Abstract
Estrogens are not only critical for sexual differentiation it is well-known for the role of 17β-estradiol (E2) in the adult brain modulating memory, learning, mood and acts as a neuroprotector. E2 exerts its actions through two classical receptors: estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). The distribution of both receptors changes from one brain area to another, E2 being able to modulate their expression. Among the classical features of aging in humans, we find cognitive impairment, dementia, memory loss, etc. As estrogen levels change with age, especially in females, it is important to know the effects of low E2 levels on ERα distribution; results from previous studies are controversial regarding this issue. In the present work, we have studied the effects of long-term E2 depletion as well as the ones of E2 treatment on ERα brain distribution of ovariectomized rats along aging in the diencephalon and in the telencephalon. We have found that ovariectomy causes downregulation and affects subcellular localization of ERα expression during aging, meanwhile prolonged estrogen treatment produces upregulation and overexpression of the receptor levels. Our results support the idea of the region-specific neuroprotection mechanisms mediated by estradiol.
Keywords: Hormonal therapy, Aging, Estrogen receptor alpha, Brain, Estradiol, Telencephalon, Diencephalon
Introduction
It is known that estrogens are not only hormones critical for sexual differentiation. The primary biologically active form of estrogens, the 17β-estradiol (E2), plays a paramount role in the brain. During development, the brain is exposed to estradiol from different sources, and the effects of this molecule are region-dependent, promoting, or preventing apoptosis, synaptogenesis, and affecting the morphology of neurons and astrocytes (for a review see McCarthy 2008). Estrogens are known trophic factors for neurons. Synapses remodeling is a phenomenon that fluctuates according to hormone levels during the estrous cycle (Olmos et al. 1987). Other areas where growth-promoting effects of the estrogens have been proven are the midbrain (Reisert et al. 1987), cortex (Garcia-Segura et al. 1989), pituitary (Chun et al. 1998), and spinal cord (VanderHorst and Holstege 1997).
In the adult brain, E2 modulates learning, memory, mood (McEwen 2002), and even auditory physiology (Tremere et al. 2009). Estrogens are also able to prevent neuronal death in several models of brain injury (Liu et al. 2010), to ameliorate brain inflammation (Pozzi et al. 2006), and to be implicated in many neurological disorders as Parkinson’s disease (Baraka et al. 2011) and Alzheimer’s disease promoting the nonamyloidogenic metabolism of the amyloid precursor protein (Jaffe et al. 1994).
Estrogens exert their actions mainly through two different receptors. Estrogen receptor alpha (ERα), the first to be discovered and cloned (Jensen 1962; Walter et al. 1985), is a 66-Kda protein in its most common form; estrogen receptor beta (ERβ) was discovered a decade later (Mosselman et al. 1996) but both of them present different splicing variants as well as natural occurring mutants.
In the rat brain, ERα is detected at high levels in areas related with sexual behavior as hypothalamic, preoptic, and limbic structures, but it is also found in olfactory regions, cerebellum, area postrema, and substantia gelatinosa of the spinal cord (Simerly et al. 1990; Laflamme et al. 1998; Belcher 1999). In spite of both classical receptors being expressed in the brain, ERβ seems to be distributed in a wider pattern (Belcher 1999; Laflamme et al. 1998; Osterlund et al. 1998, 2000). The general distribution of estrogen receptor (ER) mRNA throughout the brain is quite similar in different species, but the differences in the expression pattern are important enough for making data extrapolation inadequate. For example, human paraventricular nucleus expresses both classical receptors, with ERα being predominant, while only ERβ has been found in this area in the rat brain (Shughrue et al. 1997; Küppers and Beyer 1999; Gundlah et al. 2000; Osterlund et al. 2000).
Estrogen-mediated neuroprotection can take place through two different pathways. One way involves canonical ER activation, which is constitutively expressed in many brain regions and is able to initiate gene transcription after specifically binding to estradiol (Green and Simpkins 2000; Marin et al. 2005). The second way that has been growing interest and refers to the rapid non-genomic (or alternative) signaling pathways, involves extranuclear ER in response to physiological concentration of estrogens to elicit neuroprotection (Singer et al. 1999; Adams et al. 2002; Mendez et al. 2003; Simoncini et al. 2003). Recent studies point to a coupling between both mechanisms (for review see Vasudevan and Pfaff 2008).
Due to their neuroprotective effects, the use of estrogens has been proposed for the treatment of different neuropathologies as Alzheimer’s disease (Amtul et al. 2010), Parkinson’s disease (Baraka et al. 2011), ischemic stroke (Dang et al. 2011), schizophrenia (Kulkarni et al. 2010), and Huntington’s disease (Túnez et al. 2006). However, some epidemiological studies show no benefits on cognitive performance after hormone therapy (Coker et al. 2010; Mulnard et al. 2000; Shumaker et al. 2004), so the subject is very controversial.
It is well established that with increasing age, several aspects of brain function are impaired in rodents, non-human primates, and humans, including deficits in learning and memory, neurogenesis, neuronal, and synaptic density and alterations in dendritic architecture (Sherwin 1994, 1997, 2003). As we discussed above, ERα has a distribution that varies among the different cerebral areas studied, as well as with different situations. Because of the potential importance of this receptor in the mediating effects of changing estradiol levels with aging, many studies achieved the amount of global immunostaining and distribution of ERα in several brain areas, such as the hippocampus (Adams et al. 2002; Mehra et al. 2005) or hypothalamus (Funabashi et al. 2000). Furthermore, it was reported that not only is there an age regulation of the intensity of immunostaining, but also an evident influence of aging in the subcellular distribution of this receptor (Ishunina et al. 2000; Milner et al. 2001; Kalesnykas et al. 2005). In ovariectomized animals, the effect of the sexual hormones in the ERα level and its localization within the cell, as is described by many researchers has also been widely demonstrated (Adams et al. 2002; Wilson et al. 2002; Stirone et al. 2003).
Previous studies have produced conflicting results concerning the ability of estrogen to regulate ER protein expression in the brain. To elucidate the long-term effect of ovariectomy and 17β-estradiol treatment on the profile of ERα in the brain, we performed a study of the ERα distribution and quantified the protein signal in some selected brain areas using immunohistochemistry for ERα. These areas, cerebral cortex and diencephalon, are recognized targets of ovarian hormone stimulation. We have shown that ovariectomy causes downregulation and different subcellular localization of ERα expression during aging, meanwhile prolonged estrogen treatment produces upregulation and overexpression of the receptor levels.
Materials and methods
Animals
Virgin female Wistar rats (from the Biotery of the Faculty of Medicine, University of Oviedo), weighting 250–280 (age, 8–10 weeks), kept under standard conditions of temperature (23 ± 3°C) and humidity (65 ± 1 %), and a regular 12-h light, 12-h dark cycle (0800–2000 h) were used. The animals were fed with a standard diet (Panlab A04), and all of them had free access to water. All experimental manipulations were performed between 0930 and 1230 h. All experimental procedures which were carried out with the animals were approved by a local veterinary committee from the University of Oviedo vivarium, and subsequent handling strictly followed the European Communities Council Directive of November 24, 1986 (86/609/EEC).
Experimental design
The animals were ovariectomized through a midline incision under light anesthesia by inhalation of halothane. Ovariectomized rats were separated randomly into three groups: ovariectomized animals (OVX), ovariectomized animals treated with 17β-estradiol (E) and sham surgery animals (intact) (C), and housed individually throughout the experiment.
After surgery, all the rats began the experimental treatment exactly 1 week after ovariectomy to ensure a uniform time of estrogen depletion before replacement and to recover from surgery stress. After this, the rats were implanted subcutaneously in the posterior neck with 90-day-release 17β-estradiol pellets (25 μg/pellet; Innovative Research of America, Sarasota, FL) or placebos containing no estradiol. Every 90 days, the pellets were replaced. This dosing regimen has resulted in physiological levels of plasma estradiol and has been shown to be neuroprotective in rats (Gonzalez et al. 2000).
Groups (OVX, E, and C) were divided randomly into four subgroups (ten animals per subgroup): 6, 12, 18, and 24 (according to the month of the experimental period on which the animals were killed). Therefore, the animals were killed when they were approximately 8, 14, 20, and 26 months old. Moreover, 14 animals (seven OVX and seven C) were killed 1 week after ovariectomy (age, 9–11 weeks). Therefore, the animals included in this group did not receive any treatment and were considered as OVX-0 months and C-0 months groups.
The stage of the estrous cycle in intact rats was determined by daily examination of fresh vaginal smears. The intact animals in diestrous phase were selected for the 0 and 6 subgroups. After month 12 of the experiment, none of the intact rats showed repetitive estrous cycles, instead, 87.26 % of animals showed persistent diestrous phase.
Blood samples for the determination of 17β-estradiol plasma concentrations were collected from the jugular vein into heparinized tubes, centrifuged at 3,000 rpm for 20 min at 4°C, and plasma was immediately drawn off and stored frozen at −20°C until assayed. Plasma 17β-estradiol was measured by RIA using Immunochen kits of cover tubes (ICN Biomedicals, Inc.). The assay sensitivity was 10 pg/ml, and the intra-assay coefficient of variation was 9.45 %. All samples were measured on the same day. The sample was assayed in triplicate.
