Abstract
Regulation of sexual reproduction by estradiol involves the activation of estrogen receptors (ERs) in the hypothalamus. Of the two classical ERs involved in reproduction, ERα appears to be the critical isoform. The role of ERα in reproduction has been found to involve a nuclear ERα that induces a genomic mechanism of action. More recent a plasma membrane ERα has been shown to trigger signaling pathways involved in reproduction. Mechanisms underlying membrane-initiated estradiol signaling are emerging, including evidence that activation of plasma membrane ERα involves receptor trafficking. The present study examined the insertion of ERα into the plasma membrane of N-38 neurons, an immortalized murine hypothalamic cell line. We identified, using western blotting and PCR that N-38 neurons express full-length 66 kDa ERα and a 52 kDa ERα spliced variant missing the fourth exon - ERαΔ4. Using surface biotinylation we observed that treatment of N-38 neurons with estradiol or with a membrane impermeant estradiol elevated plasma membrane ERα protein levels, indicating that membrane signaling increased receptor insertion into the cell membrane. Insertion of ERα was blocked by the ER antagonist ICI 182,780 or with the protein kinase C (PKC) pathway inhibitor bisindolylmaleimide (BIS). Downstream membrane-initiated signaling was confirmed by estradiol activation of PKC-theta (PKCθ) and the release of intracellular calcium. These results indicate that membrane ERα levels in N-38 neurons are dynamically autoregulated by estradiol.
Keywords: N-38 neurons, membrane-initiated estradiol signaling, receptor trafficking, ERα slice variant, surface biotinylation
1. Introduction
Estradiol (17β-estradiol) influences a variety of functions including sensory processing, learning, memory, and neuroprotection. It is increasingly apparent that estradiol acts both in the nucleus to directly modulate gene expression and at the cell membrane to activate intracellular signaling pathways, some of which induce gene expression. Perhaps the most well studied estradiol action is the control of female reproduction, which requires both nuclear- and membrane-initiated estradiol signaling in neurons and astrocytes [1]. It is assumed that activation of full-length ERα mediates the majority of estradiol reproductive actions [2,3,4,5]. While other ERs have been reported in the brain, their identities and roles still remain unresolved [6,7,8].
While a great deal of attention has been directed at estradiol-mediated regulation of nuclear ERs, relatively few studies have addressed estradiol-mediated regulation of membrane ERs. A preponderance of evidence suggests that the same receptors responsible for nuclear-initiated signaling also mediate membrane-initiated signaling [1,9]. Our laboratory has demonstrated the importance of membrane ERα-initiated signaling necessitating the transactivation of metabotropic glutamate receptor 1a (mGluR1a) in arcuate nucleus (ARH) neurons in order to produce female sexual receptivity [10]. Using primary cultures of embryonic hypothalamic neurons, we demonstrated the estradiol regulation of ERα and mGluR1a trafficking to and from the cell membrane [7] and that neuropeptide-Y (NPY) neurons in the ARH have been identified as a site of membrane-initiated estradiol signaling linked to female sexual receptivity [10,11,12]. Membrane-initiated estradiol signaling activates the novel protein kinase PKCθ, a critical step for inducing sexual receptivity [12,13] but it is still not clear whether other signaling pathways are activated. For example, other investigators have shown that membrane-initiated signaling and the insertion of ERα into the cell membrane involve the activation of the ERK pathway [14,15,16,17].
In this study, we examined estradiol signaling linked to ERα trafficking in N-38 neurons, which are immortalized NPY neurons derived from mouse ARH [18,19]. Like other neural cells, N-38 cells rapidly responded to estradiol treatment with an increase in intracellular calcium concentration ([Ca2+]i), which was blocked when type I metabotropic glutamate receptors were antagonized [10,20]. N-38 neurons, like embryonic neurons and adult astrocytes expressed full-length 66 kDa as well as several ERα-immunoreactive proteins [7,21]. The most prominent was a band that migrated at approximately 52 kDa, suggesting an ERα splice variant. Other investigators have identified an isoform lacking exon 4, which appears to be most abundant in mRNA derived from brain tissue, while only trace amounts were identified in uterine tissue [8,22]. We verified by RT-PCR analysis that an mRNA species corresponding to ERαΔ4 was present, but not ERαΔ7 in N-38 neurons. Estradiol modulated levels of 66 and 52 kDa ERα in parallel by initiating signaling at the membrane in N-38 neurons.
2. Experimental
2.1. N-38 cultures
Cultures were prepared from a frozen stock of N-38 neurons (Cellutions Biosystems, Burlington, ON) and maintained in DMEM/10% FBS (high glucose, 1% penicillin/streptomycin, 7.5% NaHCO3; Invitrogen, Carlsbad, CA) at 37°C with 5% CO2 for 2 days. On day 3, cultures were washed with PBS/CM (1 mM CaCl2, 1 mM MgSO4, pH 7.4) and changed to serum-free DMEM (1% penicillin/streptomycin, 7.5% NaHCO3) 24 h prior to treatments. On day 4, experimental drugs were prepared fresh in the vehicle dimethylsulfoxide (unless otherwise indicated) and diluted directly into the medium at a final concentration of 1 nM estradiol (E2; Sigma-Aldrich, St Louis, MO), 1 μg/ml E-6-BSA (β-oestradiol-6-(O-carboxymethyl)oxime-bovine serum albumin, Sigma; diluted with sterile water). E-6-BSA stock solution was prepared using an ultracentrifugation protocol to reduce free estradiol [23]. Pretreatments for N-38 cultures were for 1 h with 1 μM of ICI 182,780, 1 μM bisindolmaleomide (Tocris Bioscience; Ellisville, MO), or the ERK/MEK inhibitor U0126 (Sigma), followed by the standard E2 stimulation 30 min later, to study their affects on estradiol-mediated ERα insertion into the cell surface.
