Abstract
Interruptions (gaps) and unfamiliar events (distracters) during a timed signal delay the timed response of humans and other animals. To explore this phenomenon, we manipulate the intensity of auditory distracters (experiment 1), and we dissociate the role of distracter intensity, distracter similarity with the inter-trial interval, and dissimilarity from the timed auditory signal (experiment 2). When the inter-trial interval and the timed signal were silent, the delay in response after an auditory distracter increased with its intensity: Rats ignored (ran through) a 40dB distracter, stopped timing during a 75dB distracter, and reset after a 100dB distracter. However, when timing was signaled by a 70dB noise, rats reset both for 40dB and 100dB distracters, stopped for both 55dB and 85dB distracters, and run for the 70dB distracter. Data are accounted for by a time-sharing model assuming two concurrent processes—time accumulation and memory decay controlled by the discriminability of the interrupting event—whose interplay results in a continuum of responses, from run to reset.
Keywords: Auditory, Distracter, Gap procedure, Intensity, Interval timing, Rat, Resource-sharing, Time-sharing, Visual
Introduction
Time is a universal presence that marks all biological and behavioral processes, and flexibility in using time and responding to changes in timed events is critical for adaptive response and survival (Buhusi & Meck, 2005). The ability to time in the seconds-to-minutes range—interval timing—was has been demonstrated in many species, including birds, rodents, and humans, and has been shown to inform fundamental processes such as foraging, rate calculation, and decision making (Gallistel, 1990). To investigate temporal regulation of behavior one may examine the response to changes in timed events, for example, by using a modified version of the peak-interval (PI) procedure (Catania, 1970) in which the to-be-timed interval is sometimes interrupted by gaps (Church, 1978) and/or distracters (Buhusi & Meck, 2006a, 2006b).
In the PI procedure with gaps and distracters, subjects are presented with a combination of four types of trials, randomly intermixed and separated by an inter-trial interval (ITI). In the fixed-interval (FI) trials, a to-be-timed signal (e.g., visual) is turned on and the subjects' first response after the criterion interval is reinforced and turns the to-be-timed signal off. In PI trials, the to-be-timed stimulus is presented for a much longer duration than the criterion, but subjects' responses are not reinforced. Trained subjects typically wait at the beginning of the trial, then start responding throughout the trial—a response called run—and quit responding towards the end of the trial, such that the average rate of response gradually increases and peaks at the expected time of reward, then gradually decreases toward the end of the trial. Gap trials are identical to PI trials, except that the to-be-timed signal is turned off at a given pre-gap interval for a given gap duration, after which the signal is turned back on for the remaining duration of the trial. In gap trials, the range of responses observed in rodents and birds is very large, depending on the experimental conditions: the response may be delayed anywhere on a continuum (Buhusi & Meck, 2006b; Buhusi, Paskalis, & Cerutti, 2006; Swearingen & Buhusi, 2010) between running (no delay, ignoring the gap) (Buhusi, et al., 2006), and resetting (a large delay indicative of restarting the entire timing process after the gap) (Aum, Brown, & Hemmes, 2004; Bateson & Kacelnik, 1998; Brodbeck, Hampton, & Cheng, 1998; Buhusi, et al., 2006; Buhusi, Sasaki, & Meck, 2002; Cabeza de Vaca, Brown, & Hemmes, 1994; W. A. Roberts, Cheng, & Cohen, 1989) . On the run - reset continuum, a delay equal to the length of the gap is sometimes referred to as stopping (timing during the gap) (Buhusi & Meck, 2006b; Church, 1978; Meck, Church, & Olton, 1984; S. Roberts, 1981; S. Roberts & Church, 1978). The same large range of responses were also reported in distracter trials (Buhusi & Meck, 2006a, 2006b), which are identical to PI trials, except that an unfamiliar stimulus (e.g., auditory distracter) is turned on for a given duration during the uninterrupted timed signal, and then turned off for the remaining duration of the timed signal.
The fact that distracters delay timing in a manner undistinguishable from gaps despite the presence of the timed stimulus (Buhusi & Meck, 2006a, 2006b) provides strong support for a time-sharing hypothesis (Buhusi, 2003; Buhusi & Meck, 2009), which can be applied in the framework of the Scalar Expectancy Theory (Gibbon, 1977; Gibbon, Church, & Meck, 1984) as follows (Figure 1A): Pulses regularly emitted by a pacemaker accumulate and are temporarily stored in working memory. During each FI trial, reinforcement is provided for the first response after the criterion interval, and the number of pulses currently in working memory is stored in the reference memory. During PI trials, subjects use a ratio rule to compare currently accumulated time (stored in working memory) and the learned number of pulses associated with the time of reinforcement (stored in reference memory), so that the average response rate reaches a peak when the two values are equal, i.e., at the expected time of reinforcement. According to the time-sharing hypothesis, during an interrupting event (gap and/or distracter), working memory for the interval preceding the interrupting event decays at a rate proportional to the salience of interrupting event due to memory and/or attentional resources being diverted toward processing the interrupting event (Buhusi, 2003; Buhusi & Meck, 2009). This view is consistent with the resource-sharing hypothesis from the human interval-timing literature (Fortin, 2003; Lejeune, 1998; Thomas & Weaver, 1975; Zakay, 1989, 2000) and is currently the focus of considerable theoretical interest (Fortin, 2003; Lejeune, 1998; Zakay, 1989, 2000) (left panel of Figure 1).
