Abstract
With aging and Alzheimer’s disease (AD), there is an increased sensitivity to stress along with declines in the memory-associated neurotrophin brain-derived neurotrophic factor in AD. We have replicated this aging phenotype in cultured neurons from aged mice despite being grown in the same environmental conditions as young neurons. This led us to hypothesize that age-related differences in epigenetic acetylation and methylation of histones are associated with age-related gene regulation. We cultured hippocampal/cortical neurons from the 3xTg-AD mouse model and from non-transgenic mice to quantify single cell acetylation and methylation levels across the life span. In non-transgenic neurons, H3 acetylation was unchanged with age, while H4 acetylation decreased with age of the donor. Compared to non-transgenic neurons, 3xTg-AD neurons had higher levels of H3 and H4 acetylation beginning at 4 months of age. In contrast to non-transgenic neurons, 3xTg-AD neurons increased acetylation with age; 3xTg-AD neurons also responded differently to inhibition of histone deacetylases at an early age. Importantly, treatment of non-transgenic neurons with the AD peptide Aβ also elevated levels of acetylation. We also examined the repressive function of histone H3 lysine 9 (H3K9) methylation. H3K9 methylation increased with age in non-transgenic neurons, which was amplified further in 3xTg-AD neurons. The dominant effect of higher H3K9 methylation was supported by lower Bdnf gene expression in non-transgenic and 3xTg-AD mice. These data show that the epigenetic states of non-transgenic and 3xTg-AD brain neurons are profoundly different and reversible, beginning at 4 months of age when the first memory deficits are reported.
Keywords: 3xTg-AD, Histone methylation, Histone acetylation, Epigenetics
Introduction
Human longevity has increased considerably over the past century accompanied by an increase in age-related diseases (Oeppen and Vaupel 2002; Olshansky and Ault 1986). Aging is the strongest etiological factor for both late-onset forms of familial and sporadic Alzheimer’s disease (AD; Kukull et al. 2002). But how aging contributes to the onset of AD is unclear. Some have hypothesized that the disease is caused by the lifelong accumulation of damage from reactive oxygen species in brain neurons, others accumulation of beta-amyloid or neurofibrillary tangles or a combination of these. But since mutations in genes associated with AD such as the amyloid precursor protein (APP) or presenilin are present from conception, why are decades needed for development of memory problems? Here, we explore an alternative hypothesis that an epigenetic shift is associated with aging upstream of increased neuronal susceptibility to stressors and memory loss.
Some consider aging as the last epigenetically controlled stage of development (Perdiguero et al. 2009; Sanosaka et al. 2009). We were led to hypothesize that aging causes epigenetic changes (EORS theory; Brewer 2010) from our work with adult neurons from young and old rodent brains that have similar viability, resting glucose uptake, and resting respiration in culture, but show increased sensitivity to stressors such as beta-amyloid (Aβ) or glutamate despite the uniform culture environment (Brewer 1998). This increased sensitivity is difficult to explain in the same uniform environment because in culture, we have removed the neurons from aging immune, hormonal, and vascular systems. Therefore, there must be some intrinsic difference in old-age neurons that causes a poor response to stressors. Here, we determined whether epigenetic changes at the histone level could explain either the aging phenotype or changes associated with AD in a transgenic mouse model. The 3xTg-AD mouse has homozygous mutations in the APP and presenilin genes that increase deposition of beta-amyloid in the brain as well as increase phosphorylation of the human tau transgene. Importantly, although these mutations are present from conception, deficits in LTP and behavioral learning don’t appear until after 4 months of age (Oddo et al. 2003). Further, intracellular Aβ is first detected in the neocortex between 3 and 4 months and in the hippocampus at 6 months, when extracellular Aβ plaque deposition is first reported in these mice (Oddo et al. 2003). Here, we determined whether epigenetic changes to histones occur before, coincident, or after the 4-month milestone.
Epigenetic marks include DNA cytosine methylation and histone methylation and acetylation, among others. These modifications regulate gene transcription and the establishment of heterochromatin and euchromatin. Of particular importance are acetylation and methylation of the amino-terminal tails of histones H3 and H4 at specific lysine residues. Histone acetylation is facilitated by histone acetyltransferases (HATs). Acetylation generally promotes gene transcription by neutralizing the positively charged N-terminal tails of histones to lessen the affinity of the tail for negatively charged DNA, which presumably relaxes the DNA and allows access to various transcription factors and/or recruits proteins based on the acetyl-lysine group (Berndsen and Denu 2008). Histone deacetylases (HDACs) remove these acetyl groups (Fischle et al. 1999). Another type of modification is mediated by histone methyltransferases (HMTs), which add mono-, di- and trimethyl groups to histones to activate or repress expression depending on the location and number of methyl groups deposited (Sarnow et al. 1981). Histone lysine acetylation and methylation usually work in an antagonistic relationship with one another. One of the most intensely studied lysine modifications is at the core histone H3 lysine 9 (H3K9) of the N-terminal tail. H3K9 mono- or dimethylation is involved in transcriptional repression whereas trimethylation is involved in gene silencing (Black and Whetstine 2011). H3K9 acetylation promotes active gene expression (Turner 2000). Through the interplay of HATs, HDACs, HMTs, and demethylases, many genes are dynamically regulated at this important chromatin domain for control of tissue-type expression with development. These epigenetic enzymes have been extensively studied from diverse biological functions such as toxic resistance and disease state (Pirola et al. 2010; Liu et al. 2011). Therapeutic applications of HDAC and histone methyltransferase inhibitors are being developed against several diseases (Fischer et al. 2010). These agents provide evidence for a key link between epigenetics and behavior (Chen et al. 2011; Zhang et al. 2010). Importantly, inhibitors of class I and III HDACs ameliorate some of the cognitive defects seen in AD mouse models (Green et al. 2008; Kilgore et al. 2010).
Since memory formation requires brain-derived neurotrophic factor (BDNF), we wanted to determine whether histone marks associated with aging or AD in our transgenic mouse model would regulate Bdnf expression. Several studies show that BDNF expression is downregulated in AD brain in humans (Phillips et al. 1991; Holsinger et al. 2000; Peng et al. 2005) and in 4 of 6 different mouse AD models (Peng et al. 2009). In neurons cultured in a uniform environment from 3xTg-AD brains across the age-span compared to non-transgenic neurons, we find increased acetylation at the N-terminal tails of the core histones H3 and H4 with age. We also find decreased acetylation on H4 with age in non-transgenic neurons compared to the 3xTg-AD neurons. Further, H3K9 methylation increased in 3xTg-AD neurons at all ages relative to non-transgenic neurons. Finally, Bdnf expression declined with age and was lower in brains from 3xTg-AD mice. The acetylation and methylation were shown to be rapidly reversible with specific inhibitors.
Methods
Rodent care and the 3xTg-AD mouse model
Mice harboring the PS1 (M146V), APPSwe (KM670/671NL), and tau (P301L) transgenes driven by the Thy1.2 promoter and non-transgenic mice on the same background (Oddo et al. 2003) were graciously provided by the LaFerla lab. These mice, on a mixed (C57BL6/129) genetic background, were housed 1–4 animals/cage and fed ad lib (lab diet # 5001, Purina, distributed by El Mel, St Louis, MO; 28.5% calories from protein, 13.5% from fat, and 58% from carbohydrates). This study used male mice exclusively to preclude cycling hormonal differences. Primers used for PCR genotyping were: PSI forward (F) = 5′-cacacgcaactctgacatgcacaggc-3´, reverse (R) = 5′-aggcaggaagatcacgtgttcaagtac-3´; APP F = 5′-gcttgcaccagttctggatgg-3′; R = 5′-gaggtattcagtcatgtgct-3′; Tau F = 5′ gaggtattcagtcatgtgct-3′; R = 5′-ttcaaagttcacctgatagt-3′. All mice were treated under NIH guidelines approved by SIUSM. Animals were anesthetized with isoflurane before being sacrificed.
Adult neuron culture
In all experiments involving adult mouse neurons in culture, cells were derived from combined hippocampus and cortex tissues. Cells were incubated at 37°C, 5% CO2 and 9% O2, and 95% humidity. Adult neurons were maintained in Neurobasal-A medium (Invitrogen #10888) with B-27 supplement 50× (1:50; Invitrogen #17504-044) and GlutaMAX 100× (1:400; Invitrogen #35050-061), FGF2 (5 ng/mL, Invitrogen # PMG0035), and PDGFbb (5 ng/mL, Invitrogen # PMG0045) during experimental conditions. Adult neurons were grown on either 15 mm or 12 mm circular glass cover slips (Assistent # 1001 15, 12 mm Carolina Biologicals, Burlington, NC). We followed the detailed protocol for adult tissue isolation, homogenization, adult neuron purification, and culture (Brewer and Torricelli 2007). All experiments on adult neurons were performed after 7–12 days in culture, but each type of experiment was performed with cells of the same number of days in vitro. Live and dead cells were determined by fluorescence microscopy using fluorescein diacetate and propidium iodide, respectively (Brewer et al. 1993).
Cell fixation, antibodies and immunofluorescence
Cover slips were placed in a 12-well tissue culture plate (Falcon # 353043) and washed in pre-warmed, 37°C 1× PBS and fixed in 95% ethanol/5% acetic acid for approximately 1 min. After the fixative was washed off with PBS, the fixed cells were blocked in a 10% BSA, 1% NGS, 0.25% TritonX-100 in 1 × PBS solution for 1 hr at room temperature. Primary antibodies (rabbit anti-pan AcH3 1:1,000 (Millipore #06-599), rabbit anti-pan AcH4 1:1,000 (Active Motif #39243), rabbit anti-H3K9Me 1:1,000 (Active Motif #39242), mouse anti-MAP2 1:400 (Sigma M4403)) were diluted in block solution and incubated overnight at 4°C. Secondary antibodies (goat anti-mouse IgG Alexa 488 1:2,000 (Invitrogen # A11001), goat anti-rabbit IgG Alexa 568 1:2,000 (Invitrogen # A-11011)) were diluted in block solution and incubated at room temperature for 1 hr in the absence of light. Slips were secured to slides (Fisher Scientific # 22-038-103) using Aqua-Mount (Lerner Lab. # 13800) and allowed to dry overnight at 4°C. All images of neurons were taken on an Olympus IX71 inverted epifluorescent microscope in a single focal plane, through a 60× objective and a Retiga EXi (Q-imaging) camera at constant gain and exposure for each antibody, using Image ProPlus 7.0 software (Media Cybernetics, Bethesda, MD). Image thresholds were equal for each genotype for analysis of cellular fluorescence intensity with Image-Pro Plus.
Trichostatin A, BIX 01294 and Aβ treatment
Adult neurons were placed in a 12-well tissue culture plate and covered in medium supplemented as described above. Experimental wells contained trichostatin A (1 ng/ml serial diluted into medium from 1 mg/ml in DMSO, Sigma #T8552), BIX 01294 (1.5 μM diluted from a 1.67 mM stock in deionized water, Sigma #B9311) or Aβ 1-42 (10 μM prepared by incubating at 500 μM in PBS for 72 h then diluted in medium, American Peptide #62-0-80); control wells only contained medium + serial diluted DMSO, water or PBS at 37°C. Immunoblots with 6E10 antibody demonstrate the presence of oligomers in our preparation as well as fibers by staining with thioflavin S (data not shown; Paula-Lima et al. 2011). Cells adhered to slips were returned to the incubator overnight. They were fixed the following day for immunostaining as described previously.
RNA isolation and RTqPCR procedure
Hippocampal and cortical tissue (~25 mg) was excised from 2-, 11- or 21-month-old non-transgenic or 3xTg-AD mice and flash frozen immediately in liquid nitrogen. Frozen samples were covered in 1 ml of QIAzol lysis reagent (supplied in the RNeasy Lipid Tissue Mini Kit; Qiagen # 74804) and homogenized with a mini-pestle in a microcentrifuge tube. After homogenization, subsequent steps were carried out as directed by the manufacturer’s protocol to yield ~18 μg RNA. One microgram of total RNA was used to create a cDNA pool of ~30 μg utilizing the High Capacity RNA-to-cDNA Kit (Applied Biosystems # 4387406) following the company’s instructions. Sixty nanograms of cDNA was used in a 20 μl multiplex Taqman reaction using primers and probes for Bdnf (within exon IX), Bdnf IV (spanning the IV-IX junction), Bdnf V (spanning the V-IX junction) and Gapdh (Applied Biosystems #’s 4230607, 0432069, 4331182 and 4352339E, respectively) with a 2× master mix (Applied Biosystems # 4369016). qPCR reactions were carried out in the AB StepOne Plus PCR system (Applied Biosystems) according to manufacturer’s optimized conditions of 10 min at 95°C for enzyme activation followed by 40 cycles of 15 s denaturation at 95°C and 1 min anneal/extend at 60°C. Resulting numerical and graphical data from qPCR runs were analyzed using the StepOne Software v2.1 (Applied Biosystems). Bdnf expression was normalized to Gapdh with fold change differences determined using the 2ΔΔCt method. Consistent with Sieber et al. (2010), we found that Gapdh expression did not vary significantly with age.
Statistical analysis and graphical interpretation of data
All statistical analysis and graphs made from data derived from immunofluorescence studies and RTqPCR results were carried out using ProStat 5.0 software (Poly Software International, Pearl River, NY). P values were derived using Student’s t test and/or one-way or two-way ANOVA.
Results
Age-related hyperacetylation of H3 and H4 N-terminal lysines in 3xTg-AD neurons relative to non-Tg neurons
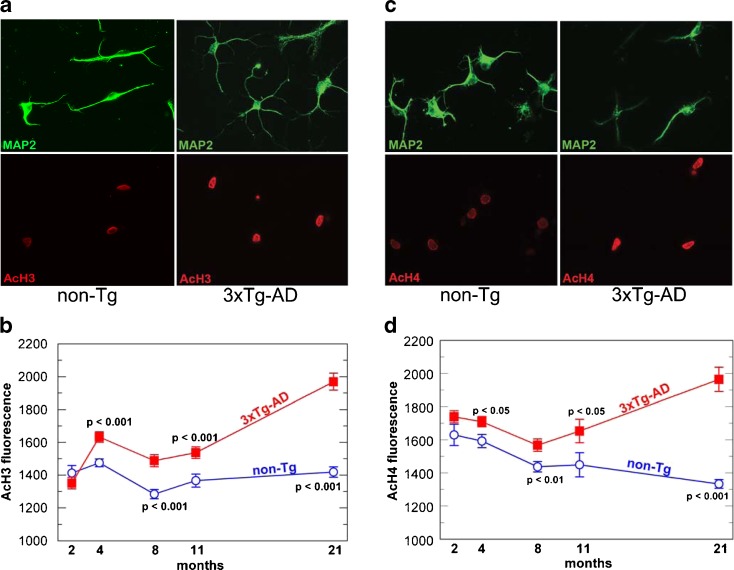
To find evidence for epigenetic regulation of brain aging, we immunostained isolated neurons with an antibody against acetylated lysines on histone H3 and H4 in cultured neurons from non-transgenic and 3xTg-AD mice. Since aging is the leading epidemiological factor in AD (Kukull et al. 2002) and aged rat neurons respond differently than embryonic neurons when assaulted with Aβ peptides (Brewer 1998), we chose to investigate the 3xTg-AD mouse model across the life span to determine whether epigenetic differences in the mouse, not only with age, but in the disease state could account for the differential susceptibility. Using the MAP2 antibody to identify neurons, which are approximately 80% of the cells in culture (Patel and Brewer 2003), we dual-stained these neurons to detect H3 lysine acetylation on the N-terminal tails (Fig. 1a). Qualitatively, it was apparent that the acetylation signal was higher in the AD neurons from 21-month-old mice, near their median life span (Fig. 1a). For neurons cultured from mice of various ages, H3 acetylation was quantitatively similar at 2 months, but by 4 months and beyond, acetylation increased in 3xTg-AD neurons compared to the non-transgenic neurons (Fig. 1b). We also observed no apparent difference between neurons from 2- to 21-month non-transgenic mice. In contrast, 3xTg-AD neurons showed elevated levels of acetylation signal that increased with age, culminating in an approximate 50% increase in signal when comparing 2-month to 21-month neurons. These data show that H3 N-terminal acetylation is profoundly different in 3xTg-AD neurons than non-transgenic mouse neurons and that this epigenetic difference switches early in adult 3xTg-AD mice compared to non-transgenic mice.
Fig. 1.
Higher H3 and H4 acetylation in neurons from 3xTg-AD brains exceeds acetylation in non-transgenic neurons with opposite effects of age. a In neurons from 21-month-old mice identified by staining for MAP2 (top panels), 3xTg-AD neurons have a higher intensity of AcH3 (bottom panels) immunoreactivity compared to non-transgenic neurons. b Increase with age in AcH3 in neurons from 3xTg-AD neurons (closed boxes) compared to steady levels in non-transgenic (non-Tg) neurons (open circles). For both genotypes n = 107–134 neurons from seven mice at 2 months; 223–261 neurons from six mice at 4 months; 146–148 neurons from five mice at 8 months; 54–57 neurons from four mice at 11 months; 85–100 neurons from five mice at 21 months. c Higher AcH4 (bottom panels) immunoreactivity in MAP2 (top panels) positive neurons compared to non-transgenic neurons. d Steady decline in AcH4 signal with age in non-transgenic neurons (open circles) contrasts with higher AcH4 signal from 3xTg-AD neurons (closed boxes) with age and genotype across the life span of mice. For both genotypes, n = 31–33 neurons from two mice at 2 months; 129 neurons from six mice at 4 months; 136–155 neurons from six mice at 8 months; 26–34 neurons from two mice at 11 months; 91–107 neurons from six mice at 21 months. Student’s t tests produced the indicated p values
To determine if increased histone acetylation in 3xTg-AD neurons was a general phenomenon, we also looked at core histone H4, which is also involved in gene regulation. As with H3 acetylation, qualitatively, we observed a more robust H4 acetylation signal in 3xTg-AD neurons (Fig. 1c). The intensity of the H4 acetylation signal across the age-span was consistently higher in 3xTg-AD neurons compared to non-transgenic neurons, beginning at 4 months (Fig. 1d). In agreement with previous studies on global histone levels (Petricevic et al. 1978), H4 acetylation steadily declined with age for the non-transgenic neurons, reaching a 25% decrease in acetylation levels from 2 to 21 months. In contrast, 3xTg-AD H4 acetylation first declined from 2 to 8 months, then increased with age after 8 months, with an approximate 11% increase from 2 to 21 months (Fig. 1d). These data show that H4 N-terminal acetylation is increased in 3xTg-AD neurons compared to non-transgenic neurons and, once again, this epigenetic difference is observed early in adult 3xTg-AD mice compared to non-transgenic mice.
HDAC inhibition reveals an intrinsic epigenetic switch at an early age
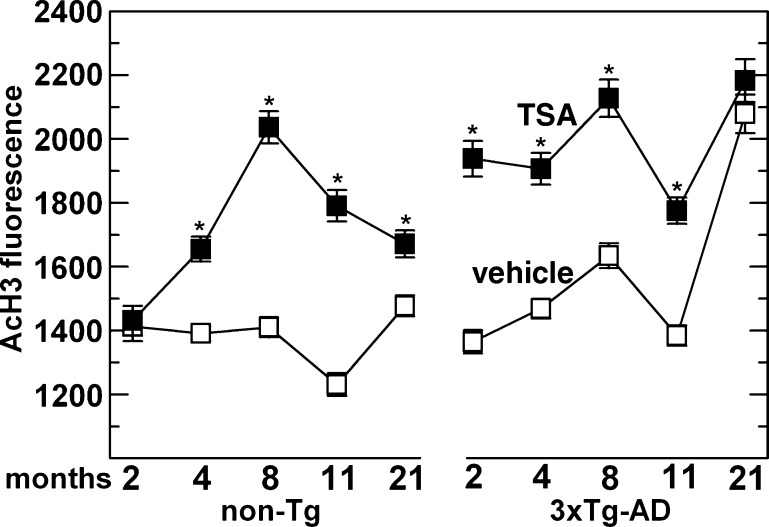
Since we observed a significant increase in H3 acetylation by 4 months of age in 3xTg-AD mouse neurons, we hypothesized that an epigenetic “switch”, pertaining to this particular chromatin modification, must occur at a relatively early age in adult mice. To test this notion, we treated both 3xTg-AD and non-transgenic neurons, either young or old, with the class I and II HDAC inhibitor TSA overnight and compared their acetylation profiles (Fig. 2). We found that non-transgenic neurons treated with TSA were unaffected at 2 months, but significantly increased their H3 acetylation up to maximum levels by 8 months. In contrast, 3xTg-AD neurons were maximally responsive to TSA at ages 2–11 months, but by 21 months, baseline acetylation was so high that TSA treatment increased acetylation no further. These observations suggest that non-transgenic neurons are TSA-insensitive at young ages, but become sensitive at middle-age, while 3xTg-AD neurons are TSA-sensitive early in life and become insensitive in old-age.
Fig. 2.
Reversal of epigenetic regulation of histone H3 acetylation by the histone deacetylase inhibitor trichostatin A (TSA). H3 acetylation was increased for all ages up to 11 months in 3xTg-AD neurons and for ages beyond 2 months in non-transgenic neurons. Indicated neurons were treated with TSA (solid boxes) or vehicle alone (DMSO; open boxes). For non-transgenic, n = 43–223 neurons; 3xTg-AD, n = 45–261 neurons from 2–7 animals for each treatment
Aβ 1–42 peptide induces hyperacetylation and TSA-reversible toxicity in non-transgenic neurons
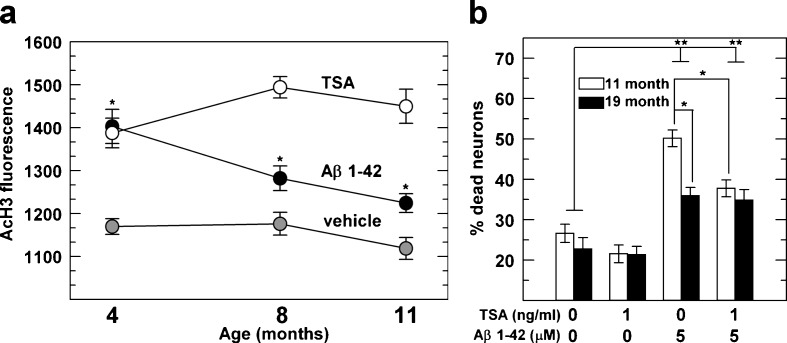
The Aβ 1–42 peptide form of β-amyloid is associated with AD. Aβ peptides can activate histone acetyltransferases (HAT; Cao and Sudhof 2001; Kinoshita et al. 2002). Therefore, we tested the hypothesis that Aβ 1–42 can increase H3 acetylation in non-transgenic neurons to phenocopy levels similar to 3xTg-AD neurons. As seen in Fig. 3a, both in young-age (4 months) and middle-age (8 and 11 months) non-transgenic neurons, Aβ 1–42 significantly increased H3 acetylation compared to non-treated neurons. TSA-treated neurons were used as a positive control. These data show that Aβ peptides redirect the epigenetic machinery to mimic the H3 hyperacetylation observed in 3xTg-AD neurons.
Fig. 3.
Treatment of non-transgenic neurons with Aβ partially increases AcH3 toward levels that are increased by TSA, which reduces Aβ 1–42 toxicity. a The AcH3 signal is significantly higher after Aβ (solid circles) or positive control of TSA treatment (open circles) compared to vehicle-treated neurons (gray circles). n = 98–169 neurons from 2–4 animals for each treatment group. b Increased toxicity from Aβ was partially reversed by TSA in non-transgenic middle-age neurons, but not in old neurons. Indicated neurons were treated with TSA, Aβ 1–42 and/or vehicle in cultures derived from 11 month (open bars) and 19 month (solid bars) mice. Percentage dead was measured as 100 × number dead / (number dead + live). Data represent 1826–3539 neurons per group from two mice. *p < 0.001, **p < 0.0001 by Student’s t test
We next tested the role of HDAC inhibition on induced Aβ 1–42 stress in non-transgenic neurons of middle (11 months) and old (19 months) ages. As expected (Brewer 1998), Aβ 1–42 treatment alone increased cell death in both adult ages (Fig. 3b), but middle-age neurons were significantly more susceptible to Aβ 1–42 stress than old-age neurons. Surprisingly, TSA treatment was able to significantly ameliorate Aβ toxicity in middle-age neurons, but not in old-age neurons, suggesting that in aged neurons increased acetylation is less effective in stress reduction. These observations lead us to conclude that there is an intrinsic epigenetic switch that occurs with age.
Bdnf mRNA expression is lower in 3xTg-AD mice
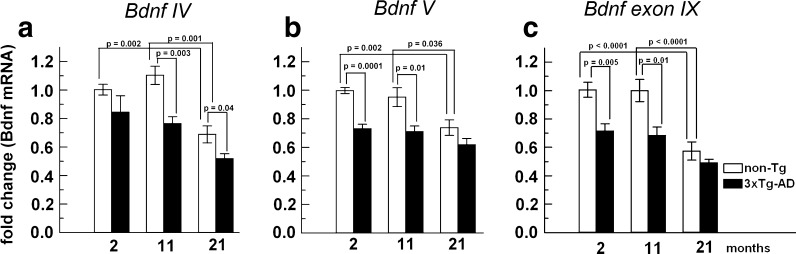
Bdnf is an essential gene involved in neuronal development, activity, and memory (Lipsky and Marini 2007). In AD brain, BDNF expression is diminished (Peng et al. 2005; Hock et al. 2000). Moreover, transcription of Bdnf decreased in four of six AD mouse models, but age was not examined independently (Peng et al. 2009). Since increased acetylation is indicative of higher gene expression (Marushige 1976), and in light of our observations of hyperacetylation on H3 and H4, we determined whether the mRNA levels of Bdnf in the 3xTg-AD mice were lower, as in AD and other mouse models or higher, if transcription was increased by higher histone acetylation. Nine major transcript variants of Bdnf exist due to alternate promoter choice with more variants involving polyadenylation sites, yet all code for the same precursor protein (Lubin et al. 2011). Bdnf IV and Bdnf V are highly expressed in the hippocampus and cortex of adult mice compared to other brain regions (Aid et al. 2007). With this in mind, we collected brain hippocampal/cortical RNA from both 3xTg-AD and non-transgenic animals and performed RT qPCR analysis on these particular Bdnf variants internally compared in each assay well to Gapdh expression, which did not change with age per ng cDNA. Relative transcript levels of Bdnf IV, V and exon IX (Fig. 4a–c) were significantly lower for 3xTg-AD animals compared to non-Tg (two-way ANOVA, p < 0.001). Each of these transcripts also declined with age (p < 0.001), with a non-significant interaction of age and genotype, indicating similar responses with age and genotype. These data suggest that the observed hyperacetylation of H3 and H4 (Fig. 1b and d, respectively), which would be expected to increase expression does not directly control the observed decrease in Bdnf expression with age or genotype.
Fig. 4.
Bdnf IV, V and exon IX expression is significantly reduced with age and more so in 3xTg-AD mouse hippocampus/cortex brain compared to extracts from non-transgenic brain. qPCR analysis of cDNA from non-transgenic (open bars) and 3xTg-AD (solid bars). a Bdnf IV significantly decreases with age and in 3xTg-AD mice. Two-way ANOVA for age and genotype: age: F(2,29) = 16.1, p < 0.0001; genotype: F(1,29) = 17, p < 0.0001; interaction: (2, 29) = 1.2, p = 0.33. b Bdnf V significantly decreases with age and in 3xTg-AD mice. Two-way ANOVA for age and genotype: age: F(2,29) = 9.6, p = 0.001; genotype: F(1,29) = 32.2, p < 0.0001; interaction: (2, 29) = 1.5, p = 0.24. c Bdnf exon IX (this amplicon is internal to exon IX to cover all Bdnf variants and should not be confused with Bdnf IXa) significantly decreases with age and in 3xTg-AD mice. Two-way ANOVA for age and genotype: age: F(2,29) = 20.1, p < 0.0001; genotype: F(1,29) = 23.7, p < 0.0001; interaction: (2, 29) = 2.4, p = 0.112. Brackets indicate Student’s t test. n = 5 animals per group
H3K9 is hypermethylated in 3xTg-AD neurons
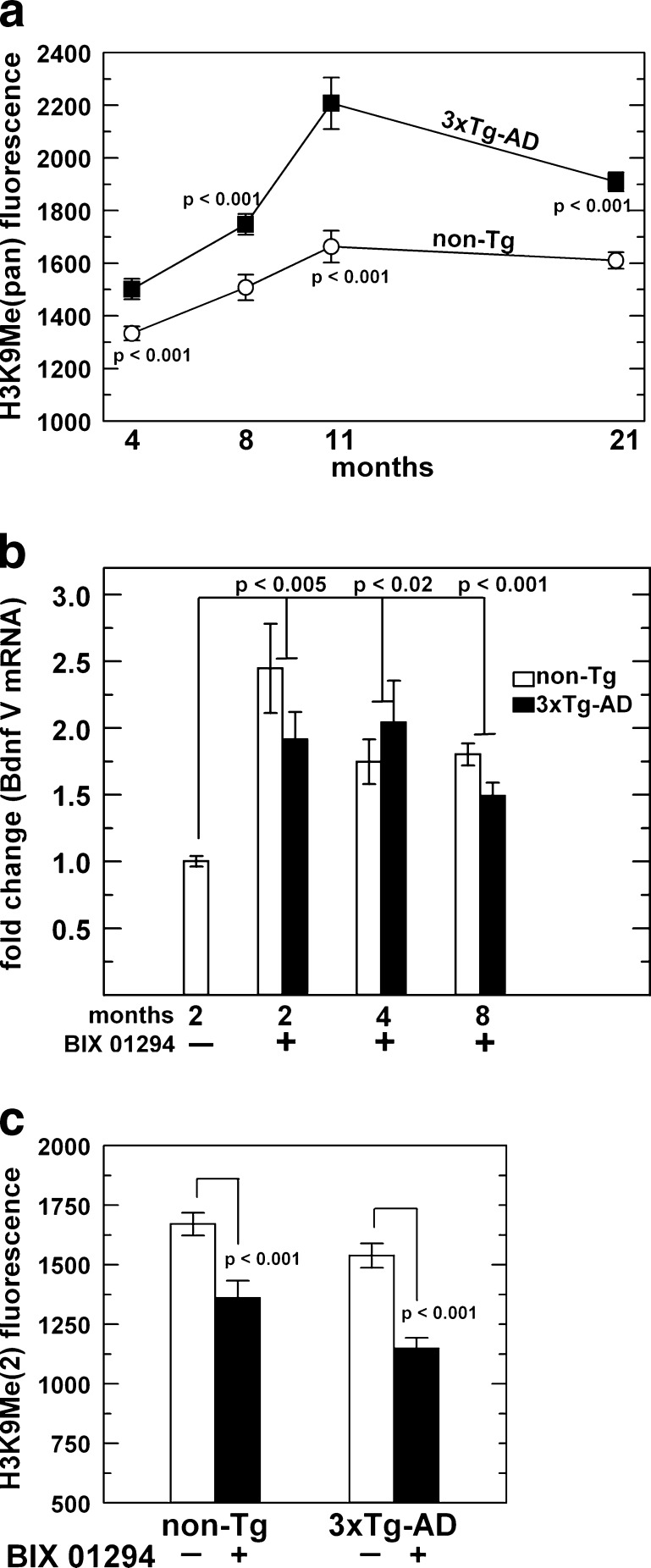
Because we did not see an increase in Bdnf expression, but in fact, observed a decrease in gene expression with age and 3xTg-AD genotype, we tested the possibility that another chromatin modification could be causing this phenomenon. The antibodies used to detect acetylation among the N-terminal tails of core histones H4 and H3 were pan specific. This could mean that while we do observe an overall increase in acetylation, indicative of promoting gene transcription, there could be more important gene repressive modifications with age and in 3xTg-AD neurons. Since methylated lysines can induce gene transcription or gene repression, we chose a specific residue known to be gene repressive, H3K9Me (Lachner et al. 2001). Utilizing the same technique to analyze acetylation levels as described above, we observed increased immunoreactivity to H3K9Me in the 21-month 3xTg-AD neurons compared to non-transgenic neurons. Analysis of the immunofluorescence per cell across the age-span revealed an age-related increase in each genotype (ANOVA, p ≤ 0.001), but H3K9 methylation was exacerbated in the 3xTg-AD neurons (Fig. 5a). This elevated level of H3K9Me could diminish Bdnf expression, more dominantly than the hyperacetylated marks in 3xTg-AD neurons.
Fig. 5.
Higher methylation at histone H3 lysine 9 (H3K9Me) with age is exacerbated in 3xTg-AD compared to non-transgenic neurons. a Quantitation of the H3K9Me signal across the life span shows significantly higher methylation signal in 3xTg-AD neurons (closed boxes) compared to non-transgenic neurons (open circles). One-way ANOVA for age: non-Tg: F(3,288) = 7.3, p = 0.001; 3xTg-AD: F(3,319) = 15, p < 0.0001, n = 79–101 neurons from two mice at 4 months; 71–89 neurons from two mice at 8 months; 22–38 neurons from two mice at 11 months; 105–110 neurons from two mice at 21 months. Treatment with methyl transferase inhibitor BIX 01294 increases Bdnf expression and lowers H3K9Me(2). b qPCR analysis of non-transgenic (open bars) and 3xTg-AD (solid bars). n = separate cultures from four mice per age group from which RNA was isolated for each treatment; each treated age was compared to the 2-month non-Tg untreated group by one-way ANOVA for treatment: 2 month: F(2,9) = 10.2, p = 0.005; 4 month: F(2,9) = 7.6, p = 0.02; 8 month: F(2,9) = 26.9, p = 0.001. c Quantitation of the H3K9Me(2) signal comparing untreated (open bars) and BIX 01294 treated (solid bars) neurons. n = 71–153 neurons from three mice. Brackets indicate Student’s t test
If H3K9 hypermethylation is a determining factor in lower Bdnf expression, then removal of this histone mark should increase Bdnf transcripts. To test this hypothesis we treated neuronal cultures from 2-, 4- and 8-month mice with a known G9a histone methyltransferase inhibitor, BIX 01294 (Kubicek et al. 2007) to determine the effect on Bdnf expression. When neurons were treated with BIX 01294, Bdnf expression increased about 2-fold across 2-, 4- and 8-month non-transgenic and 3xTg-AD adult neurons (Fig. 5b). G9a specifically targets H3 lysine 9 for mono- and/or dimethylation in euchromatin (Tachibana et al. 2001, 2002). To verify that increased Bdnf transcription was due to inhibition of G9a function, we stained neurons treated with BIX 01294 with an H3K9 dimethyl antibody. BIX treatment reduced the H3K9 dimethyl histone mark in combined neurons from 2- and 4-month mice (Fig. 5c). Together, these data indicate that G9a mediated H3K9Me is an important epigenetic mark for Bdnf regulation in adult mouse neurons.
Discussion
Since organ development is controlled by epigenetic modifications of DNA and histones and aging may be the last stage of development (Perdiguero et al. 2009; Sanosaka et al. 2009), determining the incidence of these marks and their reversibility is of paramount importance to understanding aging and age-related diseases such as Alzheimer’s disease. In this study, we compared the effect of aging on neurons from a non-transgenic mouse and a 3xTg-AD model mouse. We found that H4 acetylation was decreased in neurons from old mouse hippocampus/cortex and that middle-age neurons were susceptible to Aβ stress. Importantly, acetylation and diminished neuronal viability are reversible by treatment with the HDAC inhibitor trichostatin A. With age, global histone acetylation levels are reduced in rat brain tissue (Pina et al. 1988). Our results show that as the brain ages, there are clear histone acetylation changes in the neurons, which are reversible, and theoretically amenable to therapy. Further, the observed age-related decline in specific transcripts of the memory-essential Bdnf gene could contribute to memory deficits. Indeed, treatment of rat embryonic neurons or mouse Neuro-2a neuroblastoma cells (in medium with growth factor-rich fetal bovine serum) with the histone deacetylase inhibitors profoundly increases Bdnf expression through hyperacetylation of histones at the Bdnf promoters (Tian et al. 2010; Ishimaru et al. 2010). However, these cell culture systems are profoundly different from our adult mouse hippocampal/cortical neurons in a serum-free medium. While, these studies are in direct contrast to our observation of hyperacetylation and downregulation of Bdnf expression, it is by no means a novel phenomenon. Huysseune et al. (2009) recently showed that hyperacetylation induced by TSA treatment in mouse fibroblasts that express APP actually decreases the mRNA levels of aquaporin 1 and this downregulation is dependent on de novo protein synthesis, suggesting that a repressive element is upregulated after TSA treatment. Therefore, it is reasonable to deduce that the increased hyperacetylation we observe in cultured neurons in this study is either not substantially contributing to Bdnf promoter regulation or that some other repressive protein(s) upregulated by increased histone acetylation may be acting to diminish Bdnf mRNA levels. While we only measured global H3 and H4 N-terminal acetylation at basal levels, others have reported that with memory formation, increased histone acetylation causes a corresponding in vivo increase in Bdnf transcript levels (Bredy et al. 2007; Fuchikami et al. 2010). It will be interesting to determine whether the age and AD-related H3 and H4 hyperacetylation of hippocampal/cortical neurons change with memory formation in aged mice and in an Alzheimer mouse model.
Recently, a study in a pair of monozygotic twins revealed the importance of DNA methylation in a discordant case of AD (Mastroeni et al. 2009). The AD twin had significantly lower amounts of DNA methylation in the neocortex. It was postulated that separate environmental conditions led to the difference in epigenetic state among the twins. DNA methylation is involved in turning off gene transcription, which is important to restrict gene expression in certain tissues (Feng et al. 2010). Aberrant DNA methylation has been postulated to be a mitigating factor in AD (Fleming et al. 2011). Here we found increased acetylation levels of H3 and H4 in 3xTg-AD neurons beginning at 4 months of age that continued to increase with age. Whether this is the cause or effect of lowered DNA methylation, it remains a tantalizing question. Although not explored in this study, it is plausible that elevated histone acetylation leads to a higher APP transcription rate and consequently elevated Aβ levels in 3xTg-AD neurons. Recently, it was revealed that HAT (p300/CREB-binding protein-associated factor) knock-out mice are not susceptible to Aβ toxicity (Duclot et al. 2010). Also, APP fragments have been shown in a complex with the HAT, Tip60 (Kinoshita et al. 2002). These studies highlight the importance of interplay of APP processing, HAT activity and amyloid toxicity. We found out that Aβ treatment significantly increased H3 acetylation in non-transgenic neurons. This increase is somewhat puzzling since HDAC inhibition and subsequent increase in acetylation in the 3xTg-AD mouse model is associated with improved cognitive function (Green et al. 2008; Kilgore et al. 2010). Whether or not the observed acetylation increase in Aβ treated or 3xTg-AD neurons directly correlate with AD pathology, it is a matter of speculation at this point. Perhaps hyperacetylation in AD leads to over expression of products favoring pathology such as APP, γ-secretase components as well as others. However, it should also be noted that HAT activities target not only lysine residues of histones but also many non-histone proteins (Yang and Seto 2007), any number of which could contribute to AD pathology. For example, activation of the epigenetic HDAC SIRT3 by caloric restriction acetylates and activates SOD2 (Qiu et al. 2010).
Bdnf is an essential neurotrophin involved in neuron formation, maintenance, and memory formation. Memory formation dynamically affects acetylation of H3 and H4 N-terminal tails. For example, fear conditioning with extinction not only increases Bdnf I and IV transcript variants in the mouse prefrontal cortex but also decreases lysine acetylation of H3 and increases acetylation of H4 at the promoters of Bdnf I and IV, respectively (Bredy et al. 2007). Also, fear conditioning in post traumatic stress disorder model animals causes an increase in Bdnf I, IV and IXa variants as well as increased acetylation of H3 and H4 at the promoters of Bdnf I and IV in the rat hippocampus (Fuchikami et al. 2010). Because memory is greatly affected in AD, the role of Bdnf in AD has been widely examined (Caccamo et al. 2010; Connor et al. 1997; Peng et al. 2009). It is known that downregulation of BDNF is an early-stage indicator in the progression of AD (Peng et al. 2005). In light of our findings with increased acetylation, we examined the amount of Bdnf expression with age and in 3xTg-AD animals and found a significant decrease compared to non-transgenic controls. Also, we observed a significant decrease in Bdnf expression from 2- to 21-month-aged animals. In support of our findings, Bdnf expression is known to be downregulated in four other AD models (Peng et al. 2009). Bdnf protein levels are lower in the 3xTg-AD hippocampi, and these levels have been restored by neuronal stem cell transfer or over expression of Creb binding protein through viral delivery (Blurton-Jones et al. 2009; Caccamo et al. 2010). These animals not only increased Bdnf expression but cognitive defects seen in the untreated 3xTg-AD mice were also ameliorated, thus suggesting an important role of Bdnf expression in AD.
In contrast to our findings with age, Bdnf mRNA levels are reported to remain constant throughout adult life in rats and humans (Webster et al. 2006, 2002; Silhol et al. 2008). However, these findings are somewhat confounding in that Bdnf mRNA levels are reported relative to total RNA concentration and not mass of the brain tissue. Webster et al. (2002) reported no change in BDNF mRNA/μg of total RNA extracted from young adult, adult, and aged human dorsolateral prefrontal cortex. However, since the aged total μg RNA/mg tissue was one-half that of adults, BDNF mRNA does decline with age/mass of brain. In a follow-up study, Webster et al. (2006) examined human hippocampus and temporal cortex by autoradiography with specific probes for BDNF mRNA. No age-related changes were reported, but again the measures were made on regions of interest in autoradiographic density, which reflects concentration, not mass of BDNF mRNA/mass of total brain region. In a study of two strains of aging rats by Silhol et al. (2008), Bdnf mRNA levels were not decreased as measured by PCR relative to cyclophilin, but again if there were any cell loss in the hippocampus or if the mitochondrial cyclophilin decreased proportionally, total hippocampal mRNA could be less with age without affecting the remaining Bdnf concentration. In spite of these concerns, we also measured Bdnf mRNA as a measure of concentration relative to total RNA, and did observe a significant decrease with age. Importantly, the endogenous gene (Gapdh) we used does not change with age in rodents (Slagboom et al. 1990; Sieber et al. 2010), and we measured Gapdh levels with age, finding no significant differences. Looking at these observed differences in age, we see the result of altered Bdnf expression. While other groups have looked at Bdnf levels in mice, to our knowledge, we are the first to analyze Bdnf transcripts as a measure of age in the adult mouse with an appropriate internal control of Gapdh. Other reasons for the conflicting results observed in rats and humans compared to the mouse could be due to species disparities, environmental differences, or genetic variation or any combination of these factors.
In light of recent studies and our own findings pertaining to Bdnf, we investigated the role of the repressive H3K9 methylation mark and discovered significantly elevated levels of H3K9 methylation across the life span of both non-transgenic and even higher in 3xTg-AD neurons. The observation that H3K9 methylation was higher in the 3xTg-AD neurons at 4 months raises the possibility that this histone modification causes the learning deficits which begin at 4 months in this mouse. Several mouse studies find associations of histone methylation or acetylation with acquisition or extinction of memory (Bredy et al. 2007; Kramer et al. 2011; Maurice et al. 2008), and as with acetylation, histone methylation also actively changes with memory. Rats exposed to fear conditioning show increases in H3K4 tri-methylation, a transcriptionally active mark, at the promoter of Bdnf I in the hippocampus (Gupta et al. 2010). Mice that are exposed to environmental enrichment not only increase in H3K4 tri-methylation but also decrease in repressive transcriptional marks H3K9 and 27 tri-methylation at the promoters of Bdnf variants causing an increase in total Bdnf mRNA levels (Kuzumaki et al. 2011). The increase in H3K9 methylation explains the lower Bdnf expression that we and others observe in the hippocampus and cortex of AD-like mice but does not reveal how or why this mark is increased. Bdnf expression is under the control of the master regulator transcription factor, neuron restrictive silencer factor (NRSF, also known as REST; Hara et al. 2009). This transcription factor targets neuronal genes, including Bdnf in non-neuronal and neuronal tissues. NRSF recruits the methyltransferase G9a and its co-factor, GLP, to target genes, where it preferentially dimethylates H3K9 (Roopra et al. 2004). We showed in cultured neurons that the G9a-specific inhibitor, BIX 01294, is able to lower H3K9 dimethylation and results in an increase in Bdnf expression in non-transgenic and 3xTg-AD neurons. Currently, histone lysine methylation studies in AD are lacking (Cyr and Domann 2011). To our knowledge, this is the first report that suggests G9a’s involvement (direct or indirect) in Bdnf regulation within the hippocampus/cortex. It should be noted that G9a has been shown to directly bind to the Bdnf VI promoter and modulate H3K9 dimethylation within the mouse nucleus accumbens (Maze et al. 2010). We believe this is important because it opens the door to a less invasive pharmacological method of increasing Bdnf expression, which may be able to restore function to AD patients. Also, since BIX 01294 targets only G9a, less off-targets effects would be expected when compared to using class specific HDAC inhibitors (Tuma 2010). Finally, the decrease in Bdnf expression with age offers an exciting prospect that G9a inhibition could be used to slow the aging process, at least in terms of Bdnf levels with possible slowing of cognitive decline.
Acknowledgments
This study is supported by NIH grants R01 AG032431 and AG013435. We are especially appreciative of Kelsey LeVault for preparing cultures.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndsen CE, Denu JM. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr Opin Struct Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JC, Whetstine JR. Chromatin landscape: methylation beyond transcription. Epigenetics. 2011;6:9–15. doi: 10.4161/epