Abstract
Older individuals have impaired balance control, particularly those that are frail and/or have sensory deprivations. Obese individuals show faster body sway during upright stance than normal weight individuals, suggesting that they also have difficulty controlling balance even if they do not have the same sensory issues as the older people. Therefore, the objective of this study was to examine if obesity is associated to a decreased balance control in older women. Postural sway of normal weight (n = 15, age = 70.8 ± 5.5 years; BMI = 22.2 ± 1.9 kg/m2), overweight (n = 15, age = 71.7 ± 4.3 years; BMI = 27.3 ± 1.3 kg/m2), and obese (n = 15, age = 71.1 ± 4.3 years; BMI = 33.1 ± 3.4 kg/m2) women was measured with a force platform for normal quiet stance lasting for 30 s in opened and closed eyes conditions. The obese group oscillated at a faster speed than the normal weight group (vision 0.99 ± 0.29 cm/s vs. 0.70 ± 0.16 cm/s, p < 0.01; no vision 1.43 ± 0.50 cm/s vs. 0.87 ± 0.23 cm/s, p < 0.01). The obese group exhibited greater range in both axes without vision compared to the normal weight group (p < 0.05). When observing sway density parameters, the obese group also spent less time in stability zones (2 mm radius area in which the center of pressure is relatively stable), and the distance between these stability zones are greater than the normal weight group in both visual conditions (p < 0.01 and p < 0.05, respectively). Obesity clearly affects postural control in older women. Our results suggest that obesity has a negative impact on the capacity of older woman to adequately use proprioceptive information for posture control. As postural instability or balance control deficits are identified as a risk factor for falling, our results also suggest that obesity in older women could be considered as another potential contributing factor for falling.
Keywords: Posture, Balance control, Postural stability, Elderly, Older women, Obesity
Introduction
Human standing is a fundamental part of activities of daily living and is essentially characterized by sway of the whole body about the ankle joints (Loram et al. 2001). Maintaining upright balance is controlled primarily by the calf muscle that counteracts the destabilizing effect of gravity (Johansson et al. 1988). Recent reports indicate that passive stiffness and open loop mechanisms contribute to the generation of the muscle activity required for stance control (Loram and Lakie 2002) which depends on a coordinated effort of the sensory systems (visual, vestibular, and proprioceptive systems). Deficits in these systems result in impaired balance control. A significant body of work exists that shows older individuals have reduced balance control compared to young individuals (Prieto et al. 1996; Maki et al. 1994; Maki et al. 1990; Brauer et al. 2000; Thapa et al. 1996; Meizer et al. 2004; Gill et al. 2001; Piirtola and Era 2006; Laughton et al. 2003). These differences are normally attributed to deficits in the visual or sensorimotor systems (Prieto et al. 1996; Choy et al. 2003), and this has been clearly identified as a risk factor for falls (Horak et al. 1989; Maki et al. 1994; Muir et al. 2010).
Obese and overweight individuals have reduced functional abilities compared to normal weight individuals (Vincent et al. 2010). However, a previous study has shown that this basic functional ability is negatively affected by weight. Indeed, a strong relationship (R2 = 0.52) between weight and postural instability has been observed (Hue et al. 2007). Hue et al. (2007) observed that young individuals who are obese and overweight swayed at a faster velocity than normal weight individuals. More tellingly, when obese and overweight individuals lost weight, their balance control improved, and a strong relationship (R2 = 0.65) was noted between the amount of weight lost and the improvement in balance control (Teasdale et al. 2007). There are two proposed hypotheses for explaining the detrimental effect of weight on balance control. The first is a reduced plantar sensitivity from a hyperactivation of the plantar mechanoreceptors due to the continuous pressure of supporting a large mass (Hue et al. 2007). The second hypothesis is a greater mechanical demand due to the large body mass per se and a nonnegligible proportion of body mass further away from the axis of rotation (i.e., ankle joint assuming an inverted pendulum model) that causes a greater gravitational torque. Consequently, to maintain upright stance, this gravitational torque that accelerates the body must be countered by muscular torques (Corbeil et al. 2001; Simoneau and Corbeil 2005).
Combining the mechanical constraints and the reduced plantar mechanoreceptor sensitivity of obese individuals with the known altered sensory capabilities of older persons, we hypothesized that older overweight or obese individuals would have even greater difficulty controlling balance than normal weight older persons. Because balance control disorders have been identified as a risk factor of falling (Sturnieks et al. 2008), the objective of this study was to examine if obesity is associated to a decreased postural stability in older women.
Method
Forty-five older women aged between 65 and 80 years were separated into three groups: 15 normal weight subjects (BMI < 25 kg/m2), 15 overweight subjects (25 < BMI < 30 kg/m2), and 15 obese subjects (BMI > 30 kg/m2). Participants in this project were recruited by contacting local community social groups. Interested persons were encouraged to contact the principal investigator. All participants completed a questionnaire designed to verify their admissibility in the project. The questionnaire was developed for use in the kinesiology, podiatric and chiropractic clinics at the University of Québec at Trois-Rivières. The questionnaire contained elements to screen for exclusion of the following items: diabetes, musculoskeletal disorders, neurological disorders, cancer, 6-month postoperative condition, medication use, and cognitive dementia. The mini-mental state examination was used to verify that none of the participants suffered from mild cognitive dementia indicated by a score below 24 (Folstein et al. 1975; Tombaugh and McIntyre 1992).
All participants gave their written informed consent to participate in this study, which was approved by the University of Québec at Trois-Rivières Ethics Committee.
Balance control was evaluated with a force platform (model no. FP4060, Bertec Corporation, Columbus, OH, USA). Subjects stood barefoot on the platform with their feet 10 cm apart. Before the first trial, the position of the feet was traced on a sheet of paper secured to the platform. The subjects were asked to maintain a stable posture while fixating a reference point located at eye level (5 m in front of them). The arms were held alongside the body. They performed seven trials with vision and seven trials without vision (eyes closed). All trials lasted for 30 s and were initiated with the eyes opened. For the no-vision condition, an auditory signal, presented 5 s before the 30-s trial, indicated to the subject to close their eyes. Visual conditions were randomly presented. Subjects were able to rest midway through the experiment. An assistant helped throughout the session to ensure that procedures were adequately followed and that foot position was constant across all trials.
Anteroposterior (AP) and mediolateral (ML) coordinates of the center of pressure (CoP) were determined from the ground reaction forces recorded at 500 Hz (12-bit A/D conversion). Before computing the CoP displacement, the force data were digitally filtered (Butterworth fourth-order, 7 Hz low-pass cut-off frequency with dual pass to remove phase shift). To evaluate the ability of the participants to control their balance, CoP speed and the range of CoP displacement along both axes (Range AP and Range ML, respectively) were measured. The mean speed of the CoP corresponds to the cumulative distance over the sampling period (CoP speed). The range of the CoP displacement represents the difference between the maximum and minimum values of the CoP along the AP and ML axes. CoP speed constitutes a good index of activity required to maintain stability (Maki et al. 1994; Geurts et al. 1993) with a faster speed indicating a less stable individual. It is considered as a sensitive and discriminate variable of stability (Baratto et al. 2002; Raymakers et al. 2005).
In addition, two other parameters that are derived from a sway density plot approach, mean peaks and mean distances, were computed (Baratto et al. 2002). The mean peaks correspond to time instants in which the CoP is relatively stable and the mean distance corresponds to the distance between stability zones. Consequently, larger mean peaks and shorter mean distance between peaks indicate a more stable CoP. The discriminative power of these two sway density parameters, together with the mean speed, is greater than that of other global parameters (for instance, range of the CoP and RMS values) to distinguish among sensory and pathological conditions in the general framework of balance control (Baratto et al. 2002). All computations were performed using Matlab 7.0 (The MathWorks, Natick, MA, USA).
Statistica software 7.0 (Statsoft, Inc, Tulsa, OK, USA) was used for all analyses. The Kolmogorov–Smirnov test was used to verify if all data were normally distributed. Analyses of variance (ANOVA) were used to compare groups for age, body height, body weight, and BMI. Balance control measures were analyzed by an ANOVA with a Group (three groups: normal weight/overweight/obese) × Vision (two conditions: vision/no vision) design with repeated measures on the last factor. All results were considered to be significant at the 5% critical level (p < 0.05).
Results
Anthropometric characteristics
The anthropometric characteristics of the three groups of participants are presented in Table 1. Body weight and BMI were significantly higher in obese than normal weight participants. Values for each variable were also significantly greater for obese than overweight participants.
Table 1.
Group characteristics
| Normal weight n = 15 | Overweight n = 15 | Obese n = 15 | |
|---|---|---|---|
| Age (years) | 70.8 ± 5.5 [65–80] | 71.7 ± 4.3 [65–79] | 70.6 ± 4.3 [65–77] |
| Height (cm) | 157.0 ± 4.1 [150–164] | 158.0 ± 6.1 [142–165] | 158.0 ± 7.1 [146–173] |
| Weight (kg) | 54.5 ± 5.9 [45–64]†§ | 67.9 ± 5.6 [57.5–77]*§ | 82.4 ± 11.3 [71–109.5]*† |
| BMI (kg/m²) | 22.2 ± 1.9 [18.9–24.8]†§ | 27.3 ± 1.3 [25.5–29.1]*§ | 33.1 ± 3.4 [30.1–40.9]*† |
Values are means ± SD [min–max]
* p < 0.001, significantly different with normal weight
† p < 0.001, significantly different with overweight
§ p < 0.001, significantly different with obese
CoP speed
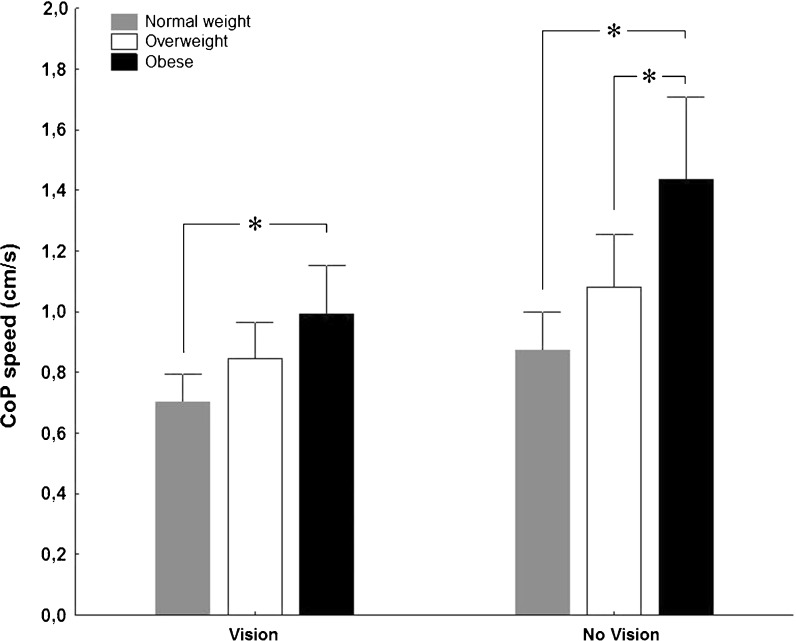
The main objective of this study was to determine if an excessive weight is associated to a decreased postural stability in older women. Figure 1 illustrates the effect of overweight and obesity on the speed of the postural sway (CoP speed) for the vision and no-vision conditions. Results of the ANOVA showed an interaction between groups and vision conditions (F(2, 42) = 9.69, p < 0.001), a main effect of group (F(2, 42) = 8.17, p < 0.01), and a main effect of vision condition (F(1, 42) = 115.20, p < 0.001). Regardless of the availability of vision, planned comparisons indicated that the obese group showed a greater CoP speed than the normal weight group (p < 0.01) and did not statistically differ from the overweight group.
Fig. 1.
CoP speed for normal weight, overweight, and obese groups. Asterisk indicates a significant difference (p < 0.05), error bars are ±95% confidence intervals
CoP ranges
The analysis of the CoP range along the AP axis revealed a main effect of vision (F(1, 42) = 68.80, p < 0.001) with all groups oscillating further (greater range) in the no-vision condition compared to the vision condition. There were no significant differences for the CoP range along the AP axis between the three groups (group effect F(2, 42) = 2.66, p < 0.09). For the ML axis, an interaction between groups and vision conditions (F(2, 42) = 5.37, p < 0.01) was revealed by the ANOVA. The magnitude of the postural sway of all groups was similar along the ML axes (group effect F(2, 42) = 1.70, p < 0.20). However, the analysis revealed a main effect of vision (F(1, 42) = 17.25, p < 0.001), and the planned comparisons indicated that the obese group showed greater oscillations along the ML axis than the normal weight group (p < 0.05). Values of CoP range along the AP and ML axes for both visual conditions are presented in Table 2.
Table 2.
Postural sway measures
| Postural parameters | Normal weight n = 15 | Overweight n = 15 | Obese n = 15 |
|---|---|---|---|
| Vision | |||
| CoP speed (cm/s) | 0.70 ± 0.16 [0.47–0.93] | 0.85 ± 0.21 [0.54–1.21] | 0.99 ± 0.29 [0.58–1.49]† |
| ML range (cm) | 0.82 ± 0.37 [0.35–1.85] | 0.85 ± 0.28 [0.51–1.45] | 0.96 ± 0.46 [0.41–1.87] |
| AP range (cm) | 1.59 ± 0.43 [0.98–2.36] | 1.84 ± 0.58 [0.86–2.86] | 1.87 ± 0.39 [1.25–2.60] |
| Mean distance (cm) | 0.27 ± 0.07 [0.19–0.42] | 0.31 ± 0.08 [0.20–0.43] | 0.33 ± 0.10 [0.21–0.52]† |
| Mean peaks (s) | 2.50 ± 0.56 [1.87–3.54] | 2.21 ± 0.48 [1.56–3.10] | 1.92 ± 0.44 [1.32–2.88]† |
| No vision | |||
| CoP speed (cm/s) | 0.87 ± 0.23 [0.56–1.30]* | 1.08 ± 0.31 [0.55–1.60]* | 1.43 ± 0.50 [0.75–2.42]*†§ |
| ML range (cm) | 0.88 ± 0.31 [0.51–1.66] | 0.92 ± 0.29 [0.52–1.54] | 1.27 ± 0.77 [0.47–3.05]*† |
| AP range (cm) | 1.81 ± 0.44 [1.23–2.49]* | 2.11 ± 0.53 [1.12–3.16]* | 2.31 ± 0.55 [1.46–3.11]*† |
| Mean distance (cm) | 0.33 ± 0.09 [0.22–0.48]* | 0.37 ± 0.09 [0.20–0.53]* | 0.46 ± 0.13 [0.28–0.67]*†§ |
| Mean peaks (s) | 2.15 ± 0.53 [1.44–3.17]* | 1.85 ± 0.49 [1.24–2.87]* | 1.47 ± 0.40 [0.90–2.19]*†§ |
Values are expressed by mean ± SD [min–max]
ML mediolateral, AP anteroposterior
* p < 0.05, significantly different between vision and no vision
† p < 0.05, significantly different between normal weight and obese groups
§ p < 0.05, significantly different between overweight and obese groups
Sway density parameters
The analysis of the mean distance revealed an interaction between groups and vision conditions (F(2, 42) = 7.00, p < 0.01), a main effect of group (F(2, 42) = 4.37, p < 0.05), and a main effect of vision condition (F(1, 42) = 114.06, p < 0.001). Planned comparisons indicated that all groups have more distance between two stability zones (greater distance) in the no-vision condition compared to the vision condition. Concerning mean peaks, there was a main effect of group (F(2, 42) = 6.90, p < 0.01) and a main effect of vision condition (F(1, 42) = 100.98, p < 0.001). Planned comparisons indicated that the obese group spent less time than the normal weight group in their stability zones (p < 0.01) with and without vision. Values of the mean distance and the mean peaks for both visual conditions are presented in Table 2.
