Abstract
Background
The C. elegans genome has been extensively annotated by the WormBase consortium that uses state of the art bioinformatics pipelines, functional genomics and manual curation approaches. As a result, the identification of novel genes in silico in this model organism is becoming more challenging requiring new approaches. The Oligonucleotide-oligosaccharide binding (OB) fold is a highly divergent protein family, in which protein sequences, in spite of having the same fold, share very little sequence identity (5–25%). Therefore, evidence from sequence-based annotation may not be sufficient to identify all the members of this family. In C. elegans, the number of OB-fold proteins reported is remarkably low (n = 46) compared to other evolutionary-related eukaryotes, such as yeast S. cerevisiae (n = 344) or fruit fly D. melanogaster (n = 84). Gene loss during evolution or differences in the level of annotation for this protein family, may explain these discrepancies.
Methodology/Principal Findings
This study examines the possibility that novel OB-fold coding genes exist in the worm. We developed a bioinformatics approach that uses the most sensitive sequence-sequence, sequence-profile and profile-profile similarity search methods followed by 3D-structure prediction as a filtering step to eliminate false positive candidate sequences. We have predicted 18 coding genes containing the OB-fold that have remarkably partially been characterized in C. elegans.
Conclusions/Significance
This study raises the possibility that the annotation of highly divergent protein fold families can be improved in C. elegans. Similar strategies could be implemented for large scale analysis by the WormBase consortium when novel versions of the genome sequence of C. elegans, or other evolutionary related species are being released. This approach is of general interest to the scientific community since it can be used to annotate any genome.
Introduction
Bioinformatics analysis of the complete genome sequence of C. elegans by the WormBase consortium initially revealed over 19000 coding genes [1]. When the genome of the closely related species C. briggsae was sequenced and a comparative analysis was performed between the two species, 6% more coding genes were predicted (20261) [2]. Since the bioinformatics annotation pipeline from the WormBase consortium is constantly evolving new protein-coding genes are being predicted and this number is increasing. The latest version of the C. elegans genome sequence (WS228) predicts 24610 coding genes. [3] Considering that twice the number of new genes has been predicted using gene prediction algorithms, novel approaches that explore different search spaces may reveal even more protein-coding genes.
Indeed, evidence suggests that more protein may exist in C. elegans in the case of old protein fold families that evolved a long time ago from divergent (or convergent) evolution [4]. Such protein family members are renowned to be difficult to identify by conventional sequence alignment software since they share very little sequence identity. The OB-fold is one example [5]. The domain is a compact structural motif frequently used for nucleic acid recognition. It is composed of a five-stranded beta-sheet forming a closed beta-barrel. This barrel is capped by an alpha-helix located between the third and fourth strands. Structural comparison and analysis of all OB-fold/nucleic acid complexes solved to date confirms the low degree of sequence similarity among members of this family arising from divergent evolution [6]. In addition, loops connecting the secondary-structure elements are highly variable in length making them difficult to compare at the sequence level. In C. elegans the number of predicted proteins containing OB-fold is remarkably low compared to other related organisms by evolution. The number of OB-fold proteins when we started this project, varied widely from 256 (human), 246 (mouse), 344 (yeast - Saccharomyces cerevisiae) to 84 (fruit fly - Drosophila melanogaster) and 46 (C. elegans). Gene loss or expansion between these different related organisms may have occurred or differences in the level of annotation for this protein family may explain these numerical discrepancies.
The identification of distant related sequences or remote homologues from functional domain families has been extensively improved this past decade. Sequence-sequence and sequence-profile alignment algorithms [7], [8], BLAST [9] and PSI-BLAST [10] have been widely adopted for this purpose. Methods that can detect intermediate sequence to connect sequences sharing insignificant BLAST scores between each other have been implemented [7], [8]. The sensitivity and alignment quality depend on the information that is used to compare proteins. The most sensitive methods use sequence-profiles or profile-profile alignments (Table 1, Sequence Discovery Module). They contain position-specific substitution scores that are computed from the frequencies of amino acids at each position of a multiple alignment of related sequences. Further improvements have been feasible by the introduction of Hidden Markov Models [11], [12] that can compute more accurately gap, insertion and deletion in the alignments compared to previous methods. Moreover, fold recognition methods that build a 3D-structural model of a protein sequence from a sequence alignment have been very efficient in their ability to align correctly sequence/profile to profile of known structures (Table 1, Structure Discovery Module). Building models that are very similar structurally to the templates structure from these alignments can be used to validate a correct alignment, especially if such alignment is between sequences that have very low sequence similarities. More recently, many bioinformatics studies suggest that consensus methods that pool together the results of different software that perform similar tasks perform better than isolated methods.