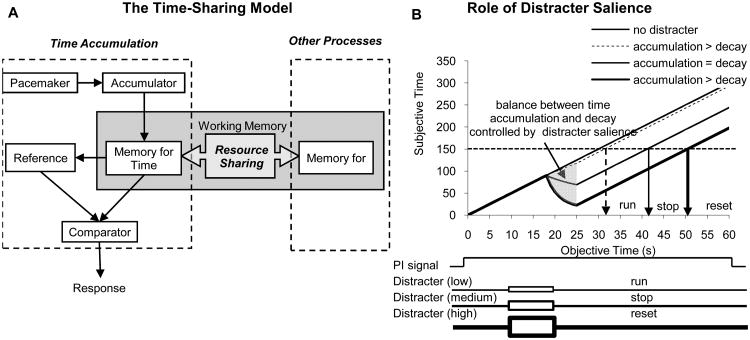
Figure 1. A model of time-sharing controlled by the discriminability (salience) of events.
A: The time-sharing model assumes limited attentional or working memory resources: time processing (left) and other processes (right) share the access to these resources. In particular, working memory for time is updated by two concurrent processes: time accumulation and active decay due to sharing of resources. The rate of memory decay is assumed to depend on the discriminability (salience) of the interrupting event. B: The interplay between time accumulation and working memory decay may result in a continuum of responses based on the discriminability of the distracter, from no delay (run response), to an apparent stop of timing (balanced time accumulation and memory decay), or a restart of timing after the distracter (reset response) (adapted from Buhusi & Meck, 2006a).
The role of salience in time-sharing is supported by studies in pigeons (Buhusi, et al., 2002) and rats (Buhusi, Perera, & Meck, 2005) showing that despite gap duration and position being kept fixed, manipulations of the contrast in brightness between the gap and the timed signal shift the response functions in a great dynamic range. According to the time-sharing hypothesis, the smaller the distracter discriminability (salience), the fewer resources would be diverted towards it, and the more working memory resources would be available to timing, resulting in a minimal effect of the distracter (Figure 1B). In contrast, the higher the distracter discriminability, the more resources would be diverted to its processing, the fewer resources would be available to timing, and the faster the accumulated time would decay during the distracter, resulting in restarting timing after the distracter (Figure 1B). For example, at 40% gap-signal contrast pigeons are minimally interrupted in their timing (run), while at 100% contrast they reset their timing after the gap (Buhusi, et al., 2002), suggesting that delays vary considerably within a continuum ranging between run and reset, with the stop rule as only an arbitrary point.
Here we address the role of discriminability (salience) of distracters in time sharing in rats by manipulating the intensity of auditory distracters (experiment 1), and by dissociating the role of distracter intensity, similarity with the inter-trial interval, and dissimilarity from the timed signal (experiment 2). While gap-signal contrast of visual stimuli is critical in pigeons (Buhusi, et al., 2002), visual intensity has minimal effects in rats (Buhusi, et al., 2002). This is not surprising, considering that rats are nocturnal animals, less sensitive to variations in the intensity of visual stimulation. Rats tend to stop for (dark) visual gaps but reset for (illuminated) reversed visual gaps as short as 1s (Buhusi & Meck, 2000), and after auditory gaps (Buhusi, et al., 2005). Therefore, in experiment 1 we examined whether in albino rats memory for time is sensitive to manipulations in the intensity of auditory signals. In experiment 2, we further examined the role of distracter intensity, but changed the setting to dissociate the role of intensity, similarity with the inter-trial interval, and dissimilarity from the timed auditory signal. Results from both experiments suggest that in rats memory for time can vary in a large dynamic range, from run to reset, with the intensity of auditory interrupting events. Computer simulations of time sharing within the framework of the Scalar Expectancy Theory (Gibbon, et al., 1984) addressed the results under the assumption that during the interrupting event memory for time is given by the balance between concurrent time accumulation and memory decay at a rate proportional to the discriminability (salience) of the distracter.
Experiment 1: Role of distracter intensity
The purpose of experiment 1 was to establish the range of auditory stimulation that affects memory for time in albino rats. The time-sharing hypothesis predicts that the higher the intensity of the distracter, the more memory for time decays, and the more rats delay their response.
Materials and methods
Subjects
Twelve naive Sprague-Dawley male rats (4 months old at the beginning of the experiment) were housed in a temperature-controlled room, under a 12/12-h light-dark cycle, with water given ad libitum. Rats were maintained at 85% of their ad libitum weight by restricting access to food (Rodent Diet 5001, PMI Nutrition International, Inc., Brentwood, MO). All experimental procedures were conducted in accordance with NIH's Guide for the Care and Use of Laboratory Animals (1996).
Apparatus
The apparatus consisted of 12 standard rat chambers (MED Associates, St. Albans, VT) housed in sound attenuating cubicles. Each chamber was equipped with three response levers (two retractable and one fixed) situated on the front wall of the chamber. All experimental procedures used only the left lever. According to the schedule, 45-mg precision food pellets (PMI Nutrition International, Inc., Brentwood, MO) were delivered in a food cup situated on the front wall, 1 cm above the grid floor, under the center lever, by a pellet dispenser. The to-be-timed visual stimulus was a house light mounted at the center-top of the front wall. The auditory distracter was a variable-intensity (40-100dB) white noise produced by a white-noise programmable generator (MED Associates, St. Albans, VT) mounted on the opposite wall from the response levers. The intensity of the distracter was measured with a sound-level meter (Realistic Radio Shack, Model 33-2050) from the center of the silent box.
Fixed-interval (FI) training
Rats were shaped to lever press, then trained in a FI procedure for 7 sessions, as follows: For each daily session all rats received 64 FI trials during which the house light was turned on for the to-be-timed interval; the first lever press 30 s after the onset of the visual signal was reinforced by the delivery of a food pellet and turned off the house light for the duration of a random 90 ± 30 s inter-trial interval (ITI). A 40dB noise was presented throughout the session, both during the FI trials and during the ITI.
Peak-interval (PI) training
For the next 15 session, rats received 32 FI trials randomly intermixed with 32 non-reinforced PI trials in which the visual signal was presented for a duration three times longer than the FI, before being terminated irrespective of responding for the random 90 ± 30 s ITI. A 40dB noise was presented throughout the session.
