. These results from visual cortex provide an important within-subject “control region” for the drug-induced effects reported within the amygdala and dlPFC ROIs of SI-smokers.
4. DISCUSSION
Modulation of hyperactive amygdala functioning during early smoking abstinence represents a putative neurobiological target underlying the efficacy of smoking cessation medications in some individuals. We assessed the behavioral and neurobiological impact of smoking abstinence and the amelioration of resulting effects by examining the impact of varenicline (vs. placebo pill) in participants concurrently wearing transdermal nicotine or placebo patches. When considering smokers as a single homogeneous group, varenicline and nicotine modulated RT, HR, and self-reported mood (Supplemental Information section “1.5 Self-report measures”) in a manner consistent with varenicline’s partial agonist/antagonist profile. When considering all smokers, drug administration had no significant impact on amygdala reactivity. However, an exploratory analysis suggested that nAChR stimulation via nicotine and varenicline dampened amygdala functioning in a subset of smokers who demonstrated greater abstinence-induced elevations in reactivity and showed stable RT improvements following pharmacological manipulations. Finally, when considering nonsmokers, drug-induced responses were observed at the physiological level (i.e., HR), but not at the behavioral (i.e., RT) or neurophysiological level (i.e., amygdala reactivity).
4.1 Differential pharmacological effects on amygdala reactivity
Whereas previous neuroimaging investigations have reported the down-regulation of amygdala functioning following cigarette smoking (Mihov and Hurlemann, 2012; Rose et al., 2003; Zubieta et al., 2005) or varenicline treatment (Franklin et al., 2011a; Loughead et al., in press; Loughead et al., 2010), our results suggest individual differences in the effects of smoking abstinence and subsequent nAChR stimulation on amygdalar responses. In the SI-smoker cohort, amygdala functioning was hyperactive in the absence of drug administration during smoking deprivation (i.e., placebo-pill/placebo patch session). Nicotine replacement and varenicline administration (as indicated by a PILL × PATCH interaction) decreased such hyperactivity. In contrast, the VI-smoker cohort presented with moderate levels of reactivity in the absence of pharmacological manipulations and drugs did not robustly modulate amygdala activity. It may be that those smokers with lower amygdala reactivity in the absence of nAChR stimulation were at or near “optimal” levels, thus providing little dynamic range for the pharmacological manipulations to reduce amygdala functioning.
Down-regulation of hyperactive amygdala functioning, which has been linked to negative emotional states accompanying drug withdrawal (Koob, 2009), may partly underlie the beneficial clinical effects of varenicline, particularly in those individuals experiencing higher degrees of affective disturbances during early abstinence (Piper et al., 2011). While the current study was underpowered to detect differences between smoker cohorts in regards to self-reported withdrawal, mood, or tobacco craving (Supplemental information section “1.5 Self-report measures”), across all sessions and smokers, self-reported withdrawal symptoms were positively correlated with amygdala reactivity. The current results may also be considered in light of other pharmacological fMRI studies that have observed decreased amygdala reactivity following administration of antidepressant (van Marle et al., 2011) or anxiolytic drugs (Paulus et al., 2005).
While ~45% of smokers in clinical trials are able to remain abstinent during varenicline treatment (Gonzales et al., 2006; Jorenby et al., 2006), the distinguishing characteristics of those individuals showing an efficacious response remain unknown. Differential effects associated with smoking abstinence and nAChR stimulation on brain activity, such as those reported herein, may prove useful for identifying those smokers who would most benefit from nAChR pharmacological treatments and thus facilitate the implementation of individualized treatment strategies for cessation. Although we did not collect reports regarding reasons for tobacco use, individuals showing elevated amygdala functioning during abstinence may be those more likely to indicate smoking for stress reduction, improved emotional coping, or relaxation (Rose et al., 2007).
4.2 Task performance
Varenicline and nicotine improved task performance in abstinent smokers but not in nonsmokers. Nicotine’s performance-enhancing properties manifest in multiple domains, particularly when considering abstinent smokers (Heishman et al., 1994). Similarly, varenicline has been shown to augment performance in preclinical models (Rollema et al., 2009) and clinical studies (Loughead et al., in press; Loughead et al., 2010; Patterson et al., 2009; Sofuoglu et al., 2009). The unique contribution of our study lies in the fact that we examined the impact of varenicline both in the presence and absence of nicotine. Using this two-drug approach, we observed not only a varenicline-induced RT reduction (consistent with a partial agonist effect), but also the attenuation of a nicotine-induced full agonist response (consistent with an antagonistic effect). While evidence for varenicline’s partial agonist/antagonist mechanism of action is largely based on cellular and animal data (Rollema et al., 2009), heretofore it has been unexamined in abstinent human smokers. Given that a core symptom of nicotine withdrawal is difficulty concentrating, amelioration of mild behavioral impairment during a quit attempt and attenuation of nicotine’s impact thereon during a smoking lapse, may contribute to varenicline’s relatively high clinical efficacy. Furthermore, indications of differential responses to pharmacological manipulations within smoker subgroups were also observed at the behavioral level. Such differential responses are consistent with the notion that nAChR stimulation most robustly improves behavior in individuals performing sub-optimally (Newhouse et al., 2004). Behavioral performance appears mediated by genetic influences on nAChR function (Greenwood et al., in press; Winterer et al., 2010) and, when assessed during early abstinence, predicts smoking relapse (Patterson et al., 2010).