Histology, immunohistochemistry, and morphometric analysis
The rats of each subgroup were perfused, under halothane inhalation anesthesia, through the left ventricle, first with 100 ml of 0.9 % NaCl and then with 300 ml of 4 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed and post-fixed by immersion in the same fixative overnight and then rinsed in PBS.
After fixation, the brains were washed in distilled water, dehydrated through successive alcohols, cleared in two baths of butyl acetate, embedded in paraffin, and blocked out in a suitable mold. Transverse sections about 10-μm thick were obtained and attached to gelatin-covered slides. Sections were deparaffined in xylene and rehydrated, and an immunocytochemical method was carried out for detection of ERα. The sections were treated sequentially with Triton X (0.1 %, 5 min) at room temperature (RT), washed in distilled water, treated with H2O2 (3 %, 5 min) in a wet chamber at RT, washed in distilled water and treated with phosphate-buffered saline (PBS) (2 min). Slides were placed in a plastic Coplin jar filled with 0.01 M sodium citrate buffer (pH 6), and incubated in a household microwave oven four times for 2 min each operating with the highest power setting (800 W). Non-specific binding was blocked by incubation with bovine serum. Incubation with a specific antibody against ERα (sc-542, Santa Cruz Biotechnology, Inc., diluted 1:1,000) was performed overnight at 4°C. After several washes in PBS, the sections were incubated for 30 min at RT using a biotinylated horse universal antibody (Vector, PK-8800) diluted 1:50. Afterwards, the sections were incubated with Extravidin (Sigma Extra-3) labeled with HRP. The peroxidase activity was visualized by incubation with 0.05 % DAB (Sigma, D5637-25 G) in 50 mM Tris buffer pH 7.6, containing 0.04 % H2O2 (33 %). The usual specificity control tests were carried out.
After the immunohistochemistry, sections were counterstained with a modification of formaldehyde thionin method (Tolivia et al. 1994), dehydrated, cleared in eucalyptol, and mounted with Eukitt (O Kindler GmbH & Co, Freiburg, Germany).
For the identification of neuronal nuclei, we followed the nomenclature described in: Atlas of The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson 1997). After acquisition with a digital camera (Nikon DN100) adapted on a Nikon Eclipse E400 microscope, images were processed according to the method described by Tolivia et al. (2006) to quantify the signal of the ERα immunostaining. After acquisition with a digital camera, images are opened in Adobe Photoshop. For subsequent comparison of the amount present in different sections, the magnification of images must be equal. To select the specific signaling, choose “Color range,” in the “Select menu” of Photoshop, and with the “Eyedropper” tool click on any object on the image displaying the desired color/chromogen and all areas with the selected color range will be highlighted in an automatically generated clone image; thus the selection process can be controlled at every step. The tolerance bar of the color range tool makes the direct visualization of the selection on the image possible, and minimal variations in the chromogen selection are clearly observed. Once chromogen selection is finished, the profile used can be archived and used in the future when similar marked sections are studied. Close the “Color range” panel, and all immunopositive objects in the original image appear highlighted. Open the “Edit” menu of Photoshop, select “Copy,” open a “New file” and “Paste” the selected image. The new image shows only the positive selected areas on a white background. Convert the image to gray; the new picture shows all selected marked areas in various gray tones, which reflects both the positive area of the signal and the intensity of it (i.e., the amount of the chromogen present in each region). This gray picture must be saved in TIF format, which can be opened without problem by the Scion Image program (other free image analysis programs, as Image J, can be used). Open the picture in this program and calculate the “Mean density” under “Uncalibrated” conditions. The measurements obtained show the strength signal of the image and can be copied and exported to a spreadsheet (i.e., Excel). The strength signal obtained varies between 0 and 255 (value 0 corresponds to a nonstained section [white], and value 255 corresponds to a fully stained section [black].
Statistical analysis
Data are expressed as mean ± standard error of mean. First, we evaluated the Gaussian distribution of each variable by a Kolmogorov–Smirnov test and the aleatoriety of the samples by a Rachas test. Thereafter, data were statistically analyzed using an analysis of variance design followed by between-group comparison using the Tuckey honestly significant difference test. Values of P ≤ 0.05 were considered significant.
Results
General characteristics of experimental animals
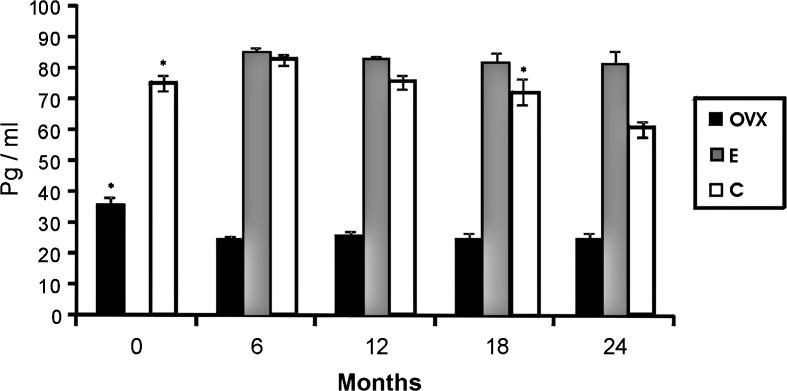
Plasma 17β-estradiol values obtained during the study were previously published in recent papers of our group (Moreno et al. 2010; Pérez et al. 2011). The plasma level of 17β-estradiol was significantly higher in groups E and C than in group O at all time points. In addition, plasma levels of 17β-estradiol were significantly higher in group E than in group C at 18 and 24 months. In the C group animals, plasma levels of 17β-estradiol increased significantly at 6 months and then decreased significantly at 24 months. In the E group, the estradiol level did not change significantly during the study. A significant decrease in the estradiol level was found in group O during the first 6 months (Fig. 1).
Fig. 1.
Levels of 17β-stradiol of ovariectomized animals (O; black bars), sham surgery animals (intact) (C; white bars) and ovariectomized animals treated with 17β-estradiol (E; gray bars). Mean ± standard error of the mean for seven animals. Significant differences are shown. *p ≤ 0.05 (month vs. next month)
ERα presence in the telencephalon
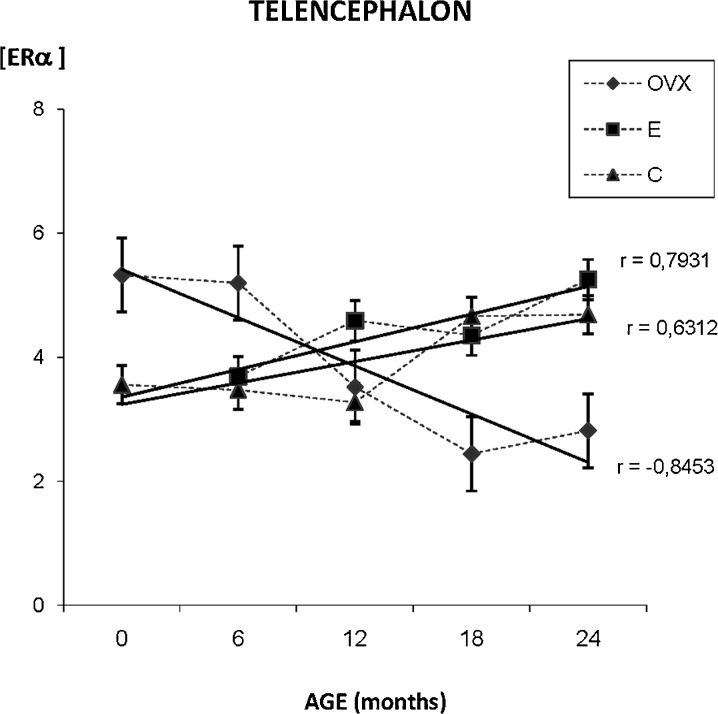
The densitometric study of the whole telencephalon shows differences in the ERα distribution pattern that can be observed among the three experimental groups. While in the younger animals of the C group ERα is mainly localized in the neuronal nucleus, perinuclear immunostaining is observed in the OVX and E groups. The C group shows a clear modification in the distribution of ERα during aging; the immunostaining appears also in the cytoplasm as well as in the nucleus. The quantification of the immunostaining noted an increase of ERα presence with aging in the C and E groups while in the OVX group a decrease in the expression of ERα was observed (Fig. 2).
Fig. 2.
Densitometric quantification of ERα immunosignal in the telencephalon along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals, in different age groups (months). Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
Due to the heterogeneity and complexity of the telencephalon and for better analysis purposes, we divide it in the following areas of study: hippocampus, olfactory cortex and motor, sensorial, visual, and auditory cortices.