2.2. Surface biotinylation
After experimental drug treatments, the treated medium was aspirated and N-38 culture dishes were placed onto ice (to prevent exocytosis and endocytosis) and incubated with a solution of 0.5 mg/ml NHS-SS-biotin (Thermo, Rockford, IL) in PBS/CM or with PBS/CM only for control (No Biotin). The biotin solution was aspirated after 10 min and the excess biotin was quenched with two incubations of TBS containing 50 mM glycine and 50 mM NH4Cl. Cells were washed with ice cold TBS and collected in a modified RIPA buffer (Triton X-100 1.25%, SDS 0.1%, 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, pH 7.6) supplemented with Halt™ protease and phosphatase inhibitor cocktails (Thermo). The suspension was sonicated while on ice and the lysate clarified by centrifugation at 14,000 rpm for 5 min at 4°C and the supernatant collected. An aliquot of the supernatant was taken for protein determination using the BCA method (Thermo) and for examination of cytoplasmic proteins PKCθ and β-actin (the latter was used for western blotting loading control). Cytoplasmic protein samples were prepared for western blot by the addition of Laemmli buffer containing β-mercaptoethanol (BioRad, Hercules, CA) and then boiled for 5 min. The remaining supernatant was added to Pierce Spin Columns containing NeutrAvidin™ beads (Thermo) and placed on an end-over-end rotator at 4°C overnight. Biotinylated proteins were eluted off the avidin beads by adding Laemmli buffer containing β-mercaptoethanol to the column tubes and boiling for 5 min. The buffer containing the eluted biotinylated proteins was collected, equilibrated, and analyzed by western blot.
2.3. Western blot
Biotinylated and cytoplasmic protein samples were loaded onto 10% SDS-PAGE gels and the gels electroblotted onto Immobilon-P™ PVDF membranes (Millipore, Billerica, MA). Membranes were washed once with TBS plus 0.1% Tween-20 (TBS-T; pH 7.5) and nonspecific immunological binding sites were blocked with 5% BSA in TBS-T for 30 min. Primary antibodies were diluted in 5% BSA in TBS-T and incubated overnight at 4°C. The following day, membranes were washed with TBS-T and incubated with a secondary antibody diluted in 5% BSA in TBS-T for 1 hr. Immunoblots were visualized with autoradiography using SuperSignal West Pico™ enhanced chemiluminescence (Thermo).
2.4. Antibodies
The antibody MC-20 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) was used to identify ERα and the antibody for phosphorylated PKCθ (pPKCθ, Thr538; 1:1,000; Cell Signaling Technology, Beverly, MA) was used to identify active PKCθ. Additionally, antibodies for β-actin (1:10,000; Sigma, St. Louis, MO) and total PKCθ (1:1,000; Cell Signaling Technology) were used as western blot loading controls. Secondary antibodies were peroxidase labeled goat anti-rabbit and anti-mouse IgG antibodies (Jackson ImmunoResearch, West Grove, PA).
2.5. Densitometric analyses
To quantify protein levels, autoradiographic films were digitally scanned and analyzed. Band densities were analyzed using ImageJ software (version 1.41). Total band intensity values were calculated by subtracting the background for each film to account for any variation in background intensity across films. Data were expressed as relative ratios, calculated as: relative optical density values for biotinylated ERα divided by the corresponding cytoplasmic β-actin value or relative optical density values for cytoplasmic pPKCθ values divided by its corresponding cytoplasmic PKCθ obtained by western blot analysis. Both sets of computed values were normalized by dividing each set by the vehicle mean (S.E.M.) value for each of the experimental data sets.
2.6. cDNA synthesis and RT-PCR
Total RNA was extracted from N-38 neurons using Trizol Reagent (Life Technologies, Carlsbad, CA) per manufacturer’s recommended protocol. To prevent DNA contamination, RNA was treated with DNAseI (Ambion Inc., Austin, TX) at 37°C for 30 min followed by inactivation with DNAse inactivation reagent (Ambion Inc). Total RNA quality and concentration was assessed using a spectrophotometer (NanoDrop 1000, Thermo). Reverse transcription (RT) was then performed using 1μg of total RNA to synthesize single-stranded cDNA in a 20 μl reaction using SuperScript III (Invitrogen Life Technologies) and a combination of random hexamers and oligo (dT)20 primers as per manufacturer’s protocol. Briefly, the RT was performed at 50°C for 50 min, reaction terminated at 85°C for 5 min, and RNA removed with 1μl of RNase H− at 37°C for 20 min. cDNAs were subjected to PCR with primers designed to span ERα (Accession NM_007956) exon 3-5, specifically targeting exon 4 (Fwd: 5- GCCAAGGAGACTCGCTACTG-3 and Rev: 5- ATAGATCATGGGCGGTTCAG-3) or ERα exon 5-8, specifically targeting exon 7 (Fwd: 5-AATGAAATGGGTGCTTCAGG-3 and Rev: 5-GCCATCAGGTGGATCAAAGT-3) in an Mx3000p Real-Time PCR System (Stratagene, Santa Clara, CA). PCR conditions used for amplification were as follows: initial denaturation at 94°C for 10 min, 40 cycles of denaturation at 94°C for 30 sec, annealing at 56°C for 1 min, and elongation at 72°C for 1 min, with a final extension at 72°C for 7 min. Amplified products were separated by electrophoresis on a 2% agarose gel with ethidium bromide and visualized under UV light. Gel images were captured digitally to confirm product size and the absence of non-specific products. Negative controls (no cDNA) and GAPDH (positive controls) were included in every PCR run.