Distracter testing
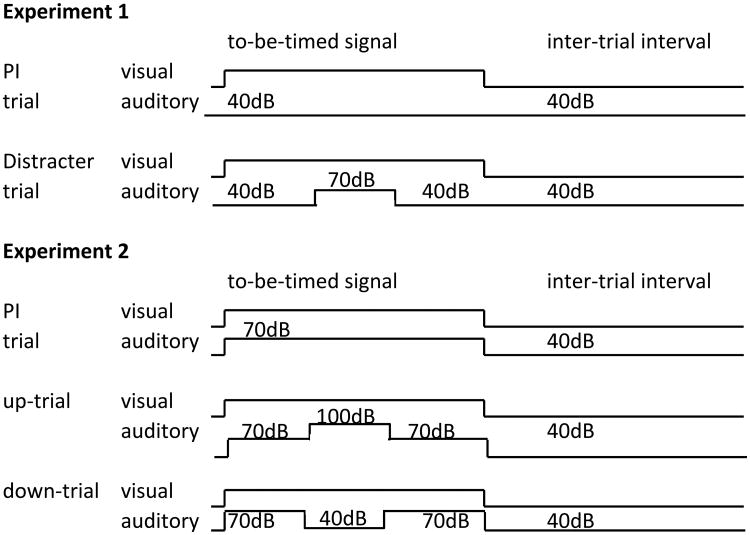
For the next 15 session, rats received 32 FI trials randomly intermixed with 27 PI trials and 5 distracter trials (1 for each distracter intensity). Distracter trials were identical to PI trials (i.e., the visual timed stimulus was not interrupted), except that 20s after the beginning of the trial the white noise was randomly set at one of the five intensities 40, 55, 70, 85, or 100dB for 5s, then set back on 40dB for the rest of the trial. Because the duration of the pre-distracter and distracter intervals were not varied during testing, temporal expectations for these events could develop during the course of testing (Aum, et al., 2004). To minimize this possibility, only 5 distracter trials were presented per session. Trials were separated by a 90 ± 30 s random ITI. A 40dB noise was presented throughout the ITI and all trials except as described above for the distracter trials. A diagram of the experimental setting is presented in Figure 2.
Figure 2. Experimental design.
In experiment 1 rats were trained to time a 30s visual signal, and were tested with 5 types of auditory distracters, 40, 55, 70, 85, and 100dB (one example of 70dB distracter is shown). In experiment 2 rats were trained to time a 30s compound visual-auditory signal, including a 70dB noise, and were tested with 5 types of auditory distracters, of which some were identical to, while some had higher (85 and 100dB) or lower (40 and 55dB) intensity than, the 70dB signal (one example of an 100dB up-trial, and one example of a 40dB down-trial are shown).
Data collection and analysis
The experimental procedures were controlled through a MED Associates interface connected to an IBM-PC compatible computer running a MED-PC software system (MED-Associates, 1999). Lever presses were recorded in real time. Lever presses recorded during distracter trials were used to estimate the peak time, peak rate, and precision of timing (width of the response functions) for each rat. The number of responses (in 5-s bins) was averaged daily over trials, to obtain a mean response rate function for each rat. Analyses were conducted on the data from an interval twice as large as the FI (i.e., 60 s), starting at the onset of the to-be-timed signal for the 40dB and 55dB distracter trials, and at the offset of the distracter for the 70, 85, and 100dB distracter trials. The mean response rate in the interval of interest was fit using the Marquardt-Levenberg iterative algorithm (Marquardt, 1963) to find the coefficients (parameters) of a Gaussian+linear equation that gave the “best fit” (least squares minimization) between the equation and the data (Buhusi & Meck, 2000):
and provided the following parameters of the response curve: the accuracy of timing (response peak time, t0), precision of timing (width of response function, b), and peak rate of response (sum of parameters a and d). These parameters were submitted to statistical analyses. All statistical tests were evaluated at a significance level of 0.05 (two tailed).
Results
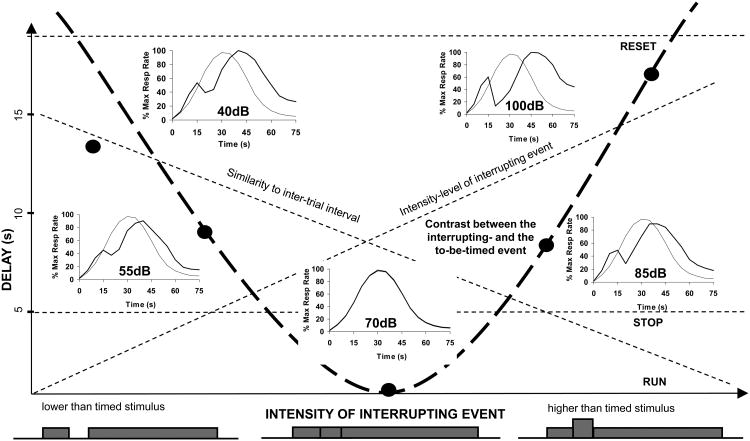
The average percent maximum response rate in distracter trials are shown in Figure 3A, which indicates that the 5-s 40- and 55-dB distracters were ignored by the rats, but that at higher intensities the response function was shifter rightward in proportion to its intensity. Moreover, data indicates that the response rate after the distracter decrease with the intensity of the distracter.
Figure 3. Distracters delay timing in proportion to their intensity.