ERα in the hippocampus (Hp)
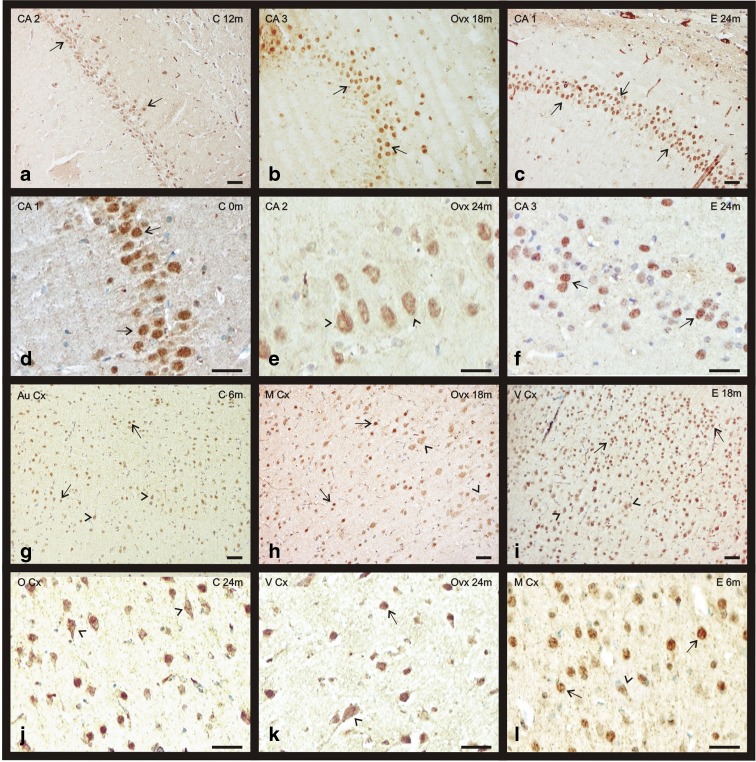
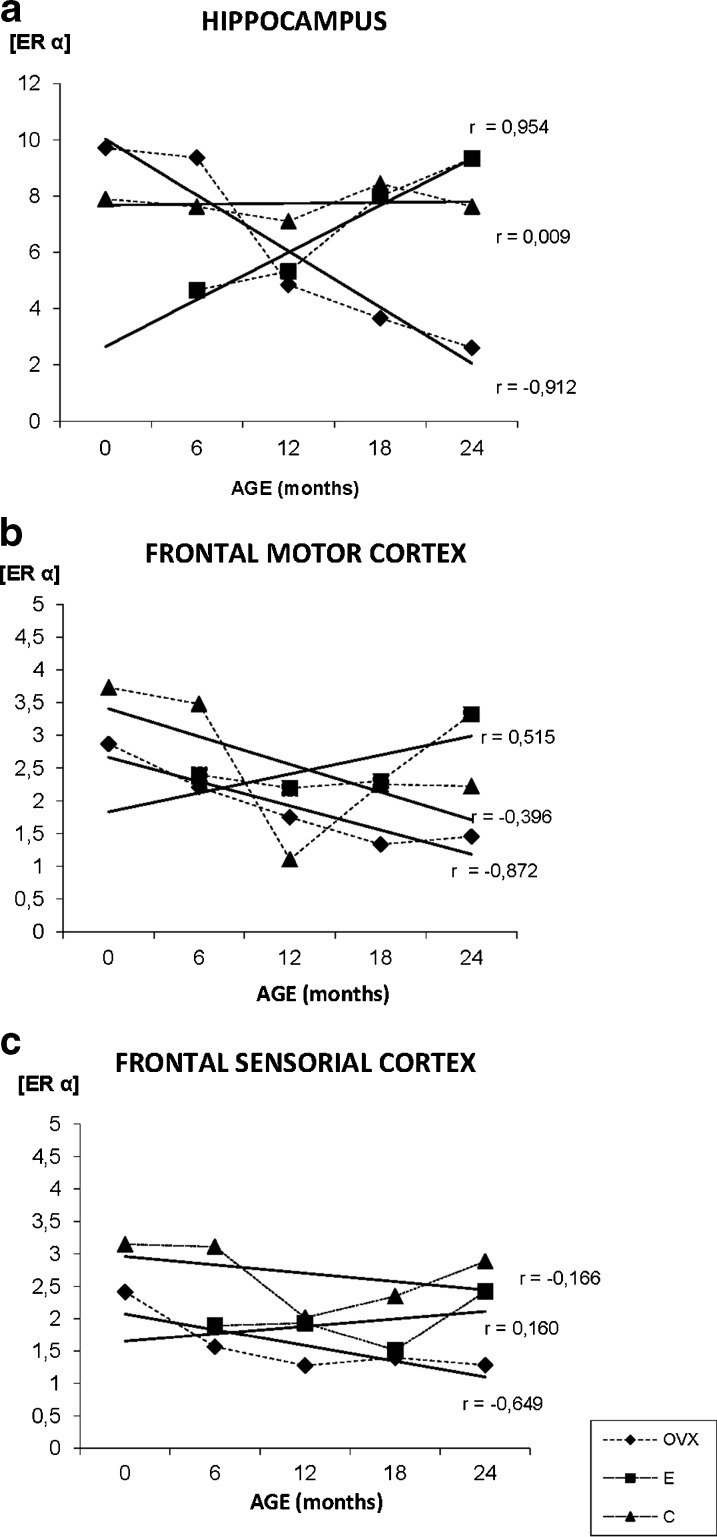
The immunopositivity for ERα was located mainly in the nucleus of pyramidal neurons of the CA1, CA2, CA3, and in the granular neurons of the dentate gyrus. Some neurons of these areas also show a slight extranuclear staining (Fig. 3a–f). During aging, the extranuclear staining for ERα mainly appears in the pyramidal neurons of the CAs of OVX and C groups (Fig. 3e). In the E group, the immunoreactivity along aging is principally located at nuclear level (Fig. 3f). The quantification of the immunostaining noted an increase of ERα presence with aging in the E group while in the OVX group a deep decrease in the expression is observed. In the C group, there is no change in receptor levels of ERα (Fig. 4a).
Fig. 3.
Immunohistochemical staining for ERα in the telencephalon of the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals during aging (0–24 months). a–f ERα presence in CAs of the hippocampus. g–l ERα presence in some cortical regions: the auditory cortex, motor cortex, visual cortex, and olfactory cortex. Nuclear location of ERα (arrows). Citoplasmatic location of ERα (arrowheads). (bar = 200 μm) (m months)
Fig. 4.
Densitometric quantification of ERα immunosignal in the hippocampus and frontal cortex along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals, in different age groups (months). Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
ERα in the frontal motor cortex
The neurons of the motor cortex show an ERα nuclear positive staining, mainly in the external pyramidal layer of all groups (Fig. 3h, l). Related with aging, the external pyramidal and granular layer neurons show a citoplasmatic staining in the youngest animals of all experimental groups. This homogeneity in ERα distribution changes with aging when the signal in the granular layer in the E and C groups virtually disappears. Group E is the only group, in which the ERα increases with age, presenting the lowest amount of receptor in the youngest animals and the highest in the oldest. The quantification of the immunostaining noted a decrease in the expression of ERα with aging in the C and OVX groups (Fig. 4b).
ERα in the frontal sensorial cortex (S Cx)
The ERα distribution in this area is quite similar to the one in the frontal motor cortex with the receptor located mainly in the nucleus of the pyramidal neurons in the three experimental groups. The main difference we found is that during aging, in the OVX group, the nuclear ERα staining adopts a granular appearance instead of the homogeneous aspect showed in the young animals. On the contrary, groups E and C do not show changes regarding the deposit shape, keeping an even look all over the experiment. In the S Cx, group C shows the highest ERα levels in all range of ages. The quantification of the immunostaining noted a decrease in the expression of ERα with aging in the C and OVX groups, while in the E group, an increase of ERα presence was observed (Fig. 4c).