2.7. Intracellular Ca2+ analysis
N-38 cells were plated onto poly-D-lysine (0.1 mg/ml) coated glass coverslips and maintained in DMEM/10% FBS at 37°C with 5% CO2 for 2 days. Media was changed to DMEM/10% charcoal stripped FBS (Sigma) for 24 h and then loaded with 4.5 μM Fluo-4 AM (Invitrogen, Carlsbad, CA) for 45 min at 37°C with 5% CO2. Cyclodextrin-encapsulated 17β-estradiol (E2, 0.1 – 1000 nM) was given as an 8 sec pulse delivered by gravity perfusion. Encapsulated estradiol was used to accommodate the volume needed for perfusion. In a subset of experiments, cells were first stimulated with estradiol to determine their responsiveness. The estradiol was washed out (2 min) and treated with 50 nM LY367385 (Tocris Biosciences, Minneapolis, MN) for 7 min and then restimulated with 10 nM estradiol. Confocal microscopy was used to determine the increase in [Ca2+]i by measuring the relative change in Fluo-4 AM fluorescence intensity. Fluo-4 AM imaging was performed using an Axioplan2-LSM 510 Meta confocal microscope with an IR-Achroplan 40×/0.80 water-immersion objective using 488 nm laser excitation and emission monitored through a low-pass filter with a cutoff at 505 nm. The relative change in Fluo-4 AM fluorescence was calculated in relative fluorescence units (RFU).
2.8. Statistical analysis
A one-way ANOVA followed by a Tukey’s or Student-Newman-Keuls post hoc test was used to determine statistical significance between experimental treatment groups. In the case of comparing two experimental treatment groups an unpaired Student’s t-test was used to determine statistical significance. Data were analyzed using GraphPad Prism 4 software (GraphPad Software, La Jolla, CA), and significance level was set at p < 0.05 for all experimental data sets.
3. Results
3.1. Immortalized hypothalamic N-38 neurons express membrane ERα
N-38 neurons expressed full-length ERα and several ERα protein variants, as previously observed in native hypothalamic cells [7,24]. Analysis of N-38 lysates using the MC-20 antibody, which targets the COOH-terminal of the ERα protein, detected low levels of 66 kDa ERα and high levels of the 52 kDa ERα (Fig 1A). Additionally, N-38 neurons expressed several ERα-immunoreactive proteins (~50, 58, 75, 80, 125 kDa) at low levels. However, after surface biotinylation, the MC-20 antibody recognized only the full-length 66 kDa ERα and a 52 kDa ERα variant on the cell membrane of N-38 neurons (Fig 1B).
Figure 1.
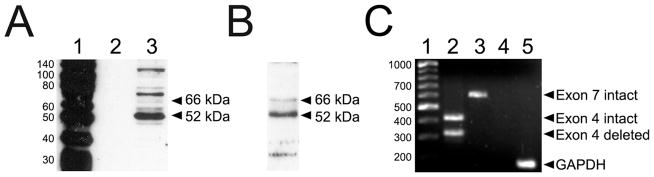
Identification of membrane ERα in immortalized hypothalamic N-38 neurons. (A) Western blot analysis of N-38 neurons using the MC-20 antibody detected full-length 66 kDa ERα protein and a prominent ERα-immunoreactive band at approximately 52 kDa, as well as several other ERα-immunoreactive bands. Lane 1 shows protein standards used to approximate immunoreactive band size; lane 2, empty and lane 3, cytoplasmic proteins extracted using RIPA buffer. (B) Western blot analysis of N-38 neurons after surface biotinylation revealed membrane localization of full-length 66 kDa ERα and the 52 kDa ERαΔ4 variant among other unidentified minor ERα-immunoreactive bands. (C) RT-PCR analysis of the expression of ERα isoforms in N-38 cells. Lane 1 shows the DNA ladder used to approximate the number of base-pairs (bp) while lane 2 shows two distinct bands corresponding to the coexistence of the wild-type ERα (445 bp; exon 4 intact) and ERα with exon 4 deleted (332 bp). Lane 3 displays only one band corresponding to wild-type ERα and no deleted exon 7 variant (640 bp; exon 7 intact). Lane 4 is a negative control (no cDNA) and lane 5, a GAPDH positive control (190 bp).
3.2. Expression of full-length ERα and ERαΔ4 mRNA in N-38 neurons
To characterize the different isoforms of the ERα expressed in immortalized N-38 neurons, total RNA was prepared from these cells and RT-PCR analysis performed using primers designed specifically flanking exons 4 and 7. Targeting exons 4 and 7 were of keen interest to us since deletions of either of these exons are the most commonly found alternatively spliced forms of ERα mRNA in the brain [25], as well as breast and ovarian tumors [26,27]. Coincidentally both splice variant mRNAs are predicted to code for proteins of ~52 kDa. After 40 cycles of amplification, PCR products were resolved on a 2% agarose gel and visualized. Two distinct bands of 449 and 332 bp were generated when using primers targeting exon 4 (Fig 1C) indicating that both intact and deleted transcripts were present in N-38 neurons. Concurrently, reaction products targeting exon 7 revealed only one band at 641 bp, suggesting that N-38 neurons do not make exon 7 deleted ERα mRNA. The integrity of the cDNA and PCR reagents were examined with the use of our negative and positive controls. These results indicate that N-38 neurons express both full-length ERα and ERαΔ4, but not ERαΔ7 or the other potential splice variant detected in the brain.