Table 1. Tools used in this study.
| Tools | Description | Reference |
| Sequence Discovery Module | ||
| PSI-BLAST | Position-Specific Iterative Basic Local Alignment Search Tool | Altschul et al. 1990 [9] |
| MEME | Motif based Hidden Markov Model of protein families | Grundy et al. 1997 [15] |
| HMMER | Bio-sequence analysis tool using profile hidden Markov Models | Eddy, 1996, 1998 [11], [12] |
| HHpred | Homology detection & structure prediction tool by HMM-HMM comparison | Soding et al. 2005 [29] |
| COMPASS | Alignment tool of multiple protein sequence profiles | Sadreyev et al. 2007 [30] |
| HHsenser | Exhaustive intermediate profile search tool using HMM-HMM comparison | Soding et al. 2006 [23] |
| Saturated-BLAST | Automated toolbox that implement the multiple intermediate sequence search method | Li et al. 2000 [7] |
| Structure Discovery Module | ||
| MetaServer | A Server that submit and collect fold recognition results from different methods andmakes 3D-prediction using a consensus approach called 3D-jury. | Bujnicki et al. 2001 [24] |
| I-Tasser | Protein 3D-structure prediction server that uses threading methods | Roy et al. 2010 [27] |
| Modeller | Protein 3D-structure modeling tool from target-template sequence alignment based on satisfaction of spatial restraints | Fiser et al. 2003 [26] |
| TM-Align | Protein 3D-structure alignment algorithm that compute the TM-Score | Zhang et al. 2005 [28] |
| Functional Discovery Module | ||
| BioGrid | Database of Protein and Genetic Interactions | Stark et al. 2006 [22] |
| STRING | Database of Functional protein association networks | Snel et al. 2000 [21] |
| Worm Interactome | A high quality yeast two-hybrid protein-protein interactions database of C. elegans | Li et al. 2004 [31] |
| WoLF PSORT | Protein sub-cellular localization predictor | Horton et al. 2007 [32] |
| Kihara PFP | Protein function predictor | Hawkins et al. 2006 [33] |
This study examines the possibility that novel OB-fold coding genes exist in the worm. We developed a consensus approach that uses the most sensitive sequence-sequence, sequence-profile and profile-profile similarity search methods followed by OB-fold 3D-structure prediction as a filter to eliminate false positive candidate remote sequences. We have predicted 18 coding gene containing the OB-fold. Remarkably, most of their corresponding genes have not been or have only been partially characterized in the worm. As expected, many of them are essential genes since their knockout produces lethal phenotypes. And it is well known that OB-fold containing proteins are frequently involved in essential nucleic-acids metabolism, such as Replication Protein A [13], tRNA synthetases [14].
Results
Using the profiles generated by MEME [15] and PSI-BLAST [10] from the 46 proteins sequences annotated as OB-fold in the C. elegans genome we obtained an additional 200 candidate proteins that may contain OB-fold (see methods). We attempted to validate these with structural alignment programs such as MetaServer, I-Tasser, Modeller and TM-align, but only two (brc-2 and pot-1) were predicted to be good structural maps to the OB-fold by any of these methods. This finding was not far from our expectation since many OB-fold family members share less than 10% sequence similarity between each other, which is consistent with the high degree of sequence divergence of this family that occurred during evolution. Therefore, even though very sensitive sequence alignment methods are used, detection of novel OB-fold proteins remained difficult.
Since very divergent sequences that do not share significant sequence identity may have the same fold, and considering the conserved structure of OB-fold, we used fold recognition methods of StrucDiM to investigate if more OB-fold proteins could be obtained directly. The underlying assumption was that if a correct model can be built by comparative modeling using a sequence alignment between a protein sequence of an OB-fold of known structure with an OB-fold candidate sequence, then the sequence alignment is significant. It allows us to put some confidence in the pairwise alignment of sequences that share a level of sequence identity below the twilight zone (18–25% identity) [16], [17], [18] since sequence alignment statistics cannot determine their significance at this level of identity. Effectively, incorrect alignments do not generate well-folded homology models. Since the C. elegans genome encodes greater than 20000 genes and many of these genes products would not be of interest, we decided to use a dataset likely to be enriched in genes containing OB-fold 3D-structure. For this purpose, we selected the 4300 genes identified by Claycomb et al. [19] that are expressed in the germline of C. elegans. We expected this dataset to be enriched in genes involved in DNA processes, including DNA repair and replication, which may contain protein coding genes with OB-fold 3D-structure and also exclude gene involved in terminal differentiation of tissues such as muscle, nerve, gut or organ that may not be relevant to this study. Each sequence was submitted directly to 3D-structure prediction using StrucDiM.