ERα in the olfactory cortex
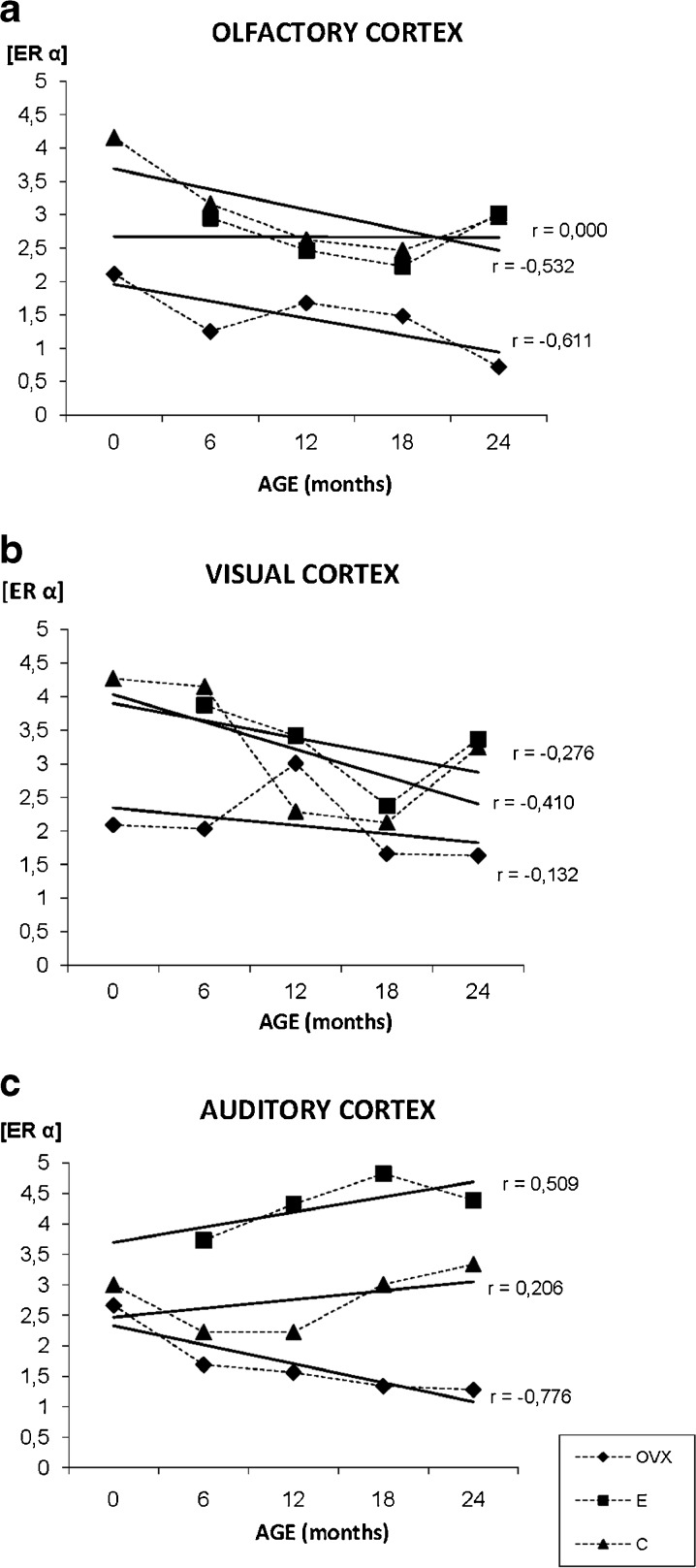
In the olfactory cortex, the ERα is mainly located in the nucleus of the neurons. During aging the staining pattern is quite homogenous in both, C and E groups, and remain at nuclear level, but in the OVX, the location of the ERα changes with age (from nuclear to citoplasmatic) and turns more heterogeneous (Fig. 3j). This experimental group shows the lowest ERα levels throughout the experiment. In both groups, OVX and C, a decrease of ERα staining with age is observed, while there is no change in receptor levels in the E group (Fig. 5a).
Fig. 5.
Densitometric quantification of ERα immunosignal in the olfactory, visual and auditory cortex along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals, in different age groups (months). Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
ERα in the visual cortex
The most reactive part of this cortex is the deepest half characterized by the presence of medium-big size granular and pyramidal neurons. The granular neurons show only nuclear staining for ERα independently of the experimental group while the big pyramidal neurons present nuclear and citoplasmatic staining for the receptor. During aging, most of ERα of the pyramidal neurons is located in the cytoplasm; especially in the E group (Fig. 3i). The OVX aging animals showed few ERα positive neurons, a common feature of different cortical areas for 24 months animals of this experimental group (Fig. 3k). The quantification of the immunostaining noted a decrease in the expression of ERα related with aging in the three experimental groups: C, OVX, and E (Fig. 5b).
ERα in the auditory cortex
The ERα staining was located again mainly in the nucleus of pyramidal neurons of the deepest layers of the cortex. During aging, and in all the experimental groups, the immunosignal for ERα extends to the cytoplasm of the neurons (Fig. 3g). An increment of the receptor, related with age, was observed in the groups C and E while the OVX ones showed a decrease in the levels of ERα. The group E shows higher ERα levels at all points of age (Fig. 5c).
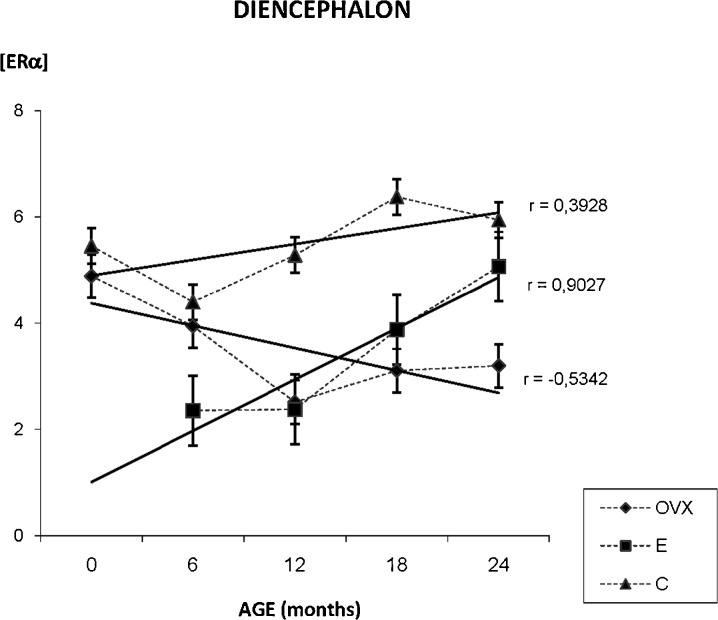
ERα presence in the diencephalon
The densitometric study of the whole diencephalon shows the next results. In the OVX group, we have found a global decrease on ERα immunostaining during aging process. In the E group, our results showed that estradiol treatment determinates an increase on ERα in diencephalon while in C group, although estrogen receptor immunostaining seems to increase during aging, the lineal correlation does not confirm dependence between age and labeling intensity (Fig. 6).
Fig. 6.
Densitometric quantification of ERα immunosignal in the diencephalon along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol-treated (E) animals in different age groups. Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
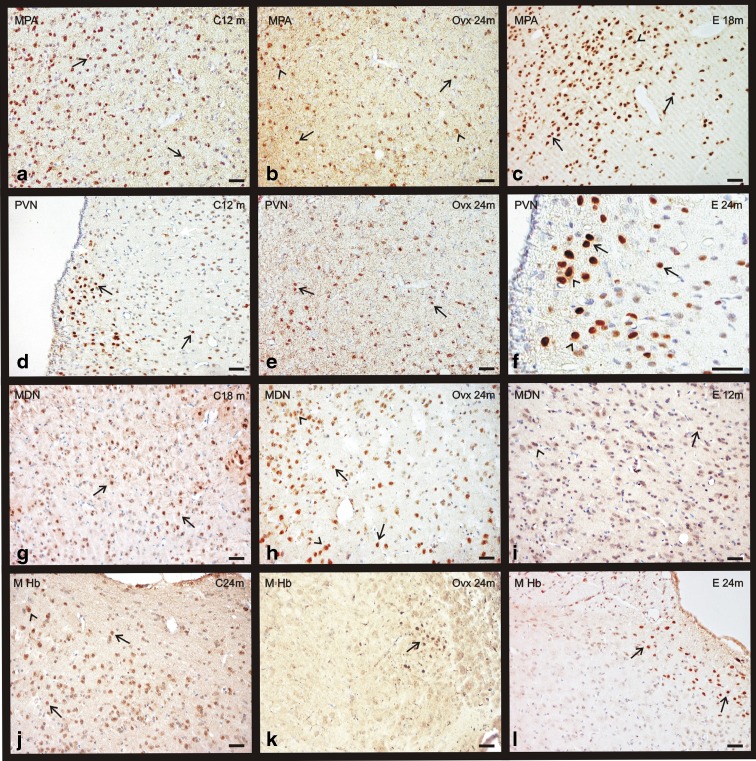
In contrast to telencephalon, ERα immunopositivity was mainly nuclear in most of the studied areas, with the exceptions that will be pointed below. As was previously achieved in the telencephalon, for better analysis purposes the diencephalon was divided in the following representative areas for the immunohistochemistry study. The medial preoptic area (MPA), the supraoptic nucleus (SON), and the paraventricular nucleus (PVN) at hypothalamic level; the medial dorsal nucleus (MDN) of the thalamus and the medial habenular nucleus (M Hb) of the epithalamus. The observation of a different immunostaing pattern in these areas raises the idea of a region-specific expression of ERα.