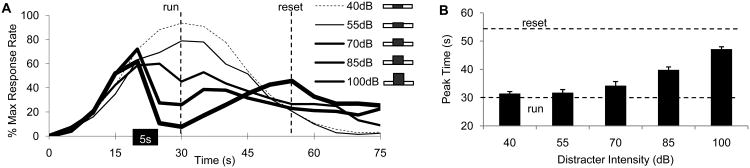
A: Average percent maximum response rate in trials with 40, 55, 70, 85, and 100dB auditory distracters during a visual to-be-timed signal. B: Low intensity distracters (40 and 55dB) minimally affect the response, medium-intensity distracters (70 and 85dB) shift the response with about the duration of the distracter (5s), while high-intensity distracters (100dB) shift the response significantly.
Figure 3B shows the estimated peak time in distracter trials. The peak time for the 40- and 55-dB distracter trials were not different from the criterion duration, 30s, ts <2.11, p>0.05, the peak time for the 70dB distracter was not different from a stop, 35s, t(11)=.51, p>0.05, while the peak time for the 85- and 100-dB distracter trials were significantly larger than a stop, ts > 4.69, p<0.001, though smaller than a reset, ts > 9.49, p<0.001. These results were further supported by a repeated measures ANOVA with factor distracter intensity, which revealed a significant effect of intensity, F(4, 44)=41.04, p<0.001. A post-hoc Sheffe test indicated that rats responded similarly for the 40-, 55-, and 70dB intensities, ps>0.05, but that they significantly delayed at the 85- and 100-dB intensities, ps<0.01. These results suggest that rats were sensitive to variations in the intensity of the auditory distracter, and delayed their response for higher-intensity distracters.
Experiment 2: Role of distracter discriminability (salience)
Results from experiment 1 may be taken to suggest that rats are sensitive to distracters of intensities larger than 85dB, but not for intensities lower than 70dB. However, because in experiment 1 a 40dB noise was presented throughout the session, it is possible that rats based their response not on the intensity level but rather on the contrast between the distracter and the background noise throughout the session, i.e., on its discriminability (salience) from either the timed signal or the ITI. To address these questions, in experiment 2 the to-be-timed signal included an auditory component, such that we varied the intensity of the distracter both higher and lower from this base level, keeping the ITI quiet. Should the delay be due to intensity level, then higher-intensity distracters will delay timing more than low-intensity ones. However, should the delay be due to similarity of the distracter with the ITI, then lower-intensity distracters will delay timing more than high-intensity ones. Finally, should the delay be due to discriminability (dissimilarity) of the distracter from the to-be-timed signal, then both low- and high-intensity distracters will delay timing more than medium-intensity ones (U-shaped effect).
Materials and methods
Sixteen naive Sprague-Dawley male rats (4 months old at the beginning of the experiment) were used as subjects in experiment 2. All details of the experiment are identical to experiment 1, except for the details shown in Figure 2: The ITI was dark and quiet (40dB noise throughout). The to-be-timed interval was signaled by both the house light and a 70-dB white noise. The 5-s distracter was presented 20s after the beginning of the to-be-timed signal for 5s. The distracter intensity was set randomly at 40, 55, 70, 85, or 100dB. Therefore, for the 70dB distracter, the trial was identical to a PI trial, for 2 “louder” trials, the 85- and 100-dB distracters were louder than the PI trial, and for 2 “softer” trials the 40- and 55-dB distracters were softer than the PI trial (see Figure 2). Analyses were conducted on the data from 6 distracter sessions, in a target interval twice as large as the FI (i.e., 60 s), starting at the offset of the distracter, as described for experiment 1.
Results
The average percent maximum response rate in distracter trials is shown in Figure 4, which also indicates the predictions based on whether the delay is based solely on the intensity level of the distracter, on the similarity of the distracter to the ITI, or the discriminability (contrast) of the distracter from the timed signal. As shown in Figure 4, while the 70dB distracter did not delay the response of the rats relative to PI trials (the PI and gap response functions practically overlap), t(15)=1.53, p>0.15, both lower- and higher-intensity distracters shifted the response function more than a stop, ts>4.28, p<0.001, but lower than a 20s reset, ts>5.49, p<0.001.
Figure 4. Distracters delay timing in proportion to their discriminability (contrast, salience) from the to-be-timed signal.
Should the effect of distracters be based on their intensity, their effect would increase with their intensity. In contrast, should their effect be based on their similarity to the ITI, their effect would decrease with their intensity. Finally, should their effect be based on their discriminability (contrast) from the timed signal, their effect would take a U-shaped curve, with both low- and high-intensity distracters having more effect than mid-level ones. The average percent maximum response rate in trials with 40, 55, 70, 85, and 100dB auditory distracters supports the latter interpretation.
These results were further supported by a repeated measures ANOVA with factor distracter intensity, which revealed a significant effect of intensity, F(4, 60)=37.99, p<0.001. A posthoc Sheffe test indicated that rats responded similarly for the 40-, 55-, and 85-dB distracters, ps>0.05, but that they responded differently for all other comparisons, ps<0.001. The leftward asymmetry in the response function may be due to the inability of the rats to differentiate the 40- and 55-dB intensities. Nevertheless, the response is clearly delayed under all conditions, suggesting that rats are sensitive to the entire range of intensities tested (from 40- to 100-dB), and that they delayed their response on the run-reset continuum in proportion to the discriminability of the distracter from the timed event (contrast), in a similar manner as found in pigeons (Buhusi, et al., 2006).
Experiment 3: Computer simulations with a time-sharing model
To evaluate the time-sharing hypothesis, we conducted simulations within the framework of the Scalar Expectancy Theory (Gibbon, et al., 1984), under the assumption that during an interrupting event (gap and/or distracter) the estimated time in working memory is the interplay of two concurrent and opposing processes, a process of time accumulation, and a process of memory decay with a decay proportional to the discriminability of the interrupting event.
Materials and methods
We assumed that before, during and after the distracter, working memory for time, wm (currently accumulated time), increases linearly with speed of accumulation set at 5 pulses per real-time second, as follows:
| (Eq. 1) |
Accordingly, the 30s criterion time was simulated as 30s × 5 pulses/s = 150 pulses.