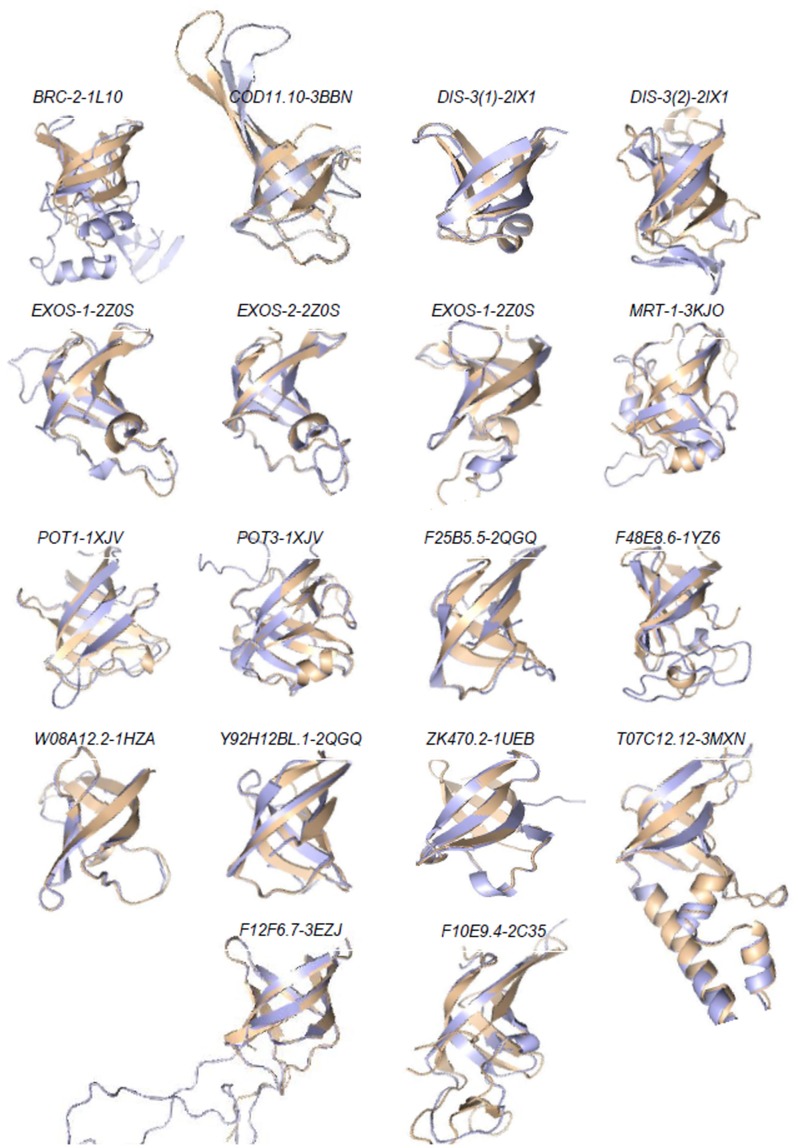
By this direct approach, we determined that 35 out of 46 previously annotated OB-fold proteins in the entire genome of C. elegans were present in the 4300 germline expressed genes set [19]. Thus, the dataset is clearly enriched in OB-fold sequences (about three fold). It also showed that the StrucDiM approach was valid and could be used to further identify novel OB-fold protein coding genes (Figure 1). Indeed, in addition to the 46 already annotated and known OB-fold proteins we identified 14 novel OB-fold candidate proteins OB-fold (Table 2). However, it should be noted that one of the member of this list, the OB-fold 3D-structure of the human homologue pot-1, has been recently deposited in the Protein Data Bank (PDB accession number: 1XJV). These results show that our approach is highly sensitive to predict novel OB-fold protein candidates. Further, structural and functional studies are needed to assess the specificity of these OB-fold prediction results.
Figure 1. Superimposition of the novel OB-fold 3D-model with their templates.
(Light blue): Predicted 3D-models, (Wheat) PDB template. (.XXXX.)-nxxx name correspond to the protein name followed by the PDB code of the template.
Table 2. Model quality of novel OB-fold protein coding genes.
| OB-fold Candidates target | Template | RMSD | TM-score | Equivalent Calpha superimposed |
| F12F6.7 | 3E0J | 0.9 | 0.91618 | 104/110 |
| F25B5.5 | 2QGQ | 1.08 | 0.79684 | 57/64 |
| exos-2 | 2Z0S | 0.39 | 0.91855 | 80/86 |
| exos-3 | 2Z0S | 1.33 | 0.93357 | 66/66 |
| exos-1 | 2Z0S | 0.97 | 0.83856 | 76/85 |
| dis-3 (First OB-fold) | 2IX1 | 2.15 | 0.77503 | 81/92 |
| dis-3 (Second OB-fold) | 1UEB | 3.66 | 0.51393 | 76/98 |
| ZK470.2 | 3BBN | 1.22 | 0.90075 | 43/45 |
| C05D11.10 | 1HZA | 1.88 | 0.8186 | 77/82 |
| W08A12.2 | 2C35 | 1.27 | 0.91183 | 58/59 |
| F10E9.4 | 1XJV | 1.98 | 0.81487 | 61/61 |
| Pot-1 | 1L1O | 1.11 | 0.89915 | 128/135 |
| brc-2 | 1XJV | 3.43 | 0.43998 | 74/115 |
| Pot-3 | 3MXN | 1 | 0.83903 | 115/133 |
| T07C12.12 | 1XJV | 1.49 | 0.90455 | 132/139 |
| Pot-2 | 3KJO | 0.4 | 0.86529 | 110/126 |
| mrt-1 | 2QGQ | 1.03 | 0.83313 | 115/135 |
| Y92H12BL.2 | 1YZ6 | 0.62 | 0.89597 | 56/60 |
| F48E8.6 | 3E0J | 2.27 | 0.64349 | 66/81 |
To further identify additional OB-fold gene coding proteins we searched for orthologues and homologues of the identified candidates in both human and C. elegans. Using the protein family orthologues, and paralogues module in the comparative genomics toolbox of ENSEMBL database we were able to identify 3 additional candidate homologues of pot-3 (pot-2, mrt-1, F48E8.6) and one homologues of F25B5.5 (Y92H12BL.2). We expected to see that these proteins also have OB-fold similar to their paralogues. In addition, we then used structDiM to verify the predicted OB-fold structure of these proteins. As expected, all candidates were confirmed to contain OB-fold. These 4 novel OB-fold proteins had not been previously predicted and annotated in the WormBase, however, for 2 of them (mrt-1 and pot-2) we found one publication mentioning that these two genes contained an OB-fold domain [20].