Discussion
The objective of this study was to assess if obesity negatively affects balance control in older women. This was a cross-sectional study and no intervention was performed with the participants. Our results show that obese older women had increased CoP speed, which could be interpreted as decreased postural stability.
Even during quiet stance, continuous adjustments of muscle activity and joint position occur in anticipation and response to the integration of sensory information from visual, vestibular, and somatosensory inputs, all of which contribute to postural balance (Horak et al. 1989; Peterka 2002; Winter 1995). For balance control in current study, there was a main interaction effect between group and vision conditions with planned comparisons revealing that the obese group was significantly more affected by the no-vision condition than the normal weight group. All older women had systematically higher values for each calculated CoP parameter while standing upright with eyes closed versus eyes open, indicating that the removal of vision had a more pronounced effect on postural balance performance. This effect of vision is more destabilizing for the obese group, especially on the ML axis. Returning to our hypotheses, this result supports the notion that there is a sensorial component to balance control that is important in maintaining stability. It is well known that when vision (or other sensory information) is removed during maintenance of normal quiet stance, there is a sensorial reweighting that occurs and other modalities (proprioceptive) are tuned in order to compensate and maintain postural stability (Peterka 2002; Teasdale and Simoneau 2001). The obese individuals swayed at a faster speed in the same boundary compared to the normal weight group as shown by the group effect for CoP speed and the lack of significant difference in CoP ranges. In addition, it should be noted that the overweight group swayed more than the normal weight group; however, this difference was not significant (i.e., Table 2). However, we have previously demonstrated that postural stability is strongly related to the amount of body weight (Hue et al. 2007; Teasdale et al. 2007). Therefore, in this study, even if our overweight group is not significantly different from the other groups, as can be clearly seen in Fig. 1, there is a linear trend between postural stability and body weight. The sway density parameters (mean peaks and mean distance) illustrate how the CoP behaves within the sampling period. For these variables, there was a significant difference between the obese group and the normal weight group, the CoP of the obese group sways further between stability zones and spends less time in these zones. The overweight group tended towards this behavior, although it was not statistically significant. There are several possible reasons why the observed distance between stability zones is greater, and the time spent in each is less in the obese group compared to the normal weight group. It could be the effect of an increased inertia (proportional to the mass); it might also be due to an increased muscular effort associated with controlling the larger mass. A reduced balance control is observed with aging and is related to an increase in the CoP speed. This has been associated with increased risk of falling (Horak et al. 1989; Maki et al. 1994; Brauer et al. 2000; Piirtola and Era 2006; Muir et al. 2010). Older indoor fallers have been identified by a reduced balance control measured with a force platform during normal quiet stance (Pajala et al. 2008). These authors reported two intrinsic risk factors for the indoor fallers—they were heavier than outdoor fallers and nonfallers (BMI 30.6 vs. 27.7 and 27.5, respectively) and also more medicated having, on average, 2.9 medications versus 1.9 for each of outdoor fallers and nonfallers. These authors noted the group differences (heavier and more medication), but they did not discuss the implications of BMI as a specific risk factor. Our force platform measures of balance control show that the obese group (mean BMI = 33.0 ± 2.8 kg/m2) swayed at a faster speed than the other groups. A study observed in 59 fallers (BMI = 25.3 ± 5.08, age 81.6 ± 6.6) has an average CoP speed in eyes open of 1.3 ± 1.37 cm/s and 1.79 ± 1.56 cm/s in eyes closed (Maki et al. 1994). In our experiment, CoP speed values of five obese participants in eyes open condition and nine obese participants in eyes closed condition were above these critical values. There are other factors that might also increase a risk of falling, for example, sarcopenia (muscle wasting) and dynapenia (muscular strength loss), have been observed in these populations, and this contributes to the deterioration in physical capacity (Baumgartner et al. 1998; Cruz-Jentof et al. 2010; Clark and Manini 2010; Visser 2011).