3.3. Estradiol-induced insertion of ERα into the plasma membrane involves the PKC pathway
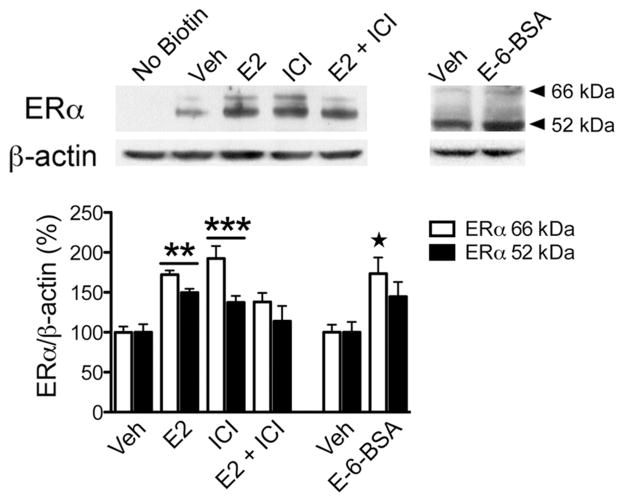
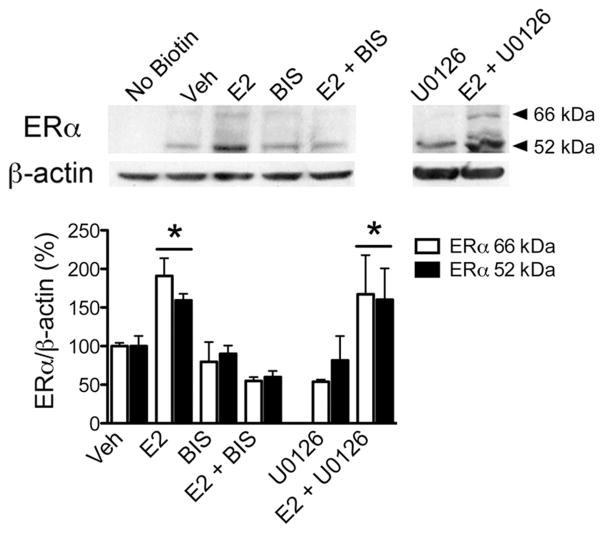
To examine whether estradiol stimulation altered membrane ERα levels, N-38 cultures were treated with estradiol, surface biotinylated and then characterized using western blotting with the MC-20 antibody. Estradiol (E2; n = 5) and unexpectedly ICI 182,780 (n = 5) treatment rapidly increased both the amounts of surface biotinylated 66 kDa (ANOVA F(3,16) = 14.52, p < 0.0001) and 52 kDa ERα (ANOVA F(3,16) = 3.66, p = 0.035) compared to their corresponding vehicles (Fig 2). However, when N-38 cultures (n = 5) were first preincubated with ICI 182,780, an ERα antagonist, followed by estradiol 30 min later (E2 + ICI), the increase in biotinylated 66 kDa and 52 kDa ERα protein was abrogated (Fig 2). Paradoxically, treatment of N-38 cultures with E-6-BSA, a cell impermeable form of estradiol, significantly increased the surface expression of full-length 66 kDa ERα only (t-test, p = 0.0173; t(6) = 3.257; R2 = 0.639), but not the 52 kDa ERα variant when compared to their corresponding vehicle controls. To further test whether the PKC signaling pathway was involved in estradiol-induced ERα insertion into the plasma membrane, N-38 cells were pharmacologically treated with the PKC inhibitor BIS or ERK/MEK inhibitor, U0126, followed by E2 stimulation. BIS treatment alone and in conjunction with E2 prevented estradiol-induced increase of both membrane 66 kDa (ANOVA F(3,16) = 11.44, p = 0.0003) and 52 kDa ERα (ANOVA F(3,16) = 16.42, p < 0.0001). However, when preincubated with the ERK/MEK inhibitor, U0126, ERα insertion into the plasma membrane was significantly increased (Fig 3) by estradiol treatment. Together these results demonstrate that estradiol-induced ERα insertion into the plasma membrane of N-38 neurons is PKC-dependent and not through the ERK pathway.
Figure 2.
Estradiol-induced increase in membrane ERα protein levels. N-38 cultures were treated with vehicle (Veh) or 1 nM estradiol (E2) for 30 min and/or pretreated for 1 h with ICI 182,780 (1 μM; top, left panel). After experimental drug treatments, the neurons were surface biotinylated, lysed, and prepared for western blot. Analysis of surface biotinylated proteins revealed that both E2 (n = 5) and ICI 182,780 (n = 5) alone increased membrane localized full-length 66 kDa ERα and 52 kDa ERα variant protein levels. When E2 was administered along with ICI 182,780 (E2 + ICI; n = 5), the increase in biotinylated ERα receptor levels was prevented. Treatment of N-38s with E-6-BSA (1 μg/ml) for 30 min increased the surface expression of 66 kDa ERα when compared with its vehicle control (top, right panel). Analysis of β-actin was performed as a loading control. Bars (mean ± S.E.M.) represent a ratio of the normalized optical density values for 66 kDa or 52 kDa ERα-immunoreactive band normalized divided by the corresponding β-actin value X 100 (%; Tukey’s post hoc, **p < 0.01. ***p < 0.001; unpaired Student’s t-test, p < 0.05).