ERα in the hypothalamus
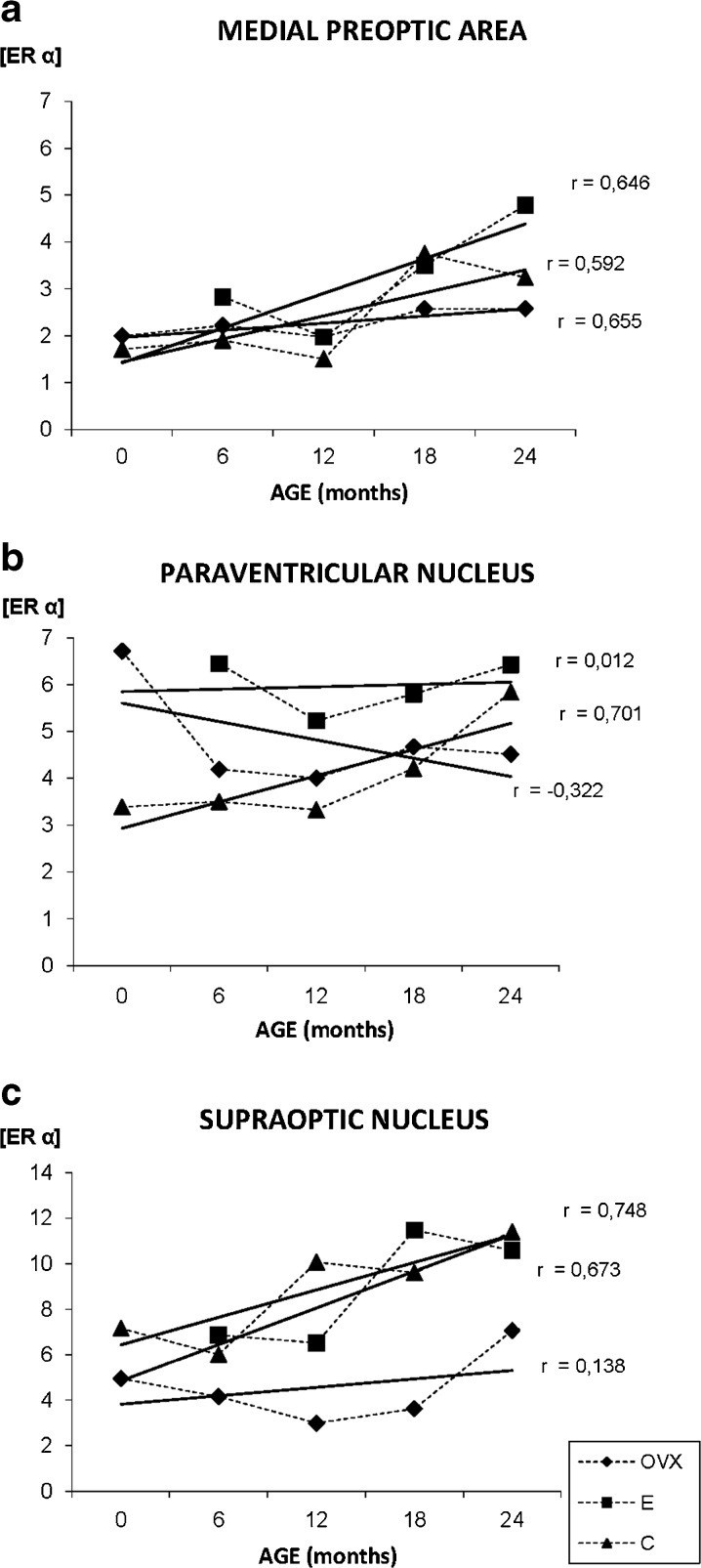
Nuclear staining for ERα is only observed in the MPA of young animals, but from 18 months to the end of the study at 24 months, the receptor is also located in the cytoplasm, especially in the group C (Fig. 7a–c). The quantification of the immunostaining noted an increase of ERα presence with aging in the three experimental groups, but this increase was not statistically significant in the OVX group that always shows the lowest levels of the receptor (Fig. 8a). The group E shows the highest ERα levels throughout the study, but in the young animals, all values are quite similar (Fig. 8a).
Fig. 7.
Immunohistochemical staining for ERα in the diencephalon of the three experimental groups: control (C), ovariectomized (OVX), and estradiol-treated (E) animals in different age groups (0–24 months). a–c ERα presence in the medial preoptic area (MPA). d–f ERα presence in the paraventricular nucleus (PVN). g–i ERα presence in the medial dorsal nucleus (MDN). j–l ERα presence in the medial habenular nucleus (M Hb). Nuclear location of ERα (arrows). Citoplasmmatic location of ERα (arrowheads). (bar = 200 μm) (m months)
Fig. 8.
Densitometric quantification of ERα immunosignal in the medial preoptic area, paraventricular, and supraoptic nuclei along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals in different age groups (months). Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
The immunosignaling for ERα, in the PVN, is located exclusively at nuclear level of the neurons, and this location does not change with aging (Fig. 7d–f). The OVX group shows few positive neurons with a weak signal, in contrast, the E group shows the highest number of immunostained cells (Fig. 7f). During aging, the quantification of the immunostaining shows an increase of ERα in the C group, while a decrease in the OVX animals is noted. The values observed in group E show a slight increase with aging (Fig. 8b).
The distribution of the immunosignaling for ERα, in the SON, is similar in all to that described for PVN, and the location of the immunostaining does not change with aging. During aging, the quantification of the immunostaining shows an increase of ERα in the C and E groups, while an absence of changes in the OVX animals was noted (Fig. 8c).
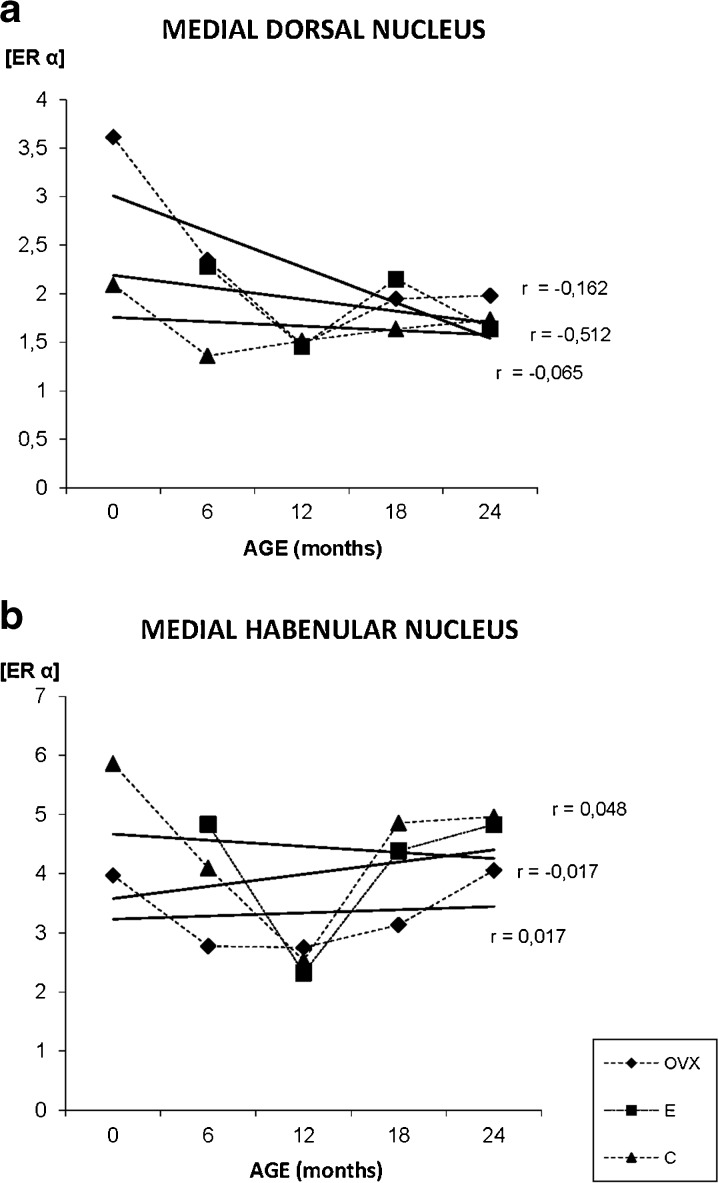
ERα in the thalamus
The number of immunopositive neurons for ERα in the MDN is lower when compared with other diencephalic studied areas (Fig. 7g–i). In the young animals, the receptor is located in the nucleus, but in the oldest, the immunoreacyivity appears located in both, neuronal nuclei and cytoplasm, especially in the OVX group (Fig. 7h). The quantification of the immunostaining shows that there is no correlation between age and ERα in both, the C and E groups and only a slight decrease of the receptor with age in the OVX animals (Fig. 9a).
Fig. 9.
Densitometric quantification of ERα immunosignal in the medial dorsal nucleus of the thalamus and habenular nucleus of the epithalamus along aging in the three experimental groups: control (C), ovariectomized (OVX), and estradiol treated (E) animals, in different age groups (months). Each point in the graph represents mean density in a × 20 field ± standard error of the mean. Regression lines and Pearson’s correlation coefficient (r) are also shown
ERα in the epithalamus
The immunostaining for ERα, in the MHb, is mainly nuclear in all experimental groups. This nuclear location does not change with aging (Fig. 7j-k). The quantification of the immunostaining shows that there is no correlation between age and ERα in any of the groups, but at 12 months, the C and E groups show an important decrease in ERα levels (Fig. 9b). The OVX group shows the lowest values for the receptor.
Discussion
Our results show that estrogen and aging have an effect in the distribution of ERα within the cell. In general, the subcellular distribution of ER was different in the three groups studied. Thus, whereas in young animals of C group most of ER immunoreactivity was concentrated into neuronal nucleus, in OVX and E groups the immunostaing not was only nuclear but also cytoplasmic. On the other hand, aging increased the cytoplasmic presence in all groups studied. As we discuss here, the subcellular distribution of ERα could be related with the different neuroprotection mechanisms mediated by estrogens.