The effect of obesity on balance control has been previously observed and described elsewhere (McGraw et al. 2000; Maffiuletti et al. 2005; Hue et al. 2007; Greve et al. 2007; Teasdale et al. 2007; Menegoni et al. 2009; Handrigan et al. 2010), and the original contribution of this study is the demonstration of the added effect of obesity on balance control in older women aged 65–80 years old. Furthermore, despite the observed differences in balance control, it is important to note that these individuals were living independently. However, Bouchard et al. (2009) have shown that in 904 physically independent older individuals aged between 67 and 84 years old, a BMI of 30.5 kg/m2 in older women (i.e., in obese older women) was identified as a threshold above which a physical capacity score, reflecting a global picture of subjects’ lower limbs performance by taking into account several tasks related to daily activities (timed up and go, chair stand, walking speed at normal and fastest pace, and one leg stand), was significantly decreased compared with a reference normal weight group. This threshold was not observed for older men. In a study investigating the association between the amount of fat mass and balance control (one legged balance), a significant correlation was found (R = −0.25) (Bouchard et al. 2007). The one-legged balance test is a timed trial of standing on one leg with eyes open (Hurvitz et al. 2000; Bohannon et al. 1984) and frequently used in older individuals (Michikawa et al. 2009) even if a recent systematic review and meta-analysis reported a moderate success at identifying fallers in older individuals with this clinical test (Muir et al. 2010). However, the obese individuals had a higher prevalence of falling and ambulatory stumbling, as well as lower quality of life in multiple health domains than the normal weight counterparts (Fjeldstad et al. 2008). Also, situations that are cognitively challenging might place additional demands on maintaining balance to reduce the risk of fall, and this has been shown to be additionally detrimental for balance control in obese persons (Mignardot et al. 2010). In older persons, postural contexts requiring a reweighting of sensory inputs could lead to an increased risk for loss of balance and falls if insufficient attentional resources are allocated to the postural task (Teasdale and Simoneau 2001).
In conclusion, balance control is an essential prerequisite in daily life. Even though it is often considered a simple task, it is the basis for most movements we perform. The results of this present study indicate an association between obesity and balance control in older women. Obese older women showed more postural instability when compared to overweight and normal weight older women. An excessive amount of fat modifies the body geometry by adding passive mass to different regions (de Souza et al. 2005), and this influences the biomechanics of activities of daily living, causing functional limitations and possibly predisposing to injury (Wearing et al. 2006). Balance control is a fundamental part of activities of daily living, and our results clearly show a decreased postural stability in older obese women. It suggests that in independent older women, when balance control is required or challenged for the execution of daily living tasks, obesity combined with age could lead to greater functional limitations and increase the possibility to predispose older obese women to injury. As postural instability or balance control deficits are identified as a risk factor for falling (Horak et al. 1989; Maki et al. 1994), our results also suggest that obesity in older women could be considered as another potential contributing factor for falling.
Acknowledgments
GH was supported by the Newfoundland and Labrador Centre for Applied Health Research (NLCAHR) and Healthy Aging Research Program (NL-HARP). PC, MS and NT were supported by NSERC and FQRNT. OH and VC were supported by FIR from UQTR. All participants are gratefully acknowledged. Finally, special thanks to Marcel Kazsap for the programming expertise and technical support.
