Abstract
The arterial endothelium is a complex organ that modulates vascular tone by release of various substances to control perfusion. Endothelial function reflects vascular ageing and health. Already at the earliest stages of atherosclerosis the delicate balance between arterial constriction and relaxation is disturbed. Therefore, non-invasive assessment of endothelial function is a means to identify patients at increased cardiovascular risk, even at levels of disease that cannot be identified with classical imaging techniques that depict arterial wall and/or lumen or with functional assessment of ischaemia. Currently, there is an increasing interest in the early recognition of endothelial dysfunction to streamline and optimise preventive therapeutic measures. In this article, several methods for the assessment of endothelial function are briefly reviewed. In particular, we discuss the fast bed-side assessment of endothelial function by the reactive hyperaemia peripheral arterial tonometry (RH-PAT) method.
Keywords: Endothelium, Endothelial function, Atherosclerosis, Diagnostic tests
Introduction
The endothelium is a huge and complex organ that modulates vascular tone to control the perfusion of tissues and organs. Already at the very early stages of atherosclerosis, the delicate balance between arterial constriction and relaxation is disturbed. Therefore, at such levels of disease which cannot be identified with classical imaging techniques or functional assessment of ischaemia, non-invasive assessment of endothelial function is a means to identify patients at increased cardiovascular risk. Currently, there is an increasing interest in the early recognition of endothelial dysfunction to streamline and optimise preventive therapeutic measures. In this article, we discuss (patho)physiological aspects of the endothelium and briefly review several methods for the assessment of endothelial function with a particular focus on the novel reactive hyperaemia peripheral arterial tonometry (RH-PAT) method.
Function and dysfunction of the endothelium
The arterial endothelium is the inner layer of the vascular wall. Its acts not only as a barrier between blood and the subintima, but is a highly productive layer of cells. A simple experiment performed by Robert Furchgott et al. [1] showed that endothelial cells play an important role in regulating vascular tone: in this experiment, the endothelial layer of isolated arteries was removed before exposing the arteries to acetylcholine, which did not lead to vasodilatation. This experiment demonstrated that the endothelial layer is required for the local control of the vasomotor tone.
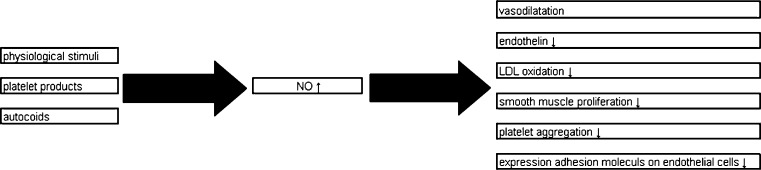
The endothelium produces endothelium-derived relaxing factor (EDRF), endothelium derived hyperpolarising factors (EDHFs) and endothelium-derived contracting factors (EDCFs) to regulate perfusion. In native endothelial cells, physiological stimuli (e.g. catecholamines, vasopressin, aldosterone), platelet products (e.g. serotonin, adenosine diphosphate) and autocoids such as histamine and bradykinin cause nitric oxide (NO) release. NO is an anti-atherosclerotic agent that causes vasodilatation, inhibits production of endothelin (a powerful vasoconstrictor), prevents LDL oxidation, and limits smooth muscle proliferation. In addition, it reduces platelet aggregation and prevents the adhesion and penetration of white blood cells into the vascular wall by inhibiting expression of adhesion molecules on endothelial cells (Fig. 1).
Fig. 1.
Physiological role of nitric oxide in healthy endothelial cells
Under certain conditions the bioavailability of NO is reduced, such as exposure to cigarette smoke, hypercholesterolaemia, obesity, sleep apnoea, arterial hypertension, diabetes mellitus, and chronic heart failure. The reduced bioavailability of NO causes an inflammatory response and can lead to endothelial dysfunction—the basic condition on which atherosclerosis develops.