Discussion
One important question regarding this study is why the annotation of these genes had been missed from the WormBase database (www.WormBase.org). The obvious lack of sequence similarity among members of this family is one possible explanation since it makes these proteins undetectable through sequence based searches. This is consistent with our inability to identify novel OB-fold protein coding genes using the SeqDiM module. On the contrary, we have showed that structural based methods are more robust at predicting OB-fold proteins. Since these methods are generally not considered in genome annotation pipelines, this may explain why many of these OB-fold containing genes have not been annotated.
Regarding the genes that have been identified, it is remarkable that most of them have not been well studied (Table 3). However, a significant fraction of their gene products perform important function during development and are essential genes since RNAi phenotype (EXOS-3) as well as knockout when available shows embryonic lethality. Those with embryonic lethality include protein coding genes involved in DNA replication and repair (F12F6.7, BRC-2) and growth rate and reproduction (EXOS-1, C05D11.10, F10E9.4) as well as the protection of telomere protein POT-3 involved in telomere maintenance. Other OB-fold candidate proteins do not seem to be essential during development since they only show no or non-lethal phenotypes. Those include gene coding proteins involved in nucleic acids and RNA binding (EXOS-2) a component of the exosome complex (with EXOS-1 and EXOS-3), DIS-3, ZK470.2, W08A12.2, T07C12.12, F25B5.5 as well as POT-1 involved in telomere maintenance. To annotate further the function of these genes, we looked at protein-protein interaction in the STRING [21] and BIOGRID [22] databases. No interactions were found for most of them with the exception of EXOS-3, C05D11.10, POT-1, and BRC-2. These interact respectively with genes products involved in cell division, nucleic-acid binding/RNA processing, IGF signaling/life span extension/longevity for POT-1 and DNA repair for BRC-2.
Table 3. Functional analysis of Novel OB folds protein coding genes.
| OB folds | WB ID | Biblio | RNAi Phenotype | Knockout | Function | Homologues Paralogues | Paralogs |
| F12F6.7 | WBGene00008722 | NA | Embryonic lethal | ok2252 | DNA replication, DNA binding,DNA-directed DNA polymerase activity | POLD2 polymerase (DNA directed),delta 2, regulatory subunit 50kDa | NA |
| F25B5.5 | WBGene00017776 | NA | NA | NA | RNA modification, iron-sulfur cluster binding,4 iron, 4 sulfur cluster binding, catalytic activity | CDK5RAP1 CDK5 regulatory subunitassociated protein 1 | Y92H12BL.2 |
| exos-2 | WBGene00022232 | [34] | late larval arrest | NA | *nucleic acid binding, RNA binding, | EXOSC2 exosome component 2 | NA |
| exos-3 | WBGene00010325 | [34]–[36] | Embryonic lethal | NA | growth,nematode larval development, receptor-mediated endocytosis | EXOSC3 exosome component 3 | NA |
| exos-1 | WBGene00012966 | [34] | Embryonic lethal, lethal | ok807 | positive regulation of growth rate,reproduction | EXOSC1 exosome component 1 | NA |
| dis-3 | WBGene00001001 | [37]–[39] | Slow growth, sick, sterile progeny | ok357 | RNA binding, ribonuclease activity, sequence-specific DNA binding, reproduction | DIS3 mitotic control homolog(S. cerevisiae) | F48E8.6 |