References
- Baratto L, Morasso PG, Re C, Spada G. A new look at posturographic analysis in the clinical context: sway density versus other parameterization techniques. Mot Control. 2002;6:246–270. doi: 10.1123/mcj.6.3.246. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallaher D, Romero L, Heymsfield SB, Ross RR, Garry PH, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Larkin PA, Cook AC, Gear J, Singer J. Decrease in timed balance test scores with aging. Phys Ther. 1984;64:1067–1070. doi: 10.1093/ptj/64.7.1067. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Beliaeff S, Dionne IJ, Brochu M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: nutrition as a determinant of successful aging (NuAge)—the Quebec longitudinal study. J Gerontol A Biol Sci Med Sci. 2007;62:1382–1388. doi: 10.1093/gerona/62.12.1382. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Dionne IJ, Payette H, Brochu M. Is there a BMI threshold value associated with a lower physical capacity in well-functioning older adults? The Quebec Longitudinal Study. Open Obes J. 2009;1:15–22. doi: 10.2174/1876823700901010015. [DOI] [Google Scholar]
- Brauer SG, Burns YR, Galley P. A prospective study of laboratory and clinical measures of postural stability to predict community-dwelling fallers. J Gerontol A Biol Sci Med Sci. 2000;55(8):M469–M476. doi: 10.1093/gerona/55.8.M469. [DOI] [PubMed] [Google Scholar]
- Choy NL, Brauer S, Nitz J. Changes in postural stability in women aged 20 to 80 years. J Gerontol A Biol Sci Med Sci. 2003;58:M525–M530. doi: 10.1093/gerona/58.6.M525. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil P, Simoneau M, Rancourt D, Tremblay A, Teasdale N. Increased risk for falling associated with obesity: mathematical modeling of postural control. IEEE Trans Neural Syst Rehabil Eng. 2001;9:126–136. doi: 10.1109/7333.928572. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentof AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis. Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza SA, Faintuch J, Valezi AC, Sant’ Anna AF, Gama-Rodrigues JJ, de Batista Fonseca IC, Souza RB, Senhorini RC. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15:1238–1242. doi: 10.1381/096089205774512627. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C, Fjeldstad AS, Acree LS, Nickel KJ, Gardner AW. The influence of obesity on falls and quality of life. Dyn Med. 2008;7(4):1–6. doi: 10.1186/1476-5918-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geurts AC, Nienhuis B, Mulder TW. Intrasubject variability of selected force-platform parameters in the quantification of postural control. Arch Phys Med Rehabil. 1993;74:1144–1150. [PubMed] [Google Scholar]
- Gill J, Allum JH, Carpenter MG, Held-Ziolkowska M, Adkin AL, Honegger F, Pierchalala K. Trunk sway measures of postural stability during clinical balance tests: effects of age. J Gerontol A Biol Sci Med Sci. 2001;56(7):M438–M447. doi: 10.1093/gerona/56.7.M438. [DOI] [PubMed] [Google Scholar]
- Greve J, Alonso A, Bordini AC, Camanho GL. Correlation between body mass index and postural balance. Clinics (Sao Paulo) 2007;62(6):717–720. doi: 10.1590/S1807-59322007000600010. [DOI] [PubMed] [Google Scholar]
- Handrigan GA, Corbeil P, Simoneau M, Teasdale N. Balance control is altered in obese individuals. J Biomech. 2010;43:383–384. doi: 10.1016/j.jbiomech.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Horak FB, Shupert CL, Mirka A. Components of postural dyscontrol in the elderly: a review. Neurobiol Aging. 1989;10:727–738. doi: 10.1016/0197-4580(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Hue O, Simoneau M, Marcotte J, Berrigan F, Doré J, Marceau P, et al. Body weight is a strong predictor of postural stability. Gait Posture. 2007;26:32–38. doi: 10.1016/j.gaitpost.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil. 2000;8:587–591. doi: 10.1016/S0003-9993(00)90039-X. [DOI] [PubMed] [Google Scholar]
- Johansson R, Magnusson M, Akesson M. Identification of human postural dynamics. IEEE Trans Biomed Eng. 1988;35:858–869. doi: 10.1109/10.7293. [DOI] [PubMed] [Google Scholar]
- Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18(2):101–108. doi: 10.1016/S0966-6362(02)00200-X. [DOI] [PubMed] [Google Scholar]