We also assumed that during the distracter, working memory for time wm is given by the balance between linear time accumulation and memory decay with rate of decay a function of the strength of interference, as follows:
| (Eq.2) |
Furthermore, according to the time-sharing hypothesis (Buhusi, 2003; Buhusi & Meck, 2009) we assumed that the decay parameter is proportional to the discriminability of the interrupting event. We assumed that both increases and decreases in the intensity of the distracter will increase its discriminability, such that the decay parameter is proportional to the contrast between the intensity of the distracter and that of the signal, 40dB in experiment 1, and 70dB in experiment 2.
| (Eq. 3) |
To test this model, we used data from experiment 1 to estimate the decay rate in Experiment 1, then use it to make predictions to be contrasted against data from experiment 2 (see Appendix for details).
Results
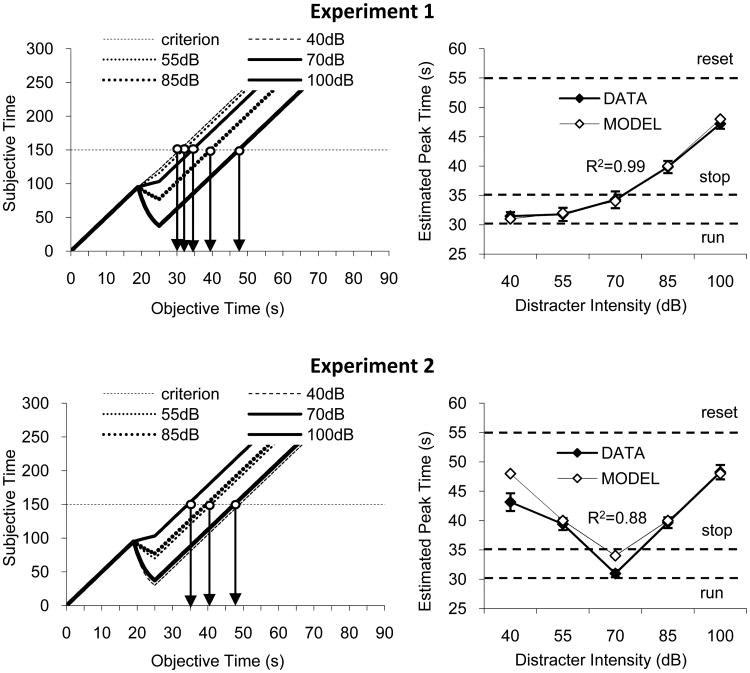
First, we estimated the (two) parameters that allows the time-sharing model to provide a good fit (R2=0.99) for data from experiment 1 (see Appendix for details). The left upper panel of Figure 5 indicates that working memory for time continues to accumulate during the 40-, 55-, and 70-dB distracters, but decays during the 85- and 100-dB distracters. The right upper panel of Figure 5 show the fit between model and data in experiment 1.
Figure 5. The time-sharing model fist the data from both experiments.
Data in the present experiments were fitted by a time-sharing model assuming concurrent time accumulation and memory decay proportional to the distracter-signal contrast. Simulations assume a rate of accumulation = 5 pulses per second. Upper panels: Data from experiment 1 were used to determine the rate of memory decay, specified by parameters alpha40dB=0.006 and beta=38, for which the model best fits the data. Lower panels: Once set, these parameters were used to determine parameter alpha70dB=0.037, and contrast predictions of the model against data from experiment 2. Left panels: Memory for time is given by the interplay between time accumulation and memory decay. Right panels: The model provides good fits to the data from both experiments.
Seconds, using the parameters extracted from data from experiment 1, we used time-sharing model to make predictions results from experiment 2. The left lower panel of Figure 5 indicates that working memory for time continues to accumulate for the 70dB distracter (for which the decay is minimal, according to data from experiment 1), but decays for all other distracters, both of lower and higher intensity than the 70dB timed signal. The right lower panel of Figure 5 indicates that with the parameters estimated from experiment 1, the time-sharing model provides remarkably good predictions for data in experiment 2, R2=0.88. However, while the prediction is symmetrical around the 70dB, the data from experiment 2 is skewed at lower distracter intensities, possibly indicating the rats inability to discriminate the 40- and 55-dB distracters, since these very soft sounds were easily masked by other sounds in the experimental setting (e.g., because the sound attenuating boxes are not 100% sound proof). Taken together, these results suggest that the time-sharing model, Eq.1 and Eq.2, provide a good fit for data from experiment 1 and 2, suggesting that the delay after a distracter is proportional to the contrast between the intensity of the distracter and that of the to-be-timed signal.
Discussion
The results from the three experiments can be interpreted within the framework of an information-processing model of timing (Gibbon, et al., 1984) (left panel of Figure 1), Within this framework, four hypotheses have been previously applied to interpret data obtained in the PI procedure with gaps and distracters: the switch hypothesis (Gibbon, et al., 1984), the instructional ambiguity hypothesis (Kaiser, Zentall, & Neiman, 2002), the memory decay hypothesis (Cabeza de Vaca, et al., 1994), and the time-sharing hypothesis (Buhusi, 2003; Buhusi, et al., 2005). According to the first hypothesis, timing is controlled by an on-off switch mechanism (Church, 1984; Gibbon, et al., 1984) that connects the pacemaker with the accumulator. In the presence of the timed signal (e.g., in PI trials), the switch is closed, pulses accumulate and rats use the run rule (Church, 1984, p. 574). However, during a gap the switch is assumed to be open and pulses from the pacemaker fail to reach the accumulator, so that during a gap the working memory stores the pre-gap duration. After the gap, the accumulation process resumes where it left off before the gap, and the moment when accumulated time becomes equal to the criterion is delayed by the duration of the gap, in accord with the stop rule (Church, 1984, p. 574). Importantly for this paper, because the timed signal is on throughout the trial, pulses should accumulate, and rats should run. Because in experiment 1 rats ignored (run through) the low-intensity distracters, but delayed their responses after the high-intensity distracters, the current experiments provide only partial support for this hypothesis.