In this work, we have studied ERα expression in two selected encephalic areas, telencephalon, and diencephalon, since this type of receptor plays a pivotal role in the maintenance of neuronal integrity in normal aging as well as in neurodegenerative diseases and injuries (Wilson et al. 2002). This function is directly related to estradiol mediated nongenomic mechanisms of action with a direct association between ERα and G proteins (Pedram et al. 2006). Confocal microscopy and Western blot studies have revealed the presence of many ERα isoforms in cerebral blood vessels (Stirone et al. 2003); among which there is an 82-KDa band corresponding to those of 80 KDa. Unlike our working group, the antibodies employed by these authors are generated against high variability regions of ERα, the H-20, which recognizes the C-terminal region, and the H-184, which binds to N-terminal region, so we are talking about monoclonal antibodies, which recognize less specific regions, which leads to different band patterns, sometimes unspecific.
According to our previous results (Alonso et al. 2008), there is an age-dependent decrease in the 67 and 47 KDa isoforms of ER during aging process in telencephalon as well as in diencephalon. Ovariectomy, with the consequent loss of ovaric estrogens, aggravates the effect of normal aging in cognitive decline and compared to hormone treated animals (Cheng et al. 2001; Oge et al. 2003; Stirone et al. 2003), raising the idea that these sexual hormones exert neuroprotective effects even once estropause has occurred (Iossa et al. 1999). Thus, we have found a different pattern of ERα localization in telencephalon and diencephalon in the three experimental treatments. In telencephalon, in young animals from the three experimental situations, the immunostaining is mainly nuclear and aging involves a change in ERα localization within the cell from the nucleus to extranuclear sites. In cortex, most of the immunoreactivity for ERα was concentrated in small and medium-sized granular and pyramidal neurons in outer layers at nuclear and even sometimes extranuclear level; in the oldest animals, the extranuclear staining was more frequent and mainly present in pyramidal neurons, in agreement with Shughrue and Merchenthaler’s results (Shughrue and Merchenthaler 2000a) and Kritzer’s studies (Kritzer 2002). A similar feature occurs in the hippocampus. During the first half of the life, ERα tends to concentrate exclusively in nuclei of CA1, CA2, and CA3 pyramidal neurons and in some granular cells from the dentate gyrus, as it has been described by some authors (Shughrue and Merchenthaler 2000a, b; Mehra et al. 2005). From 12 months of experimentation, concomitant with the estropause, the extranuclear isoform begins to be more abundant (Pappas et al. 1995; Milner et al. 2001). Ovariectomy not only involves a decrease in immunohistochemical signal, but also leads to a noticeable extranuclear immunolabeling even more intense and earlier than in the E group. In this sense, in vitro studies have demonstrated that in cells showing artificial senescence, the ERα immunostaining was mainly localized within the cytoplasm compared to controls, where the immunosignal was found only in the nuclei (Lee et al. 2004). Thus, as we can see, ovariectomy intensifies deleterious effect of aging and 17β-estradiol treatment counteracts it. This could explain the fact that the citoplasmatic isoform appeared in ovariectomized animals at earlier stages, which are physiologically more damaged because of the lack of gonadal hormones, while in estradiol-treated animals, the extranuclear immunoreactivity is not noticeable until 18 months of treatment. Regarding the levels of ER67 and ER47, 17β-estradiol treatment is capable of reverting in part the deleterious effects of the ovariectomy in the two encephalic areas studied. The treatment induce a significant increase in ER67 and ER47 levels, compared to ovariectomized animals, which fits with Stirone’s observations (Stirone et al. 2003). Our immunohistochemistry study reveals that the 17β-estradiol treatment, leads to an increase in total ERα, with the same ratio of nuclear and extranuclear isoforms as in ovariectomized animals. Although according to bibliography we could think that 17β-estradiol would repress the expression of its own receptor, what is going on may be a double hormone-mediated effect, regulating ERα expression in a dose and age-dependent manner (Funabashi et al. 2000). Therefore, the absolute level of extranuclear ERα is higher when the hormone is administrated. In this sense, non-nuclear estrogen receptor has been widely described as responsible of rapid non-genomic estrogens actions (McCarthy 2008; Vasudevan and Pfaff 2008); raising the possibility that estradiol could play a neuroprotective role through a non-genomic rapid mechanism (Singer et al. 1999; Wilson et al. 2002). The way of its participation, among many others, could be in the increase of eNOS activity in cerebral blood vessels (McEwen 2001; Milner et al. 2001; Toran-Aller et al. 2002; Li et al. 2003) or the downregulation of GSK3β (Glycogen Synthase Kinase 3β). The upregulation of this enzyme is responsible of the hyperphosphorylation of Tau protein, the main component of Alzheimer’s disease amyloid (Goodenough et al. 2003).
In the diencephalon, the change in ERα distribution in aging is not observed, but the immunostaining remains in the nuclei through the experiment in most cases, supporting some previous works (McEwen and Alves 1999; Ishunina et al. 2000; Adams et al. 2002). As it occurs in the telencephalon, ovariectomy makes the immunostaining decrease in an age-related way. We have even observed that hormonal treatment favors the increase in both ERα isoforms from 12 months of treatment, in contrast to the decrease overcome by the other groups, 17β-estradiol treatment exerts its function through the increase in ERα levels, verifying the positive effect of this sexual hormone on the estradiol signaling pathway (Alonso et al. 2008). Moreover, in the diencephalon, 17β-estradiol exposure mitigates the loss of ER67 due to aging, so the level of the protein is higher in the estradiol-treated animals than in normal aging. None of the studied areas showed a significant presence of non-nuclear ERα, with the exception of some giant neurons from the supraoptic nucleus exhibiting some extranuclear labeling in the darkest immunopositive cells. The ERα localization within the cell in the diencephalon, almost exclusive in the nuclei, reveals other mechanisms of estrogen-mediated neuroprotection in this encephalic region different from those of the telencephalon, pointing out a specific response of telencephalon and diencephalon to aging and to estradiol treatment (Alonso et al. 2008). Regarding this, the hypothalamic nuclei are very important in the maintenance of the hypophysary–hypothalamic axis, so a rigid regulation system is needed to ensure its optimum physiological functioning, so aging does not seem to affect the hypothalamus significantly. Thus, it has been described the lack of morphological changes in hypothalamic nuclei in relation with aging (Navarro et al. 1997). The exception to this general description is the thalamus where there is an intense extranuclear immunolabeling especially in ovariectomized animals since early age. A possible explanation to this phenomenon could be that, as we have previously attributed to extranuclear isoform of the receptor a crucial role in estrogen neuroprotective actions, this area might be especially sensitive to lack of estrogens, or the neuroprotective mechanisms were exclusively activated by non-nuclear estrogens receptor, which could be activated by autocrine extragonadal estrogens (Simpson 2003). However, we do not have any references that support this idea with reliability.
Another striking feature in both studied areas is that, with aging, the ERα labeling turns to acquire a heterogeneous distribution. That is, while in young animals the immunopositive neurons have a similar staining intensity as well as a uniform immunostaining distribution in the whole nucleus, aging involves huge differences in staining darkness among the immunopositive cells. Ovariectomy seems to intensify the differences in immunopositivity signal among the neurons. Related with these observations, it is possible that the extranuclear ERα level has a relationship with neuroprotective alternative non-genomic estrogen-induced mechanisms, according to the research works of some groups (Marin et al. 2005; Marin et al. 2003; Alonso et al. 2008). There is quite likelihood that neurons highly immunostained for ERα were less susceptible to neuronal death than those who lacked the receptor, being more protected by the sexual hormone, though it would be necessary the performance of more studies.
As well as this, there are some scattered little immunopositive nuclei in some telencephalic areas, which seem to belong to glial cells due to its size and localization, pointing out an involvement of glia in estrogen-mediated neuroprotection. It has been described that the presence of ERα in astrocytes, which could mediate indirect neuroprotection of ER-negative neurons, acting as an intermediate (Azcoitia et al. 2001a; Milner et al. 2001; Dhandapani and Brann 2007). From our results, we think that rapid mechanisms of action, activated by estrogens binding to it extranuclear receptor, could be a central point in the activation of the neuroprotective estrogen effects. Thus, in those animals lacking gonadal hormones from youth, extragonadal estrogens could activate the estrogens-mediated neuroprotective mechanisms prematurely (Simpson 2003), which would be reflected in a higher extranuclear ERα levels than in control animals at the same age. Among others, the group of Azcotia and collaborators has demonstrated the importance of brain aromatase in estrogen-mediated neuroprotection (Azcoitia et al. 2001b). Long-term exposure to 17β-estradiol entails a constant contribution of this sexual hormone through all the life. According to our previous results (Alonso et al. 2008), at late stages when the animals are aged, 17β-estradiol in E group remain stable, so the effectiveness in neuroprotection onset is better than in normal aging against age-induced stress. That is, taking our hypothesis as a starting point, delaying the change of ERα localization from nucleus to extranuclear sites could be a consequence of a lesser degree of neuronal stress in animals chronically exposed to 17β-estradiol.