Figure 3.
The PKC pathway is involved in estradiol-induced ERα insertion into plasma membranes. N-38 cultures were treated with vehicle (Veh) or 1 nM estradiol (E2) for 30 min and/or pretreated for 1 h with BIS (1 μM) or U0126 (1 μM; top, left panel). After experimental drug treatments, the neurons were surface biotinylated, lysed, and prepared for western blot. Analysis of cultures treated with the pathway inhibitors showed that pretreatment with BIS (E2 + BIS; n = 5) prevented the increase in ERα insertion into plasma membranes, but U0126 (E2 + U0126; n = 3) did not. BIS and U0126 alone did not affect insertion into the plasma membrane. Analysis of β-actin was performed as a loading control. Bars (mean ± S.E.M.) represent a ratio of the normalized optical density values for 66 kDa or 52 kDa ERα-immunoreactive band divided by the corresponding β-actin value X 100 (%; Tukey’s post hoc, *p < 0.05).
3.4. Estradiol-induced PKCθ pathway activation
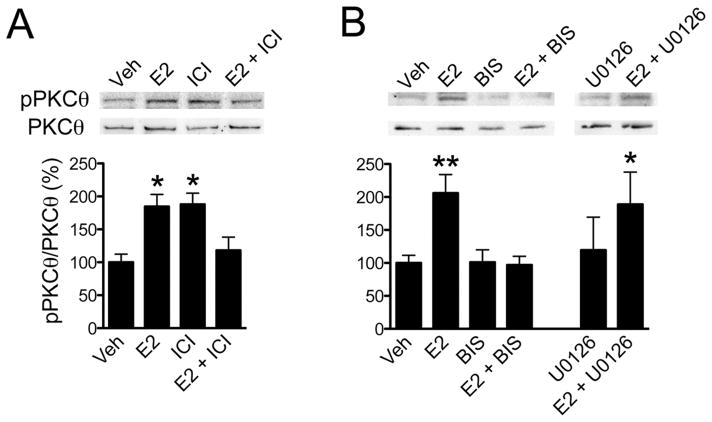
Previously, we had determined that estradiol activates PKCθ in the in vivo arcuate nucleus [12]. To determine whether estradiol leads to phosphorylation of this novel PKC in N-38 neurons, cultures were treated with estradiol and ICI 182,780. As with the surface biotinylation experiments, both E2 and ICI 182,780 (ANOVA F(3,24) = 8.689, p = 0.0004) increased the levels of phosphorylated PKCθ, but when ICI 182,780 was given prior to E2 treatment, the increase in phosphorylation was prevented (Fig 4A). To examine whether phosphorylated PKCθ was affected in ERα signaling, N-38 neurons were treated with BIS prior to E2. As predicted, BIS alone and with E2 stimulation prevented the increase in PKCθ phosphorylation (ANOVA F(3,16) = 7.815, p = 0.002; Fig 4B). Yet U0126 treatment did not significantly alter the increased PKCθ phosphorylation in N-38 cultures stimulated with E2 compared to U0126 alone. These results suggest that estradiol-induced PKCθ activation is up-stream from ERK activation (Fig 4B).
Figure 4.
Estradiol treatment increased PKCθ phosphorylation. N-38 cultures were treated with vehicle (Veh) or 1 nM estradiol (E2) for 30 min and/or pretreated for 1 h with the (A) ER antagonist ICI 182,780 (1 μM) or the (B) signal transduction pathway inhibitors BIS (1 μM) or U0126 (1 μM). After experimental drug treatments, N-38 cells were lysed and prepared for western blot analysis. Examination of lysate proteins (A, top panel) revealed that E2 (n = 7) and ICI 182,780 (n = 7) alone induced PKCθ phosphorylation (pPKCθ), while treatment with E2 and ICI 182,780 (E2 + ICI; n = 7) prevented the increase. The inhibitor of the PKC pathway BIS prevented phosphorylation of PKCθ while U0126 (n = 3) did not prevent PKCθ phosphorylation (B, top panels). Bars (mean ± S.E.M.) represent a ratio of the normalized optical density of pPKCθ value divided by the corresponding PKCθ value X 100 (%; Tukey’s post hoc, *p < 0.05, **p < 0.01).
3.5. Activation of plasma membrane ERα increased intracellular Ca2+ levels
Estradiol, in a dose-dependent fashion, increased free intracellular concentrations of calcium ([Ca2+]i; Fig 5A) (ANOVA F(5,125) = 175.4, p < 0.05). The change in fluorescence intensity between the vehicle control (ΔF = 161 ± 8 RFU, n = 18) and estradiol treated neurons demonstrated a rapid estradiol-dependent release of [Ca2+]i (0.1 nM, ΔF = 434 ± 11 RFU, n = 23; 1000 nM, ΔF = 799 ±15 RFU, n = 25). Membrane estradiol signaling is dependent on ER transactivation of mGluRs [10]. To verify this mechanism in N-38 neurons mGluR1a was blocked prior to stimulation with estradiol using the receptor antagonist LY376,385. In this experiment, the [Ca2+]i ΔF with estradiol was 604.2 + 14.5 RFU (n=15) and estradiol with LY376,385 was 178.1 ± 26.8 RFU (n = 25; P < 0.0001, paired Student’s t-Test). These data indicate that, as in astrocytes, membrane-initiated estradiol signaling can rapidly release cytoplasmic Ca2+ stores [20].