Anderson et al. [2] and Ludmer et al. [3] were the first to evaluate endothelial function in the coronary circulation. During local infusion of acetylcholine, they measured the dilatation of coronary arteries on angiography. Acetylcholine causes NO release from healthy endothelial cells but causes vasoconstriction in vascular segments with endothelial dysfunction through stimulation of muscarine receptors. Later Drexler et al. [4] refined this study by measuring coronary flow velocity and coronary resistance with intracoronary Doppler wires. Several other studies used the same technique to measure the endothelial response to a wide range of other stimuli (including cold pressor testing) and reached the same conclusions.
Non-invasive assessment of endothelial function: rationale and early experience
Tests of endothelial function measure endothelial vasodilatation in response to pharmacological agents (e.g. acethylcholine or adenosine) or to physiological stimuli such as ischaemia induced by transient arterial occlusion. Several studies have shown that peripheral endothelial dysfunction correlates well with coronary endothelial dysfunction. Accordingly, peripheral endothelial function may be considered as a surrogate marker of coronary endothelial function. As a consequence, non-invasive techniques have been developed to measure the extent and severity of peripheral endothelial dysfunction at a single point in time or during serial studies with repetitive examinations.
The purpose of such tests is to assess vascular health in large populations of subjects. Non-invasive techniques measuring endothelial dysfunction may identify patients at risk of developing premature atherosclerosis at a stage where lifestyle changes or medical interventions could prevent or postpone clinical events. Such examinations may be particularly valuable as there is a relation between impaired endothelium-dependent vasodilatation and future cardiovascular events, as demonstrated by Suwaidi et al. [5] Halcox et al. [6] even showed that an impaired endothelial function is an independent risk factor of cardiovascular events.
Celermajer et al. [7, 8] was one of the first to perform peripheral flow-mediated dilatation studies. He used ultrasound scanning of the brachial and femoral arteries at baseline, during reactive hyperaemia, and after sublingual application of nitroglycerine. He documented that there was a lower flow-mediated vasodilatation in hypercholesterolaemic study subjects, in adult smokers, and in subjects with known coronary artery disease.
Mancini et al. [9] and Anderson et al. [10] showed that endothelial dysfunction can be reversed by angiotensin-converting enzyme inhibitors and statin therapy.
While arterial ultrasound assessment, as used in the aforementioned studies, requires training and experience of the operators to provide reliable and reproducible data, reactive hyperaemia peripheral artery tonometry (RH-PAT) is an operator-independent method.
Peripheral arterial tonometry
The RH-PAT method measures endothelial function by assessing the finger pulse wave amplitude (PWA) with the EndoPAT-2000 sensing device and finger plethysmographic probes (Itamar Medical, Caesarea, Israel). The absence of major muscle mass and the good perfusion of the finger make it a suitable object for measuring changes in volume of blood flow.
With an inflatable finger cuff, the vasculature of the distal segment of the index finger is compressed to avoid blood pooling and to assure even distribution of pressure along that segment (study arm). To control for systemic effects, a cuff is placed on the contralateral finger experiencing no hyperaemia (control arm).
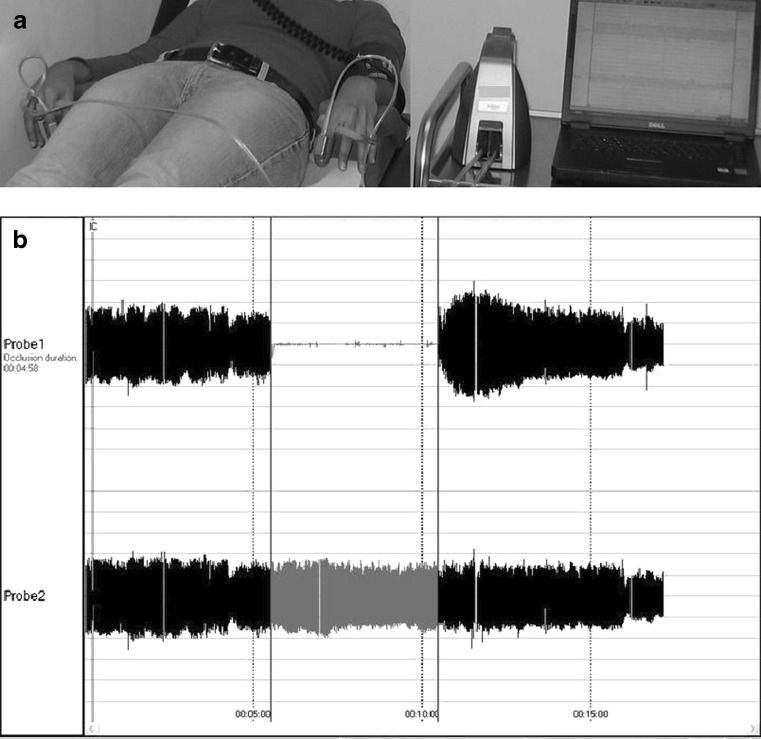
