Abstract
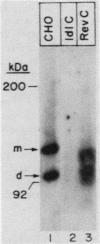
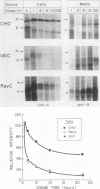
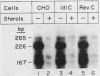
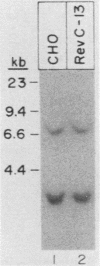
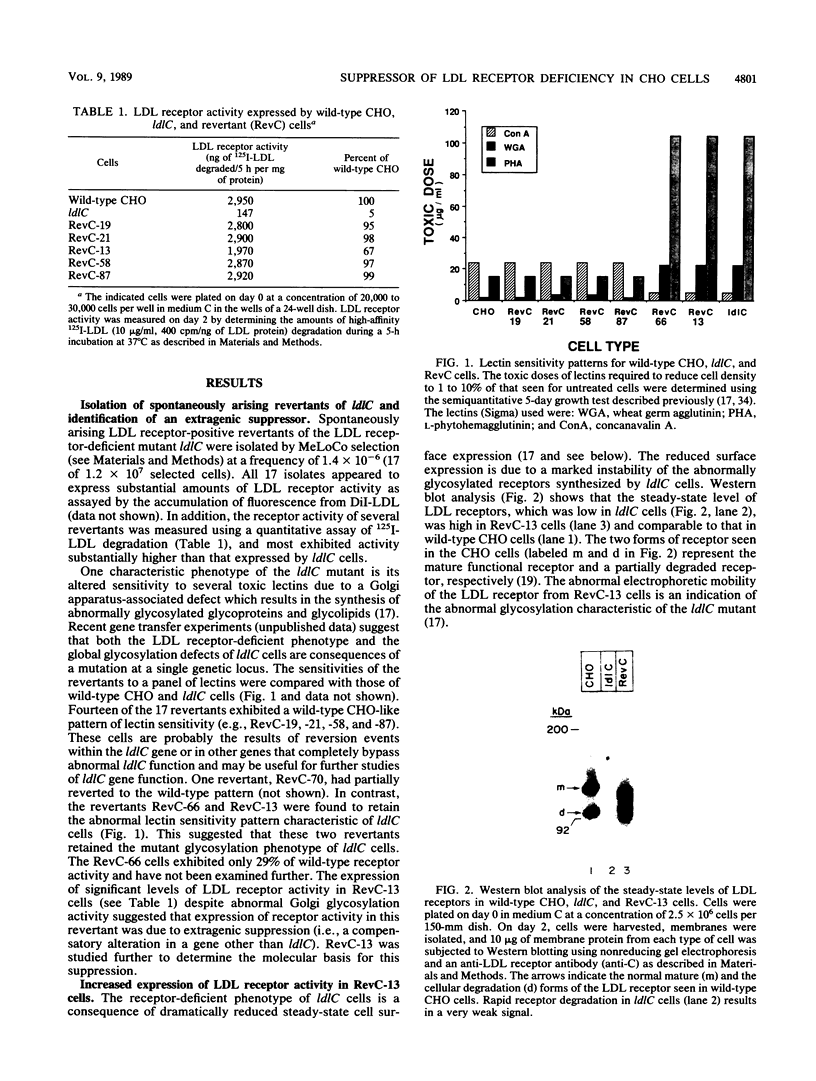
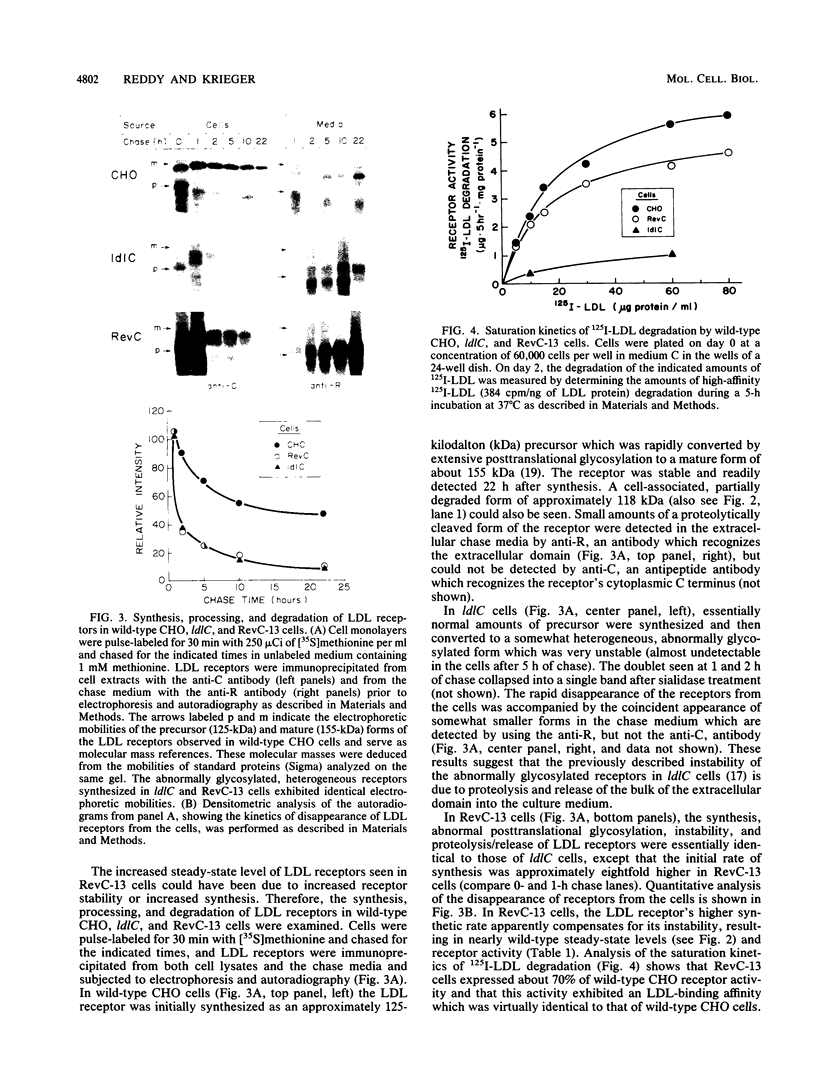
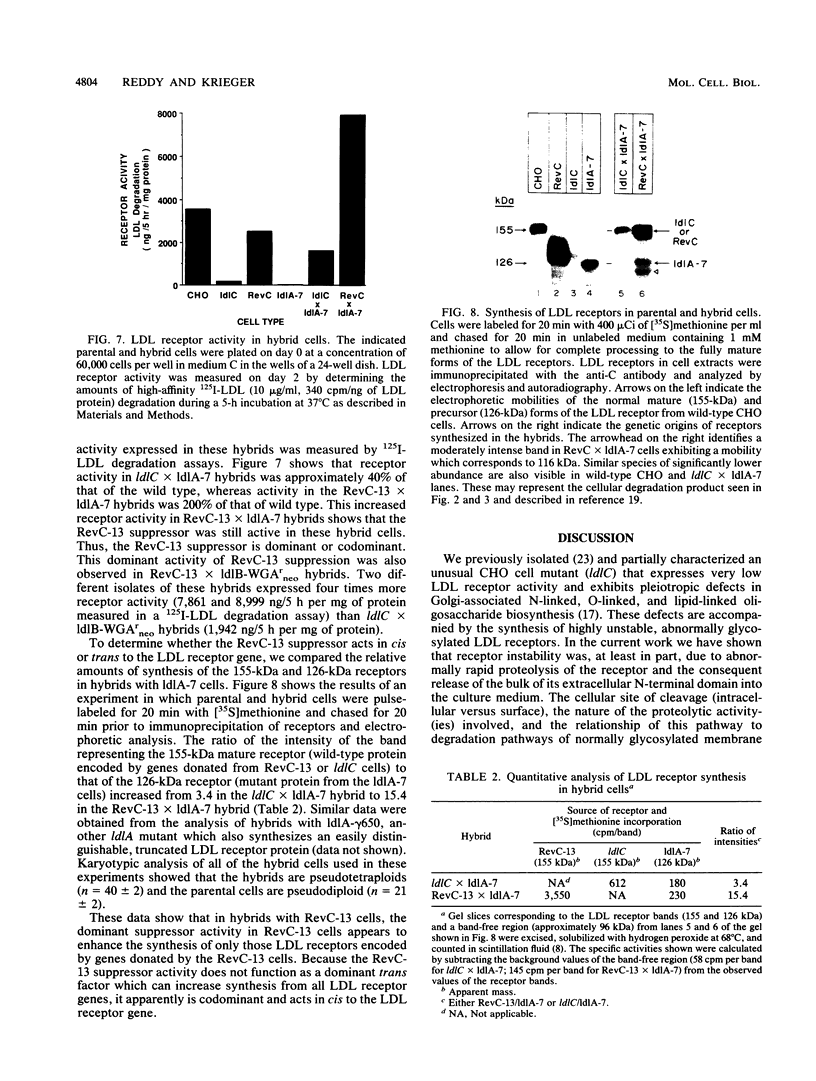
ldlC cells are low-density lipoprotein (LDL) receptor-deficient Chinese hamster ovary cell mutants which express pleiotropic defects in Golgi-associated glycosylation reactions. The dramatically reduced stability of the abnormally glycosylated LDL receptors in ldlC cells was shown to be due, in part, to rapid proteolysis and release of a large extracellular fragment of the receptor into the medium. A set of spontaneously arising LDL receptor-positive revertants of ldlC cells has been isolated. One of these, RevC-13, exhibits the glycosylation defects characteristic of the original ldlC mutant, suggesting that restoration of receptor activity was due to extragenic suppression. This suppression was due to a dramatic increase in the rate of LDL receptor synthesis rather than to an increase in the stability of the abnormally glycosylated receptors. Increased receptor synthesis was not due to receptor gene amplification. The increased LDL receptor activity was subject to normal sterol regulation. Analysis of the RevC-13 extragenic suppressor activity in a series of hybrid cells showed that RevC-13 suppression was a codominant trait that acted in cis to the LDL receptor structural gene (ldlA). Thus, the extragenic suppression in RevC-13 cells has defined a genetic element which is either part of or linked to the LDL receptor structural gene and which can control LDL receptor expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982 Jun;2(6):720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cirullo R. E., Wasmuth J. J. Isolation of Chinese hamster ovary cells that overproduce asparaginyl-tRNA synthetase. Mol Cell Biol. 1984 Sep;4(9):1939–1941. doi: 10.1128/mcb.4.9.