4.3 Heart Rate
Finally, we observed that varenicline reduced nicotine-induced HR increases in both smokers and nonsmokers. Varenicline’s ability to suppress HR increases has similarly been observed after intravenous nicotine injection (Sofuoglu et al., 2009). While the distinct nAChRs involved in the modulation of autonomic activity remain to be more fully characterized, potential candidates include α3β4, α7, and α4β2 receptors (Li et al., 2010). Although binding with lower affinity at α3β4 and α7 nAChRs (Rollema et al., 2009), varenicline’s action beyond α4β2 subtypes cannot be ruled out (Mihalak et al., 2006). Such effects may be clinically relevant, as the peripheral actions of abused drugs appear to contribute to activation of the dopaminergic system by serving as conditioned reinforcers (Wise et al., 2008). Laboratory assessments indicate varenicline decreases the subjective rewarding aspects of cigarette smoking (Patterson et al., 2009). Attenuation of nicotine-induced cardiovascular (and/or other autonomic) responses by varenicline may be one mechanism mediating subjective changes in perceived smoking satisfaction.
4.4 Limitations and conclusions
While the two-drug, within-subject, cross-over design represents a major strength of our study, the current results must also be considered in light of limitations. First, inherent in all repeated-measures designs is the potential for practice/habituation or novelty effects. Second, as we recruited smokers who were not explicitly interested in quitting smoking, the degree to which the current results generalize to a treatment-seeking sample remains indeterminate. Finally, and perhaps most critically, the post hoc parsing of participants into two cohorts based on drug-induced decreases in RT represents a quasi-experimental approach rendering those results exploratory in nature and necessitating replication. This limitation could be overcome in future studies by recruiting subgroups of smokers based on a priori characteristics. While our smoker cohorts did not significantly differ in terms of demographic or smoking characteristics (Supplemental Table S1), the literature suggests several other factors that may be worth exploring. For example, those smokers presenting with increased abstinence-induced amygdala functioning may be identified based on personality (e.g., sensation seeking or anxiety sensitivity: Evatt and Kassel, 2010; Lee et al., 2011) or genetic characteristics (e.g., dopamine or serotonin gene variants: Franklin et al., 2011b; Hariri et al., 2002a; Jasinska et al., 2012).
Despite these limitations, the current results provide a step toward fractionating the smoker phenotype by neurobiological characteristics. Pharmacotherapy administration dampened elevated amygdala functioning most robustly in those smokers appearing susceptible to abstinence-induced effects, and by inference, smoking relapse when attempting to quit. In contrast nAChR stimulation with varenicline and nicotine did not appear to modulate amygdala functioning in those individuals at or near “optimal” levels in the absence of drug administration (i.e., smokers less susceptible to abstinence-induced effects). Elucidating the differential impact of abstinence and nAChR stimulation on neural activity and behavior performance may prove useful for the development of cessation interventions tailored to the individual.
Supplementary Material
Highlights.
Varenicline attenuates nicotine’s impact on heart rate in both smokers and nonsmokers
Varenicline and nicotine augment task performance in smokers but not nonsmokers
Drugs dampen amygdala reactivity in smokers susceptible to abstinence-induced effects
ACKNOWLEDGEMENTS
This work was sponsored by the National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Department of Health and Human Services (NIDA-IRP/NIH/DHHS). We thank Eliscia Smith, Angela Neal, Kimberly Slater, Loretta Spurgeon, and the NIDA-IRP nurses, pharmacy, and recruitment staff for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Using error rates from each session, we performed a factor analysis with principal components extract to reduce the multiple error measures into a single composite value for use as a covariate.
Controlling for age and education.
Controlling for age and education. Data from two male nonsmokers were excluded from behavioral and imaging analyses.
Controlling for age, education and errors.
Controlling for age, cigarettes per day and errors.
As the REGION factor was composed of three-levels, statistical values from the multivariate approach to repeated-measures ANOVA are reported. Controlling for age, education, and errors.
FINANCIAL DISCLOSURES
The authors declare no financial conflicts of interest.