It is noteworthy that the putative properties of the switch were used to explain a range of phenomena: For example, to address data showing differences in timing stimuli in different modalities, the switch was assumed to open and close with a latency affected by the nature of the stimulus (Meck, 1984). Thus, in experiment 1 low-intensity distracters may fail to open the switch, while high-intensity distracters may open the switch in proportion to their intensity. However, in experiment 2, low-intensity distracters have similar effects as high-intensity ones, thus contradicting this interpretation. On the other hand, to address the occasional resetting as a result of manipulating the operanda (Church, 1980) or reward delivery (Matell & Meck, 1999), the switch was assumed to also be responsible for “resetting on command” (Church, 1980), a quality that seems incompatible with the positioning of the switch before the accumulator in the information-processing model of timing (Figure 1A). In contrast, a more coherent assumption was used to address data in the gap procedure following fimbria-fornix lesions in rats (Meck, et al., 1984). The use of the stop rule by normal rats was ascribed to the opening of the switch, located before the accumulator, while the resetting observed in lesioned rats was credited to the failure of working memory—positioned in the model after the accumulator—to maintain the currently accumulated time (Meck, et al., 1984, p. 21) (Figure 1A). This latter view is compatible with the time-sharing hypothesis (Buhusi, 2003; Buhusi, et al., 2005), as shown below.
A quite different account of the present results is given by the instructional ambiguity hypothesis, which assumes that subjects perceive the gap as an ambiguous, ITI-like event (Kaiser, et al., 2002; Sherburne, Zentall, & Kaiser, 1998; see also Staddon & Cerutti, 2003). This hypothesis predicts that manipulations which would render the gap to be more similar to the ITI would result in the use of the reset rule, while manipulations which would render the gap to be more dissimilar from the ITI would result in the use of the stop rule. Although the discriminability of the gap from the ITI was shown to affect the results in the gap procedure (Buhusi & Meck, 2002; Kaiser, et al., 2002), it seems unlikely that this interpretation can be applied to the present results. In experiment 1, the more intense the distracter the more rats delayed their response, despite the distracter being more and more dissimilar from the ITI (Figure 3). Moreover, in experiment 2, should the distracter delay timing based on its similarity with the ITI, one expects the distracter to delay considerably at a low intensity (similar to the ITI), and delay minimally at high intensities (dissimilar from the ITI). Instead, both low and high distracters delayed timing more than medium-low and medium-high distracters (Figure 4). These results are clearly incompatible with the ambiguity hypothesis. Together with data from previous studies that investigated the role of the perceived salience/intensity of events on timing (Buhusi & Meck, 2006a; Buhusi, et al., 2006; Buhusi, et al., 2005), the present results suggest that distracter-ITI similarity—although possibly relevant for gaps—is not a critical factor in the PI procedure with distracters.
The large dynamic range—from run to reset—found in the present experiments is also incompatible with the memory decay hypothesis which assumes that during gaps working memory undergoes (exponential) decay with a fixed rate (Cabeza de Vaca, et al., 1994). This hypothesis predicts that the delay after an intruder depends solely on the temporal parameters of the procedure. In contrast, in the present experiments the temporal parameters of procedures were not manipulated, yet rats delayed their responses in various degrees based on the intensity of distracters. Moreover, in the present experiments the to-be-timed signal was not interrupted during the distracter, yet rats delayed their responses. Therefore, this hypothesis is unable to explain the effect of distracters in these and other distracter experiments (Buhusi & Meck, 2006a, 2006b).
Within this framework, the time-sharing hypothesis (Buhusi, 2003; Buhusi & Meck, 2009; Buhusi, et al., 2005) builds and extends upon the memory decay hypothesis (Cabeza de Vaca, et al., 1994) by assuming that (a) memory decay due to intruding events is a process concurrent with timing, i.e., is not limited to gaps in the timed signal, but occurs throughout the trial, and (b) the rate of decay is dependent on salience-related features, rather than being fixed. This hypothesis seems to offer the most comprehensive explanation for the present data, particularly given the current proposal explored in experiment 3, that time accumulation and memory decay (maintenance) are concurrent processes affecting working memory, such that during the distracter there is simultaneous time accumulation and memory decay with a rate proportional to distracter-signal contrast. In the case of non-salient distracters (e.g., at small distracter-signal contrasts, e.g., 40, 55 and 70dB in experiment 1) accumulation overcomes memory decay and the response function shifts minimally (Figure 3). On the other hand, if time accumulation equals memory decay the function is delayed as if timing is stopped during the distracter (e.g., 85dB distracter in experiment 1, and the 55 and 85dB distracters in experiment 2). Finally, for highly discriminable distracters, memory decay is larger than accumulation (e.g., the 100dB distracter in experiment 1, and both 40 and 100dB distracters in experiment 2) then the response function is delayed considerably, requiring a restart of the entire timing process after the distracter (Figure 3). Most importantly, this model predicts a continuum of responses between run and reset, where the stop is only an arbitrary value in between (see Figure 1B).
Irrespective of the theoretical underpinnings, the present data suggest a continuum of response possibilities in the PI procedure with distracters, in contrast to a reduced set of response rules: run, stop, or reset. According to this time-sharing model the delay in peak time following the distracter is continuously adjusted by the balance between the timing component (resulting in time accumulation) and other components (resulting in memory decay) of the task (Figure 1). This flexible use of timing is further supported by data showing that rats reset their timing in PI trials upon presentation of reinforcement (Matell & Meck, 1999; Thorpe, Petrovic, & Wilkie, 2002), by data showing that pigeons delay their timing in a large dynamic range when the gap-signal contrast is manipulated (Buhusi, et al., 2002), by data showing that the behavior of rats in the gap procedure varies with the presence/absence of the signal (Buhusi & Meck, 2000), and with the perceptual acuity of the subject (Buhusi, et al., 2005), as well as by the present data. Together, these data support the notion that memory for time decays during distracters at a rate controlled by the discriminability (salience) of the distracter, possibly by a mechanism involving re-allocation of attentional or memory resources (Swearingen & Buhusi, 2010).