- Loram ID, Lakie M. Direct measurement of human ankle stiffness during quiet standing: the intrinsic mechanical stiffness is insufficient for stability. J Physiol. 2002;545:1041–1053. doi: 10.1113/jphysiol.2002.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram ID, Kelly SM, Lakie M. Human balancing of an inverted pendulum: is sway size controlled by ankle impedance? J Physiol. 2001;532:879–891. doi: 10.1111/j.1469-7793.2001.0879e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffiuletti NA, Agosti F, Projetti M, Riva D, Resnik M, Lafortuna CL, Sartorio A. Postural instability of extremely obese individuals improves after a body weight reduction program entailing specific balance training. J Endocrinol Invest. 2005;28(1):2–7. doi: 10.1007/BF03345521. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Fernie GR. A comparison of spontaneous and induced sway balance tests. J Am Geriatric Soc. 1990;38(1):1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.M72. [DOI] [PubMed] [Google Scholar]
- McGraw B, McClenaghan BA, Williams HG, Dickerson J, Ward DS. Gait and postural stability in obese and nonobese prepubertal boys. Arch Phys Med Rehabil. 2000;81(4):484–489. doi: 10.1053/mr.2000.3782. [DOI] [PubMed] [Google Scholar]
- Meizer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and nonfallers. Age Ageing. 2004;33(6):602–607. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- Menegoni F, Galli M, Tacchini E, Vismara L, Cavigioli M, Capodaglio P. Gender-specific effect of obesity on balance. Obesity. 2009;17(10):1951–1956. doi: 10.1038/oby.2009.82. [DOI] [PubMed] [Google Scholar]
- Michikawa T, Nishiwaki Y, Takebayashi T, Toyama Y. One-leg standing test for elderly populations. J Orthop Sci. 2009;14:675–685. doi: 10.1007/s00776-009-1371-6. [DOI] [PubMed] [Google Scholar]
- Mignardot JB, Olivier I, Promayon E, Nougier V (2010) Obesity impact on the attentional cost for controlling posture. PLoS One 20;5(12):e14387:1–6 [DOI] [PMC free article] [PubMed]
- Muir SW, Berg K, Chesworth B, Klar N, Speechley M. Quantifying the magnitude of risk for balance impairment on falls in community-dwelling older adults: a systematic review and meta-analysis. J Clin Epidemiol. 2010;63:389–406. doi: 10.1016/j.jclinepi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Pajala S, Era P, Koskenvuo M, Kaprio J, Törmäkangas T, Rantanen T. Force platform balance measures as predictors of indoor and outdoor falls in community-dwelling women aged 63–76 years. J Gerontol A Biol Sci Med Sci. 2008;63:171–178. doi: 10.1093/gerona/63.2.171. [DOI] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Piirtola M, Era P. Force platform measurements as predictors of falls among older people—a review. Gerontology. 2006;52(1):1–16. doi: 10.1159/000089820. [DOI] [PubMed] [Google Scholar]
- Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- Raymakers JA, Samson MM, Verhaar HJJ. The assessment of body sway and the choice of the stability parameter(s) Gait Posture. 2005;21:48–58. doi: 10.1016/j.gaitpost.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Simoneau M, Corbeil P. The effect of time to peak ankle torque on balance stability boundary: experimental validation of a biomechanical model. Exp Brain Res. 2005;165:217–228. doi: 10.1007/s00221-005-2290-1. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467–478. doi: 10.1016/j.neucli.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture. 2001;14(3):203–210. doi: 10.1016/S0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Teasdale N, Hue O, Marcotte J, Berrigan F, Simoneau M, Doré J, et al. Reducing weight increases postural stability in obese and morbid obese men. Int J Obes. 2007;31:153–160. doi: 10.1038/sj.ijo.0803360. [DOI] [PubMed] [Google Scholar]
- Thapa PB, Gideon P, Brockman KG, Fought RL, Raw WAS. Clinical and biomechanical measures of balance as fall predictors in ambulatory nursing home residents. J Gerontol A Biol Sci Med Sci. 1996;51(5):M239–M246. doi: 10.1093/gerona/51A.5.M239. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Visser M. Obesity, sarcopenia and their functional consequences in old age. Proc Nutr Soc. 2011;70(1):114–118. doi: 10.1017/S0029665110003939. [DOI] [PubMed] [Google Scholar]
- Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. The biomechanics of restricted movement in adult obesity. Obes Rev. 2006;7:13–24. doi: 10.1111/j.1467-789X.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]