SUPPLEMENTAL INFORMATION
Supplemental Information for this manuscript includes Supplemental Data, six figures, and two tables.
REFERENCES
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharm. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biological Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Evatt DP, Kassel JD. Smoking, arousal, and affect: The role of anxiety sensitivity. J Anxiety Disorders. 2010;24:114–123. doi: 10.1016/j.janxdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. J Clin Pharmacol. 2006;46:1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O'Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiat. 2011a;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O'Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: Confirmation in a new cohort. Addict Biol. 2011b;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Grp VPS. Varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation - A randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Espeseth T. A cognitive phenotype for a polymorphism in the nicotinic receptor gene CHRNA4. Neurosci Biobehav Rev. 36:1331–1341. doi: 10.1016/j.neubiorev.2012.02.010. in press. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002a;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage. 2002b;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence - A revision of the Fagerstrom Tolerance Questionnaire. British J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Exp Clin Psychopharm. 1994;2:345–395. [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nic Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biological Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Chua HF, Ho SS, Polk TA, Rozek LS, Strecher VJ. Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting. Neuroimage. 2012;60:766–773. doi: 10.1016/j.neuroimage.2011.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha 4 beta 2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation - A randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Eissenberg T. Effects of Transdermal Nicotine and Concurrent Smoking on Cognitive Performance in Tobacco-Abstinent Smokers. Experimental and Clinical Psychopharmacology. 2011;19:75–84. doi: 10.1037/a0022417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nature Neuroscience. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Fink GR, Hurlemann R. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;28:12868–12876. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin Attenuates Amygdala Reactivity to Fear in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Perkins KA, Zimmerman E, Robbins G, Kelly TH. Effects of 24 hours of tobacco withdrawal and subsequent tobacco smoking among low and high sensation seekers. Nic Tob Res. 2011;13:943–954. doi: 10.1093/ntr/ntr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, LaCroix C, Freeling J. Cytisine induces autonomic cardiovascular responses via activations of different nicotinic receptors. Autonomic Neuroscience-Basic & Clinical. 2010;154:14–19. doi: 10.1016/j.autneu.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, O'Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C. Brain activity and emotional processing in smokers treated with varenicline. Addict Biol. doi: 10.1111/j.1369-1600.2011.00324.x. in press. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha 4 beta 2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psych. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Molecular Psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha 4 beta 2 and a full agonist at alpha 7 neuronal nicotinic receptors. Molecul Pharm. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mihov M, Hurlemann R. Altered amygdala function in nicotine addiction: Insights from human neuroimaging studies. Neuropsychologia. 2012;50:1719–1729. doi: 10.1016/j.neuropsychologia.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Cur Op Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Palmer KJ, Buckley MM, Faulds D. Transdermal nicotine - A review of Its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs. 1992;44:498–529. doi: 10.2165/00003495-199244030-00011. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psych. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psych. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, Bolt DM, Kim SY, Kaye JT, Hefner KR, Baker TB. Tobacco withdrawal components and their relations with cessation success. Psychopharm. 2011;216:569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Vocat R, N'Diaye K, Spinelli L, Seeck M, Vuilleumier P. Errors recruit both cognitive and emotional monitoring systems: Simultaneous intracranial recordings in the dorsal anterior cingulate gyrus and amygdala combined with fMRI. Neuropsychologia. 2010;48:1144–1159. doi: 10.1016/j.neuropsychologia.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha(4)beta(2) nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, Mclean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha 4 beta 2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharm. 2007;32:2441–2452. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiat. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. Journal of Substance Abuse Treatment. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci and Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Mooney M, Waters AJ. Varenicline attenuates some of the subjective and physiological effects of intravenous nicotine in humans. Psychopharm. 2009;207:153–162. doi: 10.1007/s00213-009-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT-Subcommittee. Biochemical verification of tobacco use and cessation. Nic Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- van Marle HJF, Tendolkar I, Urner M, Verkes RJ, Fernandez G, van Wingen G. Subchronic duloxetine administration alters the extended amygdala circuitry in healthy individuals. Neuroimage. 2011;55:825–831. doi: 10.1016/j.neuroimage.2010.12.051. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Mittelstrass K, Giegling I, Lamina C, Fehr C, Brenner H, Breitling LP, Nitz B, Raum E, Muller H, Gallinat J, Gal A, Heim K, Prokisch H, Meitinger T, Hartmann AM, Moller HJ, Gieger C, Wichmann HE, Illig T, Dahmen N, Rujescu D. Risk gene variants for nicotine dependence in the CHRNA5-CHRNA3-CHRNB4 cluster are associated with cognitive performance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1448–1458. doi: 10.1002/ajmg.b.31126. [DOI] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu YJ, Koeppe RA, Ni LS, Guthrie S, Domino EF. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiat. 2005;162:567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.