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr Reversion of a mutation affecting the molecular weight of HGPRT: intragenic suppression and localization of X-linked genes. Somatic Cell Genet. 1980 Jul;6(4):477–494. doi: 10.1007/BF01539151. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Ernst V., Levin D. H. Separation and identification of type 1 and type 2 protein phosphatases from rabbit reticulocyte lysates. J Biol Chem. 1983 Feb 10;258(3):1439–1443. [PubMed] [Google Scholar]
- Gil G., Brown M. S., Goldstein J. L. Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from the hamster. II. Isolation of the gene and characterization of the 5' flanking region. J Biol Chem. 1986 Mar 15;261(8):3717–3724. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986 May;102(5):1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Krieger M. Receptor-mediated endocytosis of low density lipoprotein: somatic cell mutants define multiple genes required for expression of surface-receptor activity. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5454–5458. doi: 10.1073/pnas.81.17.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D. M., Sege R. D., Kozarsky K. F., Krieger M. DNA-mediated transfer of a human gene required for low-density lipoprotein receptor expression and for multiple Golgi processing pathways. Mol Cell Biol. 1986 Jul;6(7):2734–2737. doi: 10.1128/mcb.6.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Brush H. A., Krieger M. Unusual forms of low density lipoprotein receptors in hamster cell mutants with defects in the receptor structural gene. J Cell Biol. 1986 May;102(5):1567–1575. doi: 10.1083/jcb.102.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K., Kingsley D., Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Brown M. S., Goldstein J. L. Isolation of Chinese hamster cell mutants defective in the receptor-mediated endocytosis of low density lipoprotein. J Mol Biol. 1981 Aug 5;150(2):167–184. doi: 10.1016/0022-2836(81)90447-2. [DOI] [PubMed] [Google Scholar]