Figure 5.
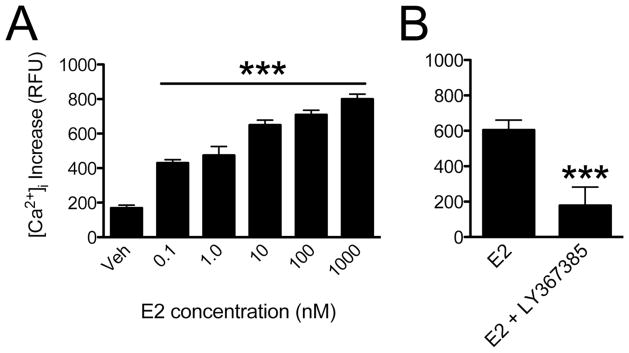
Estradiol stimulation of membrane ERα increased intracellular cytoplasmic calcium levels. N-38 cells were loaded with the calcium indicator Fluo-4 AM and rapidly pulsed with different estradiol (E2) concentrations. The change in free cytoplasmic calcium was measured by observing the change in Fluo-4 AM fluorescence intensity. (A) Analysis of images taken from vehicle (Veh) and E2 stimulated N-38 neurons (n = 18–25) revealed that intracellular calcium release increased in an E2-dependent manner. (B) The pretreatment of N-38 cultures with 50 nM LY367385 blocked the estradiol (10 nM) from increasing [Ca2+]i levels. Bars (mean ± S.E.M., n = 15) represent the relative change in Fluo-4 AM fluorescence calculated in relative fluorescence units (RFU; paired Student’s t-test, *p < 0.0001).
4. Discussion
The major findings of the present studies were that N-38 neurons respond like native cells to estradiol stimulation by initiating cell signaling and regulating ERα insertion into the plasma membrane. It has been hypothesized that activation of sexual receptivity requires estradiol action in NPY neurons of the ARH [11,28,29]. Thus N-38 neurons were selected as a model for the ARH NPY neurons. N-38 neurons were shown to have i) several ERα variants present on the cell surface of N-38 neurons, including the full-length 66 kDa and a 52 kDa [7,21,24,30]. We now show that the 52 kDa protein is coded by an ERα splice variant missing exon 4. Such splice variant mRNA was reported by Skipper et al. [31]; ii) Estradiol regulated levels of membrane ERα levels through PKC activation in N-38 neurons; iii) Estradiol increased PKCθ phosphorylation and cytoplasmic calcium levels, actions associated with membrane-initiated estradiol signaling in the hypothalamus [12,20]. These results indicate that N-38 neurons have ERα on the cell membrane and estradiol membrane signaling induces a release of intracellular stores of calcium, activates PKCθ and increases levels of ERα on the cell membrane.
Importantly, activation of PKCθ paralleled estradiol-induced ERα insertion into the cell surface, an action blocked with the PKC pathway inhibitor BIS. This same novel PKC has been implicated in regulating membrane estradiol actions that facilitate sexual receptivity [12]. PKC has been implicated in the regulation of cytosolic ERα protein levels in MCF-7 breast cancer cells [32], and in membrane ERα levels (present study). Additionally, PKC regulates the levels of other membrane receptors. For example, stimulating PKC induces a rapid delivery of N-methyl-d-aspartate (NMDA) glutamate receptors to the cell membrane via soluble NSF-associated protein SNAP-dependent exocytosis [33]. Presently, it is not clear whether the activation of plasma membrane ERα triggers exocytosis by a mechanism involving the PKC pathway. Our results do show that insertion of ERα into the plasma membrane, like the NMDA receptor, is regulated by PKC pathway activation (see Fig 3). We also examined the involvement of the ERK activation to determine whether the signaling pathway is involved in the insertion ERα into the plasma membrane. The ERK pathway has been described to be involved in several membrane-initiated estradiol actions [16,32,34,35,36] but it still remains unclear in N-38 neurons whether the ERK pathway has a role in ERα membrane trafficking. Further studies will be needed to address this concern.
Estradiol increases intracellular levels of Ca2+ in dose-dependent manner. The [Ca2+]i increase in N-38 neurons was observed in Ca2+-free media indicating that estradiol induces the release of Ca2+ ions from cytoplasmic stores. The mechanism in N-38 neurons appears similar to that defined in astrocytes, where a phospholipase C/inositol trisphosphate receptor (PLC/IP3R) linked pathway mediated the membrane-initiated estradiol elevation of [Ca2+]i [37]. This activation of intracellular cell signaling required an membrane ER-mGluR interaction; the mGluR1a antagonist LY367385 abrogated the estradiol actions that included preventing the increase in [Ca2+]i [10,21,38,39]. One idea to explain the ERα trafficking is that estradiol-stimulated calcium release mobilizes ERα-immunoreactive vesicle, which fuse to the cell membrane and deliver ERα to the cell membrane [16,40,41,42] as well as internalize receptors via endosomes [43]. Such activity-induced membrane insertion is analogous to the opioid-induced increase of membrane δ-opioid receptors (DORs) [44]. In the non-stimulated condition, DORs are primarily associated with exocytotic vesicles, rather than on the cell membrane. Activating surface DORs releases intracellular Ca2+ stores that induce exocytosis resulting in the insertion of DORs into the cell membrane. How ERα proteins becomes inserted into the membranes of vesicles is not completely known but recent studies provide evidence that the hinge (D domain) and ligand-binding (E/F domain) regions interact with lipid bilayer to form transmembrane structures [45,46].