Importantly, the time-sharing model based on concurrent time-accumulation and memory-decay processes, is also amenable to addressing neurophysiological manipulations in the gap procedure. For example, the accumulation component was shown to be dependent on the dopaminergic system (Buhusi & Meck, 2010; Meck, 1986, 1996), whose manipulation results in differential effects during PI trials and gap trials (for a review see Buhusi, 2003; Buhusi & Meck, 2002, 2009). On the other hand, lesions of the hippocampal system interfere with working memory for time (Meck, et al., 1984), such that lesioned rats reset after the gap. The effect of such manipulations can be directly addressed by the proposed concurrent time-accumulation memory-decay model as shown elsewhere (Buhusi, 2003), because the putative locus of action of the hippocampal lesion (working memory) is different from the putative locus of action of dopaminergic drugs (clock speed) (Buhusi & Cordes, 2011; Oprisan & Buhusi, 2011).
In summary, the current body of evidence suggests that the notion that simple behavioral rules are employed in the PI procedure with gaps and distracters needs to be replaced by a more general notion of a continuous shift in the response function, varying with temporal and non-temporal parameters of the procedure. The results reported here support an integrative approach to the data patterns obtained after interruptions of interval timing and other tasks in rodents, birds, and humans, in contrast to possible species-specific differences in task-specific strategies (Bateson & Kacelnik, 1998; Brodbeck, et al., 1998; W. A. Roberts, et al., 1989).
Acknowledgments
This work was supported by grants MH65561 and MH73057 from the National Institute of Mental Health to CVB. Correspondence concerning this article should be addressed to Catalin V. Buhusi, Dept. Neurosciences, Medical University of South Carolina, 173 Ashley Ave, 403 Basic Science Bldg., Charleston, SC 29425, buhusi@musc.edu.
Appendix.
Simulations for Experiment 1 (upper panel of Figure 5)
Because the decibel (dB) scale is a logarithmic scale, the perceived intensity of a distracter, intensitydistracter can be estimated from the experimentally measured auditory intensity of the sound on the dB scale, dB(distracter), as follows:
where alpha40dB and beta are fixed parameters (same values for all distracters). Because in experiment 1 memory decay is proportional to distracter's discriminability from the 40dB background, the decay parameter becomes
where abs is the absolute value of the difference between the distracter and the background, on the dB scale. Eq.4 shows that alpha40dB represents the memory decay for a 40dB distracter:
Substituting in Eq.2, we obtain that for experiment 1, working memory during the distracter varies as follows:
| (Eq. 2′) |
Parameters alpha40dB and beta that allow Eq.1 and Eq.2′ to fit the data from experiment 1 were estimated as follows: alpha40dB = 0.006 and beta = 38. The model fits data from experiment 1 with R2=0.99.
Simulations for Experiment 2 (lower panel of Figure 5)
While memory decay in experiment 1 is assumed proportional to the discriminability of the distracter relative to the 40dB background (Eq.2′), in experiment 2 memory decay is assumed proportional to the discriminability of the distracter relative to the 70dB timed signal. Therefore, to simulate data from experiment 2, we first estimated the memory decay for a 70dB sound, decay70dB = alpha70dB, based on data from experiment 1:
With alpha40dB = 0.006 and beta = 38, one obtains alpha70dB = 0.037, such that Eq.2′ becomes:
| (Eq.2″) |
To test this model, we contrasted the results based on Eq.2″ against data from experiment 2, and obtained an estimated goodness of fit R2=0.88.
References
- et al. Guide for the Care and Use of Laboratory Animals. The National Academies Press; 1996. [PubMed] [Google Scholar]
- Aum SW, Brown BL, Hemmes NS. The effects of concurrent task and gap events on peak time in the peak procedure. Behavioural Processes. 2004;65(1):43. doi: 10.1016/S0376-6357(03)00152-9. [DOI] [PubMed] [Google Scholar]
- Bateson M, Kacelnik A. Risk-sensitive foraging: Decision making in variable environments. In: Dukas R, editor. Cognitive ecology: The evolutionary ecology of information processing and decision making. Chicago: Chicago University Press; 1998. pp. 297–341. [Google Scholar]
- Brodbeck DR, Hampton RR, Cheng K. Timing behaviour of blackcapped chickadees (Parus atricapillus) Behavioural Processes. 1998;44:183–195. doi: 10.1016/s0376-6357(98)00048-5. [DOI] [PubMed] [Google Scholar]
- Buhusi CV. Dopaminergic mechanisms of interval timing and attention. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 317–338. [Google Scholar]
- Buhusi CV, Cordes S. Time and number: the privileged status of small values in the brain. Front Integr Neurosci, 5. 2011;5:67. doi: 10.3389/fnint.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Timing for the absence of a stimulus: the gap paradigm reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26(3):305–322. doi: 10.1037/0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116(2):291–297. doi: 10.1037/0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Interval timing with gaps and distracters: evaluation of the ambiguity, switch, and time-sharing hypotheses. J Exp Psychol Anim Behav Process. 2006a;32(3):329–338. doi: 10.1037/0097-7403.32.3.329. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Time sharing in rats: A peak-interval procedure with gaps and distracters. Behav Processes. 2006b;71(2-3):107–115. doi: 10.1016/j.beproc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: new findings and an extension of the resource allocation model of temporal processing. Philos Trans R Soc Lond B Biol Sci. 2009;364(1525):1875–1885. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Timing Behavior. In: Stolerman IP, editor. Encyclopedia of Psychopharmacology. Berlin Heidelberg: Springer; 2010. pp. 1171–1172. [Google Scholar]