| ZK470.2 | WBGene00022745 | [40] | NA | ok5876 | *single-stranded telomeric DNA binding, ion binding, monosaccharide metabolism | NA | NA |
| C05D11.10 | WBGene00015487 | NA | Embryonic lethal, lethal, slow growth | ok5298 | growth, nematode larval development,positive regulation of growth rate, reproduction | NA | NA |
| W08A12.2 | WBGene00021079 | NA | NA | NA | *purine nucleotide binding, adenyl nucleotide binding, cellular macromolecule metabolism | NA | NA |
| F10E9.4 | WBGene00017356 | NA | Slow growth, larval lethal | NA | growth, nematode larval development, positive regulation of growth rate, reproduction | NA | NA |
| Pot-1 | WBGene00015105 | [20], [41]–[42] | organism development variant, telomere homeostasis variant | NA | *cAMP-dependent protein kinase activity, transition metal ion binding, ion binding | Pot1 Protection Of Telomeres 1 | NA |
| brc-2 | WBGene00020316 | [43]–[55] | Embryonic lethal, lethal,embryonic arrest | ok1629 | strand invasion, double-strand break repair, reproduction, single-stranded DNA and protein binding | Brca1 Breast Cancer type 1 susceptibility protein | NA |
| Pot-3 | WBGene00007065 | [22], [44] | lethal | ok1530 | *cation binding, adenyl nucleotide binding, heterocycle metabolism | Pot1 Protection Of Telomeres 1 | pot-2, mrt-1 |
| T07C12.12 | WBGene00011576 | [56] | Embryonic lethal | NA | *adenyl nucleotide binding,rRNA (adenine) methyltransferase activity, purine nucleotide binding | RMI1, RecQ mediated genomeinstability 1 | NA |
| Pot-2 | WBGene00010195 | [20], [42] | NA | NA | *cation binding, adenyl nucleotide binding, heterocycle metabolism | NA | pot-3, mrt-1 |
| MRT-1 | WBGene00045237 | [20,42,56–59] | Sterile, lethal | oK758 | Nuclear excision repair, telomere maintenancevia telomerase, reproduction, Singlestranded DNA binding | NA | pot-2, pot-3 |
| Y92H12BL.2 | WBGene00022363 | NA | NA | NA | Iron-sulfur cluter binding | CDKAL1, CDK5 regulatory subunitassociated protein 1-like 1 | F25B5.5 |
| F48E8.6 | WBGene00018612 | NA | NA | NA | RNA binding, ribonuclease activity | DIS3L2, DIS3 mitotic control homolog(S. cerevisiae)-like 2 | dis-3 |
refers to predicted functions. Homologues and paralogues referred to human.
We have shown that comparative modelling approaches are powerful tools to identify novel protein coding genes with interesting and uncharacterized functions even in a genome and proteome of a model organism as extensively annotated as C. elegans. Such approach is of general interest to the scientific community since it can be applied to any genome.
Materials and Methods
Input Sequences
Protein sequences used in this study to identify novel OB-fold proteins were obtained from the 46 OB-fold known proteins in WormBase and an enriched data set of 4300 expressed genes in the germ line of C.elegans [19]. This dataset should be enriched in novel genes containing OB-fold since OB-fold proteins are generally involved in many DNA transaction and repair processes highly actives in the C. elegans germline.
Consensus Discovery Pipeline
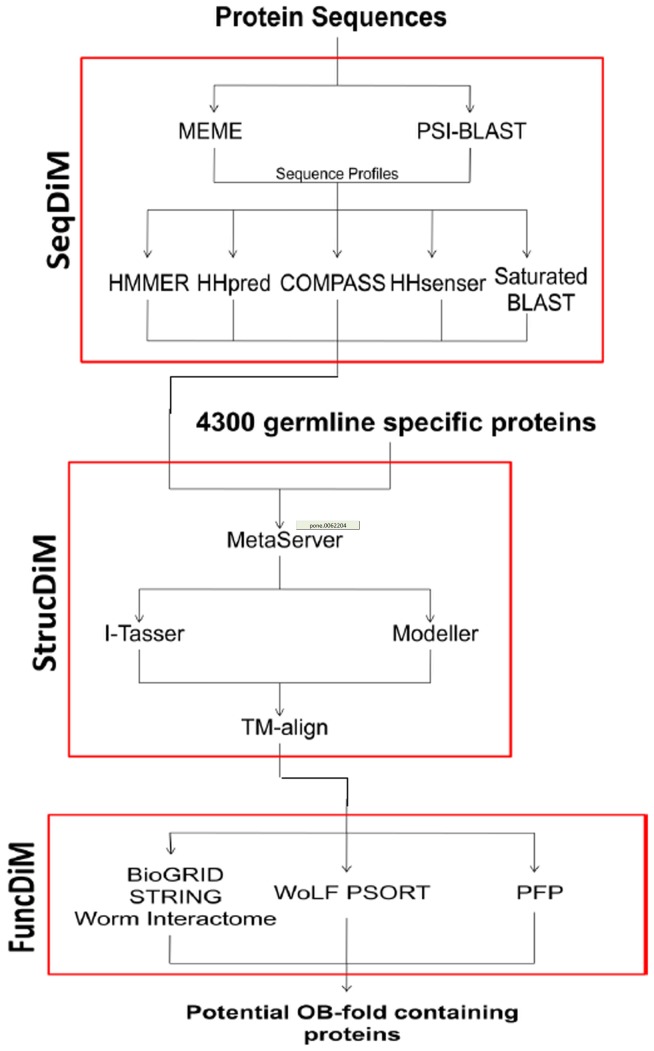
The pipeline (Figure 2, Table 1) has 3 modules (i) Sequence based Discovery Module (ii) Structure based Discovery Module and filtering (iii) Functional Discovery Module:
Figure 2. Discovery Pipeline of novel OB fold protein coding genes.