These present studies underscore that different ERα isoforms are associated with the cell membrane, an observation previously reported in neurons, glia, and breast cancers [7,21,24,32,47]. In addition to the full-length ERα, these proteins appear to be alternative splicing products of the ERα gene [48,49,50,51,52]. In the ERα knockout (ERKO) mouse, neither the 52 nor the 66 kDa ERα were expressed [21]. Since two ERα splice variant mRNA, ERαΔ4 and ERαΔ7, code for proteins with a similar predicted size, we examined mRNA from native and N-38 neurons. The ERαΔ7 is missing the 7th exon and because of an internal stop codon, the predicted protein is missing the E/F domain, which constitutes the COOH terminal of the full-length ERα [51]. The ERαΔ4 is missing exon 4, which compromises the hinge region, part of the ligand-binding domain and the nuclear localization sequence. In the present study, primers targeting exon 3-5 and 5-8 demonstrated the presence of ERαΔ4 but not ERαΔ7 mRNA in N-38 neurons. We had similar results in cultured primary hypothalamic neurons (P. Dewing, personal communication). Thus, we concluded that ERαΔ4 protein is the same 52 kDa ERα-immunoreactive that is found in primary cultures of hypothalamic neurons [7,53] and astrocytes [21].
Various studies indicate that ERαΔ4 is the most prominent splice variant, brain while others suggest that it is the ERαΔ7 mRNA [25,31]. Our studies support ERαΔ4, which contains the E domain, a portion of the receptor that is critical for membrane localization and is missing in the ERαΔ7 protein [54,55,56]. Moreover, antibodies directed at the amino and carboxy terminals recognize the 52 kDa protein suggesting that the carboxy terminal is intact [21,57]. While other ERα variants (e.g., ER46) have been reported to be biologically active and are known to be involved in regulating estradiol action in the vascular and skeletal systems [58,59], the role of ERαΔ4 in membrane-initiated estradiol signaling is still not clear [27,49]. Future experiments will be necessary to test the function of the 52 kDa ERαΔ4.
While unexpected, the “full ER antagonist” ICI 182,780 elicited cell signaling and receptor trafficking when given in the absence of exogenous estradiol. While unexpected agonist actions have been reported previously in neurons and non-neuronal cells [60,61,62]. However, when N-38 neurons were sequentially treated with ICI 182,780 and estradiol, both effects were attenuated. One possibility is that ICI 182,780 is activating another ER or that the antagonist structurally alters ERα protein to influence signaling [63]. In hippocampal neurons, it has been suggested that GPR30 (also called GPER) is the ER being activated [62]. It is not known whether N-38 neurons express GPR30, but in native hypothalamic neurons and astrocytes surface biotinylation does not reveal GRP30, suggesting that GPR30 is not present in cell membranes of hypothalamic neurons or astrocytes [21,24,39]. Alternatively, ICI 182,780 has been reported to be an ER-X agonist [64], but we could not identify ER-X in N-38 neurons.
In summary, we demonstrated that in N-38 neurons, the insertion of full-length ERα and the ERαΔ4 splice variant into the cell membrane is dependent upon membrane ER activation of cell signaling pathways that include PKC and elevation of [Ca2+]i. This data suggest the membrane-initiated estradiol mechanism present in N-38 neurons is similar to the mechanism present in different neuronal populations in the central nervous systems and that N-38 neurons provide a workable model system with which to examine membrane-initiated estradiol signaling and receptor trafficking at the cellular level. We hypothesize that insertion into the cell membrane involves fusing of ERα-containing vesicles under the control of estradiol activated PKC activation and increase in cytoplasmic calcium levels.
Highlights.
Different ERα splice variants are found on the cell surface of N-38 neurons.
Estradiol increases membrane ERα levels by activation of PKC in N-38 neurons.
Estradiol rapidly increases PKCθ phosphorylation and intracellular calcium.