Another possibility is that the key of neuroprotective effects of estradiol is related to the ER67/ER47 ratio, which is supported by Flouriot et al. (Flouriot et al. 2000). Our previous results showed that this ratio depends on the region of the brain that is studied. Regarding our results, in the telencephalon, there is a ratio ER67/ER47 in ovariectomized animals significantly higher than in the rest of the groups all throughout the study. However, the value experiments a decrease in normal aged and in ovariectomized animals from 12 months of treatment (Alonso et al. 2008). In this sense, in cell cultures it has been described as an ER67/ER47 ratio lower in proliferating cells than in quiescent or non-proliferating cultures, so when the culture is young and healthy the value of the ratio is low (Flouriot et al. 2000). There is a strong possibility that a compensatory mechanism was activated by the low levels of estrogen; 17β-estradiol treatment induces a progressive increase of this parameter from the beginning to the end of the experimental period, though the value never reaches up to those of the OVX group. The E group ratio, similar to that which belongs to normal-aged animals, verifies that long-term 17β-estradiol exposure eases ovariectomy effects by the modulation of ER67/ER47 ratio, but not those aging-associated (Alonso et al. 2008). Not only ER47 is a powerful inhibitor of ER67 AF-1 transactivation activity, but this N-terminal truncated isoform seems to have a stronger binding affinity for the estrogen responsive element (ERE) in DNA than in ER67 (Xing et al. 1995).
Several evidences have suggested that the neuroprotective effects of estrogen may be mediated by the activation of phosphatidylinositol-3 kinase (PI3K) signaling pathways and that ERα but not ERβ is involved of this intracellular signaling mechanism (Cardona-Gomez et al. 2002a, 2002b; Mendez et al. 2003; D’Astous et al. 2006). The odds are that non-nuclear ERα is the isoform responsible of downstream activation of PI3K signaling pathway (Gundlah et al. 2000). Further investigations point ER47 to have a crucial role in mediating eNOS activation specifically (Li et al. 2003). Taking into account these observations, the higher proportion of ER47 in estradiol-treated animals at the end of the study might be favoring PI3K signaling pathway activation, being therefore a hallmark of 17β-estradiol-mediated neuroprotection effectiveness.
In the diencephalon, 17β-estradiol treatment tends to increase the ER67/ER47 ratio, even over those of the normal aged animals. Three hypotheses can explain these results: firstly, in this encephalic area, estradiol is hardly neuroprotective; secondly, the action and regulation of estrogen-dependent mechanisms is region-specific and finally yet importantly, the estrogen-mediated neuroprotection is performed in other different points of the signaling pathway. A vast majority of immunohistochemical labeling is concentrated in cellular nuclei. To the best of our knowledge, many authors support the idea that extranuclear ERα is responsible for most of the neuroprotective actions of estrogens, which initially do not involve genic transcription (Pappas et al. 1995; Marin et al. 2005). Moreover, during artificially induced senescence of PC12 pheochromocytoma cells, the subcellular localization of ERα, most of immunoreactivity was dramatically shifted from the nucleus into the cytoplasmic compartment (Lee et al. 2004). While artificial senescence is not identical to the physiological aging process, normal diploid cells in culture may achieve a non-dividing state termed “senescence” after they divide a certain amount of times, concomitant with culture aging, being a stressing state similar to physiological aging. In these situations, estrogens must activate its neuroprotective mechanisms, among which, promoting non-nuclear receptor-mediated rapid signaling pathways activation. Since a low ratio ER67/ER47 could be associated with a better functioning of short-term estradiol-mediated neuroprotective mechanisms, the fact that in the diencephalon the ratio value was the highest, as well as a predominant nuclear immunostaining could indicate that in the diencephalic area 17β-estradiol treatment was not enough to restore the loss of neuroprotection effectiveness. Thus, according to our results and previous works (Alonso et al. 2008), estradiol-mediated effects are mostly exerted in the telencephalon where 17β-estradiol is capable of downregulating ER67/ER47 ratio, with a presence of extranuclear receptor more frequent than in normal aging animals. The diencephalon is an encephalic region that carries out very important homeostatic functions, such as the regulation of the hypothalamic–hypophysial axis, needing reliable previous neuroprotective mechanisms, so neuroprotection could be exerted by other pathways different from estrogen-activated systems.
Although none of the hypotheses expressed in this discussion can be completely discarded, our results support the idea of the existence of region-specific estrogen-mediated neuroprotective mechanisms, above all exerted in the telencephalon rather in the diencephalon in the case of non-genomic pathways. Hence, a previous work of our group (Alonso et al. 2008) demonstrates that chronic estradiol treatment improves brain homeostasis during aging in female rats through the activation of the PI3K signaling pathway in both telencephalon and diencephalon. In summary, our findings indicate that estradiol treatment during aging in ovariectomized rats may ease ovariectomy effect on ERα levels. As well as this, during aging and with 17β-estradiol treatment there is an increase in ERα immunoreactivity, with a global decrease in total immunolabeling in ovariectomized animals and, on the contrary, with an increase in estradiol-treated animals. As well as this, long-term treatment with 17β-estradiol seems to regulate ERα in a region-specific manner. Our findings are a starting point to look through the relationship between subcellular localization of ERα and the specific function in each region of the brain.
Our immunohistochemical results agree with Western blot studies in a vast majority; however, there are some faint differences between them. We cannot forget that immunohistochemistry does not allow to discern between ER67 and ER47 isoforms, and Toran-Aller and colleges have identified a novel plasma membrane-associated putative ER in neurons, called ER-X, different from classical estrogen receptors α and β (Toran-Aller et al. 2002). The new isoform, whose apparent molecular weight is of 62–63 KDa, reacts with antibodies to ERα LBD, such as our MC-20 from Santa Cruz. Moreover, immunohistochemistry and Western blot are different scientific techniques that require a differential tissue processing procedure. While for the molecular assays, the samples are stored in liquid N in the aim to guarantee the optimum conservation, the p-formaldehyde fixation before immunohistochemistry induces a modification of the three-dimensional protein conformation, with the possibility of ER-X LBD region could be accessible to the antibody and therefore recognized by immunohistochemistry but not by Western blot. In consequence, in Western blot, we are maybe underestimating total ER level, or it is possible that the 62- and 67-KDa bands were covered up. As well as this, this alternative ER-X might be mostly expressed in some exceptional situations, such as ovariectomy, which would justify the high levels of ERα detected in the OVX group by immunohistochemistry. Nevertheless, whatever technique was used, as a direct consequence of ovariectomy, there is a decrease in ERα, intensifying the aging-associated loss of protein (Adams et al. 2002; Mehra et al. 2005), while 17β-estradiol treatment is capable of restoring the effect of ovariectomy at later stages of the experiment.
Acknowledgments
This work was supported by MEC and FEDER SAF2007-64076 grants; FIS PI020324 grant and MEC grants AP20040854, AP20053815, and FICYT grant BP05-064 to CP, EM, and CO.