Probes on the index fingers of both hands (study and control arm) are inflated to a pressure of 10 mmHg below the diastolic pressure, while sensors integrated in the cuffs continuously record pressure in both fingers. After an acclimatisation period of 10 min in a room controlled for temperature and light, baseline measurements are recorded during a period of 5 min prior to inducing an ischaemic stimulus: a blood pressure cuff on the upper arm (study arm) is inflated for 5 min to suprasystolic pressures. This leads to NO release from the endothelium and consecutively to vasodilatation that is recorded by the sensors in the finger cuff through beat-to-beat finger pulsed wave analysis (PWA). Signals from both cuffs (study and control arm) are graphically displayed on a computer screen after amplification and filtering. After release of the blood pressure cuff from the upper arm (study arm), the ratio of the pulse amplitude to baseline of the hyperaemic finger is calculated for each 30-second interval. Consecutively, that ratio is divided by the corresponding ratio obtained in the control arm (Fig. 2).
Fig. 2.
RH-PAT testing. Finger probes are placed on both hands and attached to the device by sealed pneumatic tubing. The beat to beat plethysmographic data are displayed. The upper tracing is from the side undergoing hyperaemia and the lower tracing is from the contralateral arm
Nohria et al. [11] demonstrated that the hyperaemic response in healthy volunteers in the RH-PAT assessment depends on endothelial NO release, which occurs between 60 to 120 s after deflation of the blood pressure cuff. Recently, Hamburg et al. [12] showed that the maximum hyperaemic response can be measured 90 to 120 s after cuff deflation. The reactive RH-PAT index is calculated as the ratio of the mean hyperaemic PWA over a period of 1 min, beginning at 60 s after cuff deflation, divided by the baseline PWA (mean baseline measurements during 3.5 min) and normalised to the concurrent measurements from the control arm. By use of ROC curve analysis, an RH-PAT index of 1.35 was identified as the best threshold to discriminate between normal and abnormal endothelial function with a sensitivity and specificity for detection of coronary endothelial dysfunction of 80% and 85%, respectively.
Peripheral arterial tonometry and vascular function
Bonetti et al. [13] measured coronary endothelial dysfunction invasively with acetylcholine and compared measurements with data obtained from RH-PAT, demonstrating a lower RH-PAT index in patients with proven coronary endothelial dysfunction.
A direct correlation with ultrasound assessment of flow-mediated dilatation (FMD) of the brachial artery—a well-established endothelial function test—was reported by Kuvin et al., who examined 89 patients by both brachial FMD and RH-PAT [14]. This study revealed a significant correlation between brachial FMD and RH-PAT index (r = 0.55, P < 0.0001). This and other studies show that the RH-PAT index correlates with the number of cardiovascular risk factors, similar to relations that have been shown for brachial artery FMD [14, 15]
Hamburg et al. [12] assessed the relation between traditional and metabolic risk factors and RH-PAT in 1957 study subjects from the Framingham Third Generation Cohort Study; they found a lower RH-PAT index to be related with diabetes mellitus, increased body mass index, hypercholesterolaemia, smoking and increased age, but not to arterial hypertension. The aforementioned studies established RH-PAT as a valuable method for the assessment of endothelial function, even in large study cohorts. The method is currently being used in various studies, among others a subpopulation of the RESPONSE trial [16, 17], at the Thoraxcentrum Twente in Enschede.