- Buhusi CV, Paskalis JP, Cerutti DT. Time-sharing in pigeons: Independent effects of gap duration, position and discriminability from the timed signal. Behav Processes. 2006;71(2-3):116–125. doi: 10.1016/j.beproc.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, Meck WH. Memory for timing visual and auditory signals in albino and pigmented rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31(1):18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Sasaki A, Meck WH. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) Journal of Comparative Psychology. 2002;116(4):381–390. doi: 10.1037/0735-7036.116.4.381. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Brown BL, Hemmes NS. Internal clock and memory processes in animal timing. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:184–198. doi: 10.1037/0097-7403.20.2.184. [DOI] [PubMed] [Google Scholar]
- Catania AC. Reinforcement schedules and psychophysical judgements: A study of some temporal properties of behavior. In: Schoenfeld WN, editor. The theory of reinforcement schedules. New York: Appleton-Century-Crofts; 1970. pp. 1–42. [Google Scholar]
- Church RM. The internal clock. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 277–310. [Google Scholar]
- Church RM. Short-term memory for time intervals. Learning and Motivation. 1980;11:208–219. doi: 10.1016/0023-9690(80)90013-2. [DOI] [Google Scholar]
- Church RM. Properties of an internal clock. In: Gibbon J, Allan LG, editors. Annals of the New York Academy of Sciences: Timing and time perception. Vol. 423. New York: New York Academy of Sciences; 1984. pp. 566–582. [DOI] [PubMed] [Google Scholar]
- Fortin C. Attentional time-sharing in interval timing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. 235–260. [Google Scholar]
- Gallistel CR. The organization of behavior. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psychological Review. 1977;84(3):279–325. doi: 10.1037/0033-295X.84.3.279. [DOI] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. In: Gibbon J, Allan LG, editors. Timing and time perception. Vol. 423. New York: The New York Academy of Sciences; 1984. pp. 52–77. [DOI] [PubMed] [Google Scholar]
- Kaiser DH, Zentall TR, Neiman E. Timing in pigeons: Effects of the similarity between intertrial interval and gap in a timing signal. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(4):416–422. doi: 10.1037/0097-7403.28.4.416. [DOI] [PubMed] [Google Scholar]
- Lejeune H. Switching or gating? The attentional challenge in cognitive models of psychological time. Behavioural Processes. 1998;44:127–145. doi: 10.1016/S0376-6357(98)00045-X. [DOI] [PubMed] [Google Scholar]
- Marquardt DW. An algorithm for least squares estimation of parameters. Journal of the Society of Industrial and Applied Mathematics. 1963;11:431–441. doi: 10.1137/0111030. [DOI] [Google Scholar]
- Matell MS, Meck WH. Reinforcement-induced within trial resetting of an internal clock. Behavioral Processes. 1999;45:157–171. doi: 10.1016/S0376-6357(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Meck WH. Attentional bias between modalities: effect on the internal clock, memory, and decision stages used in animal time discrimination. Ann N Y Acad Sci. 1984;423:528–541. doi: 10.1111/j.1749-6632.1984.tb23457.x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacology Biochemistry and Behavior. 1986;25(6):1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Brain Research and Cognitive Brain Research. 1996;3(3-4):227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci. 1984;98(1):3–22. doi: 10.1037/0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- MED-Associates. WMPC software (Version 1.15) St. Albans, VT: 1999. [Google Scholar]
- Oprisan SA, Buhusi CV. Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Front Integr Neurosci. 2011;5:52. doi: 10.3389/fnint.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:242–268. doi: 10.1037/0097-7403.7.3.242. [DOI] [PubMed] [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:318–337. doi: 10.1037/0097-7403.4.4.318. [DOI] [Google Scholar]
- Roberts WA, Cheng K, Cohen JS. Timing light and tone signals in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:23–35. doi: 10.1037/0097-7403.15.1.23. [DOI] [PubMed] [Google Scholar]
- Sherburne LM, Zentall TR, Kaiser DH. Timing in pigeons: The choose-shortb effect may result from a confusion between delay and intertrial intervals. Psychonomic Bulletin and Review. 1998;5:516–522. doi: 10.3758/BF03208831. [DOI] [Google Scholar]
- Staddon JER, Cerutti DT. Operant behavior. Annual Review of Psychology. 2003;54:114–144. doi: 10.1146/annurev.psych.54.101601.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearingen JE, Buhusi CV. The pattern of responding in the peak-interval procedure with gaps: an individual-trials analysis. J Exp Psychol Anim Behav Process. 2010;36(4):443–455. doi: 10.1037/a0019485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EAC, Weaver WB. Cognitive processing and time perception. Perception and Psychophysics. 1975;17:363–367. doi: 10.3758/BF03199347. [DOI] [Google Scholar]
- Thorpe CM, Petrovic V, Wilkie DM. How rats process spatiotemporal information in the face of distraction. Behavioral Processes. 2002;58:79–90. doi: 10.1016/S0376-6357(02)00062-1. [DOI] [PubMed] [Google Scholar]
- Zakay D. Subjective time and attentional resource allocation: An integrated model of time estimation. In: Levin I, Zakay D, editors. Time and human cognition: A life-span perspective. Amsterdam: North-Holland; 1989. pp. 365–397. [Google Scholar]
- Zakay D. Gating or switching? Gating is a better model of prospective timing (a repsonse to ‘switching or gating?’ by Lejeune) Behavioural Processes. 2000;50:1–7. doi: 10.1016/S0376-6357(00)00141-8. [DOI] [PubMed] [Google Scholar]