It contains 3 Discovery Modules. SeqDIM: Sequence alignment DIscovery Module; StrucDIM:3D Structure prediction Discovery Module; and a Functional prediction Discovery Module FuncDIM.
Sequence based Discovery Module
From the 46 OB-fold known proteins sequences in C. elegans a position-specific scoring matrix of OB-fold motifs was built using PSI-BLAST [10] as well as a Hidden Markov Model using MEME [11], [12], [15] Each of the profiles were subsequently submitted to different database scanning software using sequence-profile based alignment methods against the wormpep210 protein sequence database. For the HHSenser profile-profile methods [23] the database was made-up of sequence profiles of all the known protein families. For each method the default threshold of significance were used to select for novel candidate OB-fold protein sequences (see Text S1, Figure S1 and S2, Table S1 and S2).
Structural Discovery Module
The 4300 sequences from claycomb et al. [19] as well as the 200 sequence OB-fold candidates obtained from SeqDiM were submitted to the consensus fold recognition metaserver [24] to perform and confirm fold prediction. This method collects and scores many different fold prediction results using the 3D jury consensus method from a protein sequence [25]. Model building for the predicted OB-fold motif in candidate genes were further performed by the modeller algorithm [26] from meta-server sequence alignment results as well as re-submitting candidate sequences to the 3D-structure prediction server I-tasser [27]. Model quality and validation were further performed using TM-align [28]. A TM-score <0.2 indicated that there was no similarity between two structures; a TM-score >0.5 meant that the structures shared the same fold (Text S1, Figure S3).
Functional Discovery Module
To gain insight into the function of the novel OB-fold candidates discovered, protein-protein interaction databases, subcellular localization and gene ontology predictors were interrogated (Table 1, Function Discovery Module).
Supporting Information
Generation of PSI-BLAST profiles using the 46 C. elegans OB fold protein sequences.
(TIF)
Profile based search to identify novel OB fold protein sequences.
(TIF)
Direct fold recognition prediction to identify novel OB fold protein.
(TIF)
Parameters explored for profile generation using PSI-BLAST
(DOCX)
Parameters of sequence similarity search tools used on step 4. Mostly default parameters were used otherwise specified.
(JPG)
Supporting methods.
(DOC)
Funding Statement
No current external funding sources for this study.
References
- 1. C. elegans Sequencing Consortium (1998) Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 282(5396): 2012–2018. [DOI] [PubMed] [Google Scholar]
- 2. Stein LD, Bao Z, Blasiar D, Blumenthal T, Brent MR, et al. (2003) The genome sequence of caenorhabditis briggsae: A platform for comparative genomics. PLoS Biol 1(2): E45 10.1371/journal.pbio.0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magrane M, Consortium U (2011) UniProt knowledgebase: A hub of integrated protein data. Database (Oxford) 2011: bar009 10.1093/database/bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murzin AG (1998) How far divergent evolution goes in proteins. Curr Opin Struct Biol 8(3): 380–387. [DOI] [PubMed] [Google Scholar]
- 5. Murzin AG (1993) OB(oligonucleotide/oligosaccharide binding)-fold: Common structural and functional solution for non-homologous sequences. EMBO J 12(3): 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Theobald DL, Cervantes RB, Lundblad V, Wuttke DS (2003) Homology among telomeric end-protection proteins. Structure 11(9): 1049–1050. [DOI] [PubMed] [Google Scholar]
- 7. Li W, Pio F, Pawlowski K, Godzik A (2000) Saturated BLAST: An automated multiple intermediate sequence search used to detect distant homology. Bioinformatics 16(12): 1105–1110. [DOI] [PubMed] [Google Scholar]
- 8. Soding J, Remmert M (2011) Protein sequence comparison and fold recognition: Progress and good-practice benchmarking. Curr Opin Struct Biol 21(3): 404–411 10.1016/j.sbi.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3): 403–410 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 10. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25(17): 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eddy SR (1996) Hidden markov models. Curr Opin Struct Biol 6(3): 361–365. [DOI] [PubMed] [Google Scholar]
- 12. Eddy SR (1998) Profile hidden markov models. Bioinformatics 14(9): 755–763. [DOI] [PubMed] [Google Scholar]
- 13. McJunkin K, Mazurek A, Premsrirut PK, Zuber J, Dow LE, et al. (2011) Reversible suppression of an essential gene in adult mice using transgenic RNA interference. Proc Natl Acad Sci U S A. 108(17): 7113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, et al. (2003) Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 100(8): 4678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grundy WN, Bailey TL, Elkan CP, Baker ME (1997) Meta-MEME: Motif-based hidden markov models of protein families. Comput Appl Biosci 13(4): 397–406. [DOI] [PubMed] [Google Scholar]