Advantages and limitations of RH-PAT
Assessment with RH-PAT has advantages over the ultrasound assessment of brachial FMD (Table 1). It requires minimal training and is largely operator independent as automated calculation of RH-PAT index minimises interoperator variability. The day to day variability is small, as reported by Bonetti et al. [18] in a study including 28 patients with coronary artery disease. Reisner et al. [19] also preformed a similar study in which 113 healthy volunteers underwent vascular function testing on two consecutive days under similar controlled conditions. The mean hyperaemia indices were 0.56 ± 0.38 and 0.54 ± 0.37 respectively for days 1 and 2 (p = 0.53). Repeated RH-PAT measurements, performed 4 weeks apart by Haller et al. [20] in 44 children with diabetes mellitus, demonstrated an acceptable reproducibility (p = 0.0025).
Table 1.
Peripheral arterial tonometry: advantages and disadvantages
| Advantages | Disadvantages |
|---|---|
| • Non-invasive technique is applied without risk for patients | • Autonomic nervous system and fever could influence measurements |
| • Compact analysis system can be used in ambulatory settings | • Environmental conditions (e.g. room temperature) could be of influence |
| • Highly reproducible measurements are obtained | • Pulsatile changes (and not volume changes) are measured |
| • Minimal operator training is required | • Sensitive probes are required (single-use) |
The vascular tone of the fingers is highly responsive to the state of the autonomous nervous system as well as the temperature of the study room. Therefore, RH-PAT examinations have to be performed in a well-controlled environment (study room). However, there is limited knowledge about the effect of circadian variation or food intake on RH-PAT measurements.
Conclusions
Endothelial function reflects vascular ageing and health, and can be studied non-invasively to identify patients at increased risk of cardiovascular events. As RH-PAT is an operator-independent, highly reproducible method that correlates well with other established non-invasive methods for the assessment of endothelial function, it may facilitate surrogate endpoint studies that address changes in endothelial function.
Acknowledgments
Conflict of interest & funding
None
References
- 1.Vanhoutte PM, Shimokawa H, Tang EHC, et al. Endothelial function and vascular disease. Acta Physiol. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HV, Roubin GS, Leimgruber PP, et al. Measurement of transtenotic pressure gradient during percutaneous transluminal coronary angioplasty. Circulation. 1986;73:1223–1230. doi: 10.1161/01.CIR.73.6.1223. [DOI] [PubMed] [Google Scholar]
- 3.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 4.Drexler H, Zeiher AM, Wollschlager H, et al. Flow-dependent coronary artery dilatation in humans. Circulation. 1989;80:466–474. doi: 10.1161/01.CIR.80.3.466. [DOI] [PubMed] [Google Scholar]
- 5.Suwaidi JA, Hamasaki S, Higano ST, et al. Long term follow up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 6.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilatation in systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 9.Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) study. Circulation. 1996;94:258–265. doi: 10.1161/01.CIR.94.3.258. [DOI] [PubMed] [Google Scholar]
- 10.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 11.Nohria A, Gerhard-Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonetti PO, Pumper GM, Higano ST, et al. Non-invasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial dysfunction with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 15.Kuvin JT, Mammen A, Mooney P, et al. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 16.Jørstad HT, Alings AMW, Lim AH, et al. RESPONSE study: randomised evaluation of secondary prevention by outpatient nurse specialists. Study design, objectives and expected results. Neth Heart J. 2009;17:322–328. doi: 10.1007/BF03086277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jørstad HT, von Birgelen C, Alings M, et al. Effect of a nurse coordinated prevention program on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE trial. 2010—submitted. [DOI] [PMC free article] [PubMed]
- 18.Bonetti PO, Barness GW, Keelan PC, et al. Enhanced external counterpulsation for ischemic heart disease: what’s behind the curtain? J Am Coll Cardiol. 2003;41:1918–1925. doi: 10.1016/S0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- 19.Reisner Y, Lusky R, Shay-El Y, et al. Reproducibility of endothelial function and arterial stiffness using finger peripheral arterial tonometry. Eur Heart J. 2008;29(suppl 1):255–503. [Google Scholar]
- 20.Haller MJ, Stein J, Shuster J, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type I diabetes. Pediatr Diabetes. 2007;8:193–198. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]