- Krieger M. Complementation of mutations in the LDL pathway of receptor-mediated endocytosis by cocultivation of LDL receptor-defective hamster cell mutants. Cell. 1983 Jun;33(2):413–422. doi: 10.1016/0092-8674(83)90423-3. [DOI] [PubMed] [Google Scholar]
- Krieger M. Isolation of somatic cell mutants with defects in the endocytosis of low-density lipoprotein. Methods Enzymol. 1986;129:227–237. doi: 10.1016/0076-6879(86)29072-2. [DOI] [PubMed] [Google Scholar]
- Krieger M., Martin J., Segal M., Kingsley D. Amphotericin B selection of mutant Chinese hamster cells with defects in the receptor-mediated endocytosis of low density lipoprotein and cholesterol biosynthesis. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5607–5611. doi: 10.1073/pnas.80.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Reddy P., Caras I., Krieger M. Effects of O-linked glycosylation on the cell surface expression and stability of decay-accelerating factor, a glycophospholipid-anchored membrane protein. J Biol Chem. 1989 Oct 15;264(29):17329–17336. [PubMed] [Google Scholar]
- Sege R. D., Kozarsky K. F., Krieger M. Characterization of a family of gamma-ray-induced CHO mutants demonstrates that the ldlA locus is diploid and encodes the low-density lipoprotein receptor. Mol Cell Biol. 1986 Sep;6(9):3268–3277. doi: 10.1128/mcb.6.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege R. D., Kozarsky K., Nelson D. L., Krieger M. Expression and regulation of human low-density lipoprotein receptors in Chinese hamster ovary cells. Nature. 1984 Feb 23;307(5953):742–745. doi: 10.1038/307742a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stanley P. Selection of specific wheat germ agglutinin-resistant (WgaR) phenotypes from Chinese hamster ovary cell populations containing numerous lecR genotypes. Mol Cell Biol. 1981 Aug;1(8):687–696. doi: 10.1128/mcb.1.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H. Mutant isolation. Methods Enzymol. 1979;58:308–322. doi: 10.1016/s0076-6879(79)58147-6. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]