- 16.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al.. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25(17): 3389–402. Review. PubMed PMID: 9254694; PubMed Central PMCID: PMC146917. [DOI] [PMC free article] [PubMed]
- 17.Rost B (1999) Twilight zone of protein sequence alignments. Protein Eng. 12(2): 85–94. PubMed PMID: 10195279. [DOI] [PubMed]
- 18.Doolittle RF (1981) Similar amino acid sequences: chance or common ancestry. Science, 214, 149–159. [DOI] [PubMed]
- 19. Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, et al. (2009) The argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell 139(1): 123–134 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meier B, Barber LJ, Liu Y, Shtessel L, Boulton SJ, et al. (2009) The MRT-1 nuclease is required for DNA crosslink repair and telomerase activity in vivo in caenorhabditis elegans. EMBO J 28(22): 3549–3563 10.1038/emboj.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snel B, Lehmann G, Bork P, Huynen MA (2000) STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res 28(18): 3442–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stark C, Breitkreutz BJ, Reguly T, Boucher L, Breitkreutz A, et al.. (2006) BioGRID: A general repository for interaction datasets. Nucleic Acids Res 34(Database issue): D535–9. 10.1093/nar/gkj109. [DOI] [PMC free article] [PubMed]
- 23.Soding J, Remmert M, Biegert A, Lupas AN (2006) HHsenser: Exhaustive transitive profile search using HMM-HMM comparison. Nucleic Acids Res 34(Web Server issue): W374–8. 10.1093/nar/gkl195. [DOI] [PMC free article] [PubMed]
- 24. Bujnicki JM, Elofsson A, Fischer D, Rychlewski L (2001) Structure prediction meta server. Bioinformatics 17(8): 750–751. [DOI] [PubMed] [Google Scholar]
- 25. Ginalski K, Elofsson A, Fischer D, Rychlewski L (2003) 3D-jury: A simple approach to improve protein structure predictions. Bioinformatics 19(8): 1015–1018. [DOI] [PubMed] [Google Scholar]
- 26. Fiser A, Sali A (2003) Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol 374: 461–491 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- 27. Roy A, Kucukural A, Zhang Y (2010) I-TASSER: A unified platform for automated protein structure and function prediction. Nat Protoc 5(4): 725–738 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Skolnick J (2005) TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res 33(7): 2302–2309 10.1093/nar/gki524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soding J, Biegert A, Lupas AN (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33(Web Server issue): W244–8. 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed]
- 30.Sadreyev RI, Tang M, Kim BH, Grishin NV (2007) COMPASS server for remote homology inference. Nucleic Acids Res 35(Web Server issue): W653–8. 10.1093/nar/gkm293. [DOI] [PMC free article] [PubMed]
- 31. Li S, Armstrong CM, Bertin N, Ge H, Milstein S, et al. (2004) A map of the interactome network of the metazoan C. elegans . Science 303(5657): 540–543 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, et al.. (2007) WoLF PSORT: Protein localization predictor. Nucleic Acids Res 35(Web Server issue): W585–7. 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed]
- 33. Hawkins T, Luban S, Kihara D (2006) Enhanced automated function prediction using distantly related sequences and contextual association by PFP. Protein Sci 15(6): 1550–1556 10.1110/ps.062153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gallo CM, Munro E, Rasoloson D, Merritt C, Seydoux G (2008) Processing bodies and germ granules are distinct RNA granules that interact in C. elegans embryos. Dev Biol 323(1): 76–87 10.1016/j.ydbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 35. Chen D, Pan KZ, Palter JE, Kapahi P (2007) Longevity determined by developmental arrest genes in caenorhabditis elegans. Aging Cell 6(4): 525–533 10.1111/j.1474-9726.2007.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Haaften G, Romeijn R, Pothof J, Koole W, Mullenders LH, et al. (2006) Identification of conserved pathways of DNA-damage response and radiation protection by genome-wide RNAi. Curr Biol 16(13): 1344–1350 10.1016/j.cub.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 37. Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, et al. (2009) Multiple ERK substrates execute single biological processes in caenorhabditis elegans germ-line development. Proc Natl Acad Sci U S A 106(12): 4776–4781 10.1073/pnas.0812285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xue H, Xian B, Dong D, Xia K, Zhu S, et al. (2007) A modular network model of aging. Mol Syst Biol 3: 147 10.1038/msb4100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coghlan A, Wolfe KH (2004) Origins of recently gained introns in caenorhabditis. Proc Natl Acad Sci U S A 101(31): 11362–11367 10.1073/pnas.0308192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andachi Y (2008) A novel biochemical method to identify target genes of individual microRNAs: Identification of a new caenorhabditis elegans let-7 target. RNA 14(11): 2440–2451 10.1261/rna.1139508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lowden MR, Meier B, Lee TW, Hall J, Ahmed S (2008) End joining at caenorhabditis elegans telomeres. Genetics 180(2): 741–754 10.1534/genetics.108.