Abbreviations
- Au Cx
Auditory cortex
- CA
Cornu Amonis region
- CNS
Central nervous system
- DAB
Diaminobenzidine
- E2
17β-estradiol
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- H2O2
hydrogen peroxide
- M Cx
Motor cortex
- MDN
Medial dorsal nucleus
- M Hb
Medial habenular nucleus
- MPA
Medial preoptic area
- O Cx
Olfactory cortex
- PBS
Phosphate-buffered saline
- PVN
Paraventricular nucleus
- RIA
Radioimmuno assay
- S Cx
Sensorial cortex
- SON
Supraoptic nucleus
- V Cx
Visual cortex
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen-receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22(9):3808–3814. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Moreno M, Ordoñez P, Fernandez R, Perez C, Diaz F, Navarro A, Tolivia J, Gonzalez C. Chronic estradiol treatment improves brain homeostasis during aging in female rats. Endocrinology. 2008;149(1):57–72. doi: 10.1210/en.2007-0627. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Garcia-Ovejero D, Chowen JA, Garcia-Segura LM. Astroglia play a key role in the neuroprotective actions of estrogen. Prog Brain Res. 2001;132:469–478. doi: 10.1016/S0079-6123(01)32096-4. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Veiga S, Honda SI, Harada N, García-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47(4):318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- Amtul Z, Wang L, Westaway D, Rozmahel RF. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer's disease. Neuroscience. 2010;169(2):781–786. doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Baraka AM, Korish AA, Soliman GA, Kamal H. The possible role of estrogen, selective estrogen receptor modulators in a rat model of Parkinson's disease. Life Sci. 2011;88(19–20):879–885. doi: 10.1016/j.lfs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Belcher SM. Regulated expression of estrogen receptor alpha, beta mRNA in granule cells during development of the rat cerebellum. Brain Res Dev Brain Res. 1999;115(1):57–69. doi: 10.1016/S0165-3806(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen, insulin-like growth factor-I in the brain: molecular mechanisms, functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/S0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol, insulin-like growth factor-I in the activation of PI3K/Akt signalling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80–88. doi: 10.1016/S0169-328X(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Wang J, Bondy CA. Estrogen augments glucose transporter, IGF1 expression in primate cerebral cortex. FASEB J. 2001;15:907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- Chun TY, Gregg D, Sarkar DK, Gorski J. Differential regulation by estrogens of growth, prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci U S A. 1998;95(5):2325–2330. doi: 10.1073/pnas.95.5.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker LH, Espel MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy, cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118(4–5):304–1032. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage, stimulate behavioral recovery after transient middle cerebral artery occlusion in male, female rats. Brain Behav Immun. 2011;25(4):715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- D'Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69(4):1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Flouriot G, Brand H, Denger S, Metivier R, Los M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-α) that is encoded by distinct transcripts, that is able to repress hER-α activation function 1. EMBO J. 2000;19(17):4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Kleopoulos SP, Brooks PJ, Kimura F, Pfaff DW, Shinohara K, Mobbs CV. Changes in estrogenic regulation of estrogen receptor α mRNA, progesterone receptor mRNA in the female hypothalamus during aging: an in situ hybridization study. Neurosci Res. 2000;38:85–92. doi: 10.1016/S0168-0102(00)00150-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Olmos G, Robbins RJ, Hernez P, Meyer JH, Naftolin F. Estradiol induces rapid remodelling of plasma membranes in developing rat cerebrocortical neurons in culture. Brain Res. 1989;498(2):339–343. doi: 10.1016/0006-8993(89)91113-X. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Alonso A, Alvarez N, Diaz F, Martinez M, Fernandez S, Patterson AM. Role of 17β-estradiol and/or progestorone on insulin sensitivity in the rat: implications during pregnancy. J Endrocrinol. 2000;166:283–291. doi: 10.1677/joe.0.1660283. [DOI] [PubMed] [Google Scholar]
- Goodenough S, Schaffer M, Behl C. Estrogen-induced cell signalling in a cellular model of Alzheimer’s disease. J Steroid Biochem Mol Bio. 2003;84:301–305. doi: 10.1016/S0960-0760(03)00043-8. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18(4–5):347–358. doi: 10.1016/S0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76(2):191–204. doi: 10.1016/S0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Energy intake and utilization vary during development in rats. J Nutr. 1999;129:1593–1596. doi: 10.1093/jn/129.8.1593. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Kruijver FPM, Balesar R, Swaaf DF. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab. 2000;85(9):3283–3291. doi: 10.1210/jcem.85.9.6826. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Toran-Aller CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269(18):13065–13068. [PubMed] [Google Scholar]
- Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47–54. doi: 10.1353/pbm.1963.0005. [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Roschier U, Puoliväli J, Wang J, Miettinen R. The effect of aging on the subcellular distribution of estrogen receptor-alpha in the cholinergic neurons of transgenic and wild-type mice. Eur J Neurosci. 2005;21(5):1437–1442. doi: 10.1111/j.1460-9568.2005.03953.x. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Regional, laminar and cellular distribution of immunoreactivity for ERα and ERβ in the cerebral cortex of hormonally intact adult male and female rats. Cereb Cortex. 2002;12(2):116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- Kulkarni J, Gurvich C, Lee SJ, Gilbert H, Gavrilidis E, de Castella A, Berk M, Dodd S, Fitzgerald PB, Davis SR. Piloting the effective therapeutic dose of adjunctive selective estrogen receptor modulator treatment in postmenopausal women with schizophrenia. Psychoneuroendocrinology. 2010;35(8):1142–1147. doi: 10.1016/j.psyneuen.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Küppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276(2):95–98. doi: 10.1016/S0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36(3):357–378. doi: 10.1002/(SICI)1097-4695(19980905)36:3<357::AID-NEU5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lee E, Mun GH, Oh CS, Chung YH, Lee YS, Shin DH. A subcellular distribution of estrogen receptor-alpha is changed during artificially induced senescence of PC12 pheochromocytoma cells. Neurosci Lett. 2004;372(1–2):80–84. doi: 10.1016/j.neulet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the oestrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva Endocrinol. 2010;35(2):127–143. [PMC free article] [PubMed] [Google Scholar]
- Marin R, Guerra B, Morales A, Diaz M, Alonso R. An oestrogen membrane receptor participates in estradiol actions for the prevention of amyloid-β-peptide1-40 induced toxicity in septal-derived cholinergic SN56 cells. J Neurochem. 2003;85:1180–1189. doi: 10.1046/j.1471-4159.2003.01767.x. [DOI] [PubMed] [Google Scholar]
- Marin R, Guerra B, Alonso R, Ramirez CM, Diaz M. Estrogen activates classical and alternative mechanisms to orchestrate neuroprotection. Curr Neurovasc Res. 2005;2:287–301. doi: 10.2174/156720205774322629. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol-3 kinase in the adult brain. Brain Res Mol Brain Res. 2003;112:170–176. doi: 10.1016/S0169-328X(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056(1):22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. doi: 10.1002/1096-9861(20010115)429:3<355::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Moreno M, Ordóñez P, Alonso A, Díaz F, Tolivia J, González C. Chronic 17β-estradiol treatment improves skeletal muscle insulin signaling pathway components in insulin resistance associated with aging. AGE. 2010;32:1–13. doi: 10.1007/s11357-009-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERbeta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:269–277. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer's Disease Cooperative Study JAMA. 2000;283(8):1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Alvarez-Uria M. The magnocellular neurosecretory system of the hamster hypothalamus: an ultrastructural and morphometric study during lifetime. Mech Ageing Dev. 1997;97(2):143–161. doi: 10.1016/S0047-6374(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Oge A, Sezer ED, Ozgonull M, Bayraktar F, Sozmen EY. The effects of estrogen and raloxifene treatment on the antioxidant enzymes and nitrite-nitrate levels in brain cortex of ovariectomized rats. Neurosci Lett. 2003;38(3):217–220. doi: 10.1016/S0304-3940(02)01416-7. [DOI] [PubMed] [Google Scholar]
- Olmos G, Aguilera P, Tranque P, Naftolin F, Garcia-Segura LM. Estrogen-induced synaptic remodelling in adult rat brain is accompanied by the reorganization of neuronal membranes. Brain Res. 1987;425(1):57–64. doi: 10.1016/0006-8993(87)90483-5. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54(1):175–180. doi: 10.1016/S0169-328X(97)00351-3. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85(10):3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labelling and impeded-lig, binding. FASEB J. 1995;9(5):404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. New York: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pérez C, Navarro A, Martínez E, Ordóñez C, Del Valle E, Tolivia J (2011) Age-related changes of apolipoprotein D expression in female rat central nervous system with chronic estradiol treatment. Age (Dordr) (in press) [DOI] [PMC free article] [PubMed]
- Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- Reisert I, Han V, Lieth E, Toran-Aller D, Pilgrim C, Lauder J. Sex steroids promote neurite growth in mesencephalic tyrosine hydroxylase immunoreactive neurons in vitro. Int J Dev Neurosci. 1987;5(2):91–98. doi: 10.1016/0736-5748(87)90054-2. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogenic effects on memory in women. Ann NY Acad Sci. 1994;743:213–231. doi: 10.1111/j.1749-6632.1994.tb55794.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:21–26. doi: 10.1212/WNL.48.5_Suppl_7.21S. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endoc Rev. 2003;24:131–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99(4):605–612. doi: 10.1016/S0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Rabkin E, Liao JK. Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3-kinase in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:198–203. doi: 10.1161/01.ATV.0000053846.71621.93. [DOI] [PubMed] [Google Scholar]
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/S0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirone C, Duckless SP, Krause DN. Multiple forms of estrogen receptor-α in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:184–192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Tolivia J, Navarro A, Tolivia D. Differential staining of nerve cells and fibres for sections of paraffin-embedded material in mammalian central nervous system. Histochemistry. 1994;102(2):101–104. doi: 10.1007/BF00269013. [DOI] [PubMed] [Google Scholar]
- Tolivia J, Navarro A, del Valle E, Pérez C, Ordóñez C, Martínez E. Application of Photoshop and Scion Image analysis to quantification of signals in histochemistry, immunocytochemistry and hybridocytochemistry. Anal Quant Cytol Histol. 2006;28(1):43–53. [PubMed] [Google Scholar]
- Toran-Aller CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov A. ER-X: a novel, plasma membrane-associated putative estrogen receptor that is regulated during development and following ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29(18):5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Túnez I, Collado JA, Medina FJ, Peña J, Del C, Muñoz M, Jimena I, Franco F, Rueda I, Feijóo M, Muntané J, Montilla P. 17 beta-Estradiol may affect vulnerability of striatum in a 3-nitropropionic acid-induced experimental model of Huntington’s disease in ovariectomized rats. Neurochem Int. 2006;48(5):367–373. doi: 10.1016/j.neuint.2005.11.011. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Holstege G. Estrogen induces axonal outgrowth in the nucleus retroambiguus-lumbosacral motoneuronal pathway in the adult female cat. J Neurosci. 1997;17(3):1122–1136. doi: 10.1523/JNEUROSCI.17-03-01122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29(2):238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor alpha and estrogen receptor beta gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/S0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Xing H, Mattick S, Lew D, Shapiro DJ. An N-terminal deletion mutant of estrogen receptor exhibits increased synergism with upstream activators and enhanced binding to the estrogen response element. Biochemistry. 1995;34(12):3956–3963. doi: 10.1021/bi00012a013. [DOI] [PubMed] [Google Scholar]