Acknowledgments
Supported by DA013185 (PM), by NS079064 (RD), and the Reproductive Scientist Development Program (JK). We thank Ms. Amy Christensen for her assistance with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 4.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 5.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin ER. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1425–1430. doi: 10.1152/ajpregu.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30:12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiology of aging. 2007;28:1670–1681. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Mermelstein PG, Micevych PE. Nervous system physiology regulated by membrane estrogen receptors. Rev Neurosci. 2008;19:413–424. doi: 10.1515/revneuro.2008.19.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, et al. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbati C, Pierdominici M, Gambardella L, Malchiodi Albedi F, Karas RH, et al. Cell Surface Estrogen Receptor Alpha Is Upregulated during Subchronic Metabolic Stress and Inhibits Neuronal Cell Degeneration. PLoS One. 2012;7:e42339. doi: 10.1371/journal.pone.0042339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F. Palmitoylation regulates 17beta-estradiol-induced estrogen receptor-alpha degradation and transcriptional activity. Molecular endocrinology. 2012;26:762–774. doi: 10.1210/me.2011-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez S, Sosa L, Petiti JP, Mukdsi JH, Mascanfroni ID, et al. 17beta-Estradiol stimulates the translocation of endogenous estrogen receptor alpha at the plasma membrane of normal anterior pituitary cells. Molecular and cellular endocrinology. 2012;355:169–179. doi: 10.1016/j.mce.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Watson CS, Jeng YJ, Hu G, Wozniak A, Bulayeva N, et al. Estrogen- and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-alpha interactions with G protein-alphai and caveolin I. Steroids. 2012;77:424–432. doi: 10.1016/j.steroids.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, et al. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. doi: 10.1210/en.2003-0946. [DOI] [PubMed] [Google Scholar]
- 19.Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- 20.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, et al. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proc Natl Acad Sci U S A. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taguchi Y, Koslowski M, Bodenner DL. Binding of estrogen receptor with estrogen conjugated to bovine serum albumin (BSA) Nucl Recept. 2004;2:5. doi: 10.1186/1478-1336-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Ishunina TA, Swaab DF. Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol. 2008;24:93–98. doi: 10.1080/09513590701705148. [DOI] [PubMed] [Google Scholar]
- 26.Fuqua SA, Fitzgerald SD, Allred DC, Elledge RM, Nawaz Z, et al. Inhibition of estrogen receptor action by a naturally occurring variant in human breast tumors. Cancer Res. 1992;52:483–486. [PubMed] [Google Scholar]
- 27.Park W, Choi JJ, Hwang ES, Lee JH. Identification of a variant estrogen receptor lacking exon 4 and its coexpression with wild-type estrogen receptor in ovarian carcinomas. Clin Cancer Res. 1996;2:2029–2035. [PubMed] [Google Scholar]
- 28.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micevych PE, Dewing P. Membrane-initiated estradiol signaling regulating sexual receptivity. Frontiers in endocrinology. 2011;2:26. doi: 10.3389/fendo.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153:3872–3877. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, et al. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zivadinovic D, Watson CS. Membrane estrogen receptor-alpha levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2005;7:R130–144. doi: 10.1186/bcr959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 34.Minano A, Xifro X, Perez V, Barneda-Zahonero B, Saura CA, et al. Estradiol facilitates neurite maintenance by a Src/Ras/ERK signalling pathway. Molecular and cellular neurosciences. 2008;39:143–151. doi: 10.1016/j.mcn.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain research. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 37.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 38.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 42.Sosa L, Gutierrez S, Petiti JP, Palmeri CM, Mascanfroni ID, et al. 17beta-Estradiol modulates the prolactin secretion induced by TRH through membrane estrogen receptors via PI3K/Akt in female rat anterior pituitary cell culture. American journal of physiology Endocrinology and metabolism. 2012;302:E1189–1197. doi: 10.1152/ajpendo.00408.2011. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- 45.Byrne C, Khemtemourian L, Pelekanou V, Kampa M, Leclercq G, et al. ERalpha17p, a peptide reproducing the hinge region of the estrogen receptor alpha associates to biological membranes: A biophysical approach. Steroids. 2012;77:979–987. doi: 10.1016/j.steroids.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Kim KH, Toomre D, Bender JR. Splice isoform estrogen receptors as integral transmembrane proteins. Molecular biology of the cell. 2011;22:4415–4423. doi: 10.1091/mbc.E11-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shupnik MA. Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic-pituitary axis. J Neuroendocrinol. 2002;14:85–94. doi: 10.1046/j.0007-1331.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- 48.Shupnik MA, Pitt LK, Soh AY, Anderson A, Lopes MB, et al. Selective expression of estrogen receptor alpha and beta isoforms in human pituitary tumors. J Clin Endocrinol Metab. 1998;83:3965–3972. doi: 10.1210/jcem.83.11.5236. [DOI] [PubMed] [Google Scholar]
- 49.Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol. 2000;14:634–649. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- 50.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 51.Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, et al. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 52.Ishunina TA, Swaab DF. Hippocampal estrogen receptor-alpha splice variant TADDI in the human brain in aging and Alzheimer’s disease. Neuroendocrinology. 2009;89:187–199. doi: 10.1159/000158573. [DOI] [PubMed] [Google Scholar]
- 53.Gorosito SV, Cambiasso MJ. Axogenic effect of estrogen in male rat hypothalamic neurons involves Ca(2+), protein kinase C, and extracellular signal-regulated kinase signaling. J Neurosci Res. 2008;86:145–157. doi: 10.1002/jnr.21466. [DOI] [PubMed] [Google Scholar]
- 54.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 55.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, et al. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, et al. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denger S, Reid G, Kos M, Flouriot G, Parsch D, et al. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001;15:2064–2077. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- 59.Longo M, Brama M, Marino M, Bernardini S, Korach KS, et al. Interaction of estrogen receptor alpha with protein kinase C alpha and c-Src in osteoblasts during differentiation. Bone. 2004;34:100–111. doi: 10.1016/j.bone.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Dudley MW, Sheeler CQ, Wang H, Khan S. Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action. Proc Natl Acad Sci U S A. 2000;97:3696–3701. doi: 10.1073/pnas.040558197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson JA, Zhang Y, Ing NH. ICI 182,780 acts as a partial agonist and antagonist of estradiol effects in specific cells of the sheep uterus. J Steroid Biochem Mol Biol. 2001;77:281–287. doi: 10.1016/s0960-0760(01)00061-9. [DOI] [PubMed] [Google Scholar]
- 62.Zhao L, O’Neill K, Brinton RD. Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J Pharmacol Exp Ther. 2006;319:1124–1132. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 63.Lupien M, Jeyakumar M, Hebert E, Hilmi K, Cotnoir-White D, et al. Raloxifene and ICI182,780 increase estrogen receptor-alpha association with a nuclear compartment via overlapping sets of hydrophobic amino acids in activation function 2 helix 12. Mol Endocrinol. 2007;21:797–816. doi: 10.1210/me.2006-0074. [DOI] [PubMed] [Google Scholar]
- 64.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]