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raices M, Verdun RE, Compton SA, Haggblom CI, Griffith JD, et al. (2008) C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell 132(5): 745–757 10.1016/j.cell.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 43. Lemmens BB, Tijsterman M (2011) DNA double-strand break repair in caenorhabditis elegans. Chromosoma 120(1): 1–21 10.1007/s00412-010-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Youds JL, Barber LJ, Boulton SJ (2009) C. elegans: A model of fanconi anemia and ICL repair. Mutat Res 668(1–2): 103–116 10.1016/j.mrfmmm.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 45. Ko E, Lee J, Lee H (2008) Essential role of brc-2 in chromosome integrity of germ cells in C. elegans . Mol Cells 26(6): 590–594. [PubMed] [Google Scholar]
- 46. Kruisselbrink E, Guryev V, Brouwer K, Pontier DB, Cuppen E, et al. (2008) Mutagenic capacity of endogenous G4 DNA underlies genome instability in FANCJ-defective C. elegans . Curr Biol 18(12): 900–905 10.1016/j.cub.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 47. Youds JL, Barber LJ, Ward JD, Collis SJ, O'Neil NJ, et al. (2008) DOG-1 is the caenorhabditis elegans BRIP1/FANCJ homologue and functions in interstrand cross-link repair. Mol Cell Biol 28(5): 1470–1479 10.1128/MCB.01641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodyer W, Kaitna S, Couteau F, Ward JD, Boulton SJ, et al. (2008) HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev Cell 14(2): 263–274 10.1016/j.devcel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 49. Pispa J, Palmen S, Holmberg CI, Jantti J (2008) C. elegans dss-1 is functionally conserved and required for oogenesis and larval growth. BMC Dev Biol 8: 51 10.1186/1471-213X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Min J, Park PG, Ko E, Choi E, Lee H (2007) Identification of Rad51 regulation by BRCA2 using caenorhabditis elegans BRCA2 and bimolecular fluorescence complementation analysis. Biochem Biophys Res Commun 362(4): 958–964 10.1016/j.bbrc.2007.08.083. [DOI] [PubMed] [Google Scholar]
- 51. Ward JD, Barber LJ, Petalcorin MI, Yanowitz J, Boulton SJ (2007) Replication blocking lesions present a unique substrate for homologous recombination. EMBO J 26(14): 3384–3396 10.1038/sj.emboj.7601766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petalcorin MI, Galkin VE, Yu X, Egelman EH, Boulton SJ (2007) Stabilization of RAD-51-DNA filaments via an interaction domain in caenorhabditis elegans BRCA2. Proc Natl Acad Sci U S A 104(20): 8299–8304 10.1073/pnas.0702805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petalcorin MI, Sandall J, Wigley DB, Boulton SJ (2006) CeBRC-2 stimulates D-loop formation by RAD-51 and promotes DNA single-strand annealing. J Mol Biol 361(2): 231–242 10.1016/j.jmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 54. Garcia-Muse T, Boulton SJ (2005) Distinct modes of ATR activation after replication stress and DNA double-strand breaks in caenorhabditis elegans. EMBO J 24(24): 4345–4355 10.1038/sj.emboj.7600896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Martin JS, Winkelmann N, Petalcorin MI, McIlwraith MJ, Boulton SJ (2005) RAD-51-dependent and -independent roles of a caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25(8): 3127–3139 10.1128/MCB.25.8.3127-3139.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boerckel J, Walker D, Ahmed S (2007) The caenorhabditis elegans Rad17 homolog HPR-17 is required for telomere replication. Genetics 176(1): 703–709 10.1534/genetics.106.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang W, Hekimi S (2010) Two modes of mitochondrial dysfunction lead independently to lifespan extension in caenorhabditis elegans. Aging Cell 9(3): 433–447 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 58. Harris J, Lowden M, Clejan I, Tzoneva M, Thomas JH, et al. (2006) Mutator phenotype of caenorhabditis elegans DNA damage checkpoint mutants. Genetics 174(2): 601–616.10.1534/genetics.106.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of PSI-BLAST profiles using the 46 C. elegans OB fold protein sequences.
(TIF)
Profile based search to identify novel OB fold protein sequences.
(TIF)
Direct fold recognition prediction to identify novel OB fold protein.
(TIF)
Parameters explored for profile generation using PSI-BLAST
(DOCX)
Parameters of sequence similarity search tools used on step 4. Mostly default parameters were used otherwise specified.
(JPG)
Supporting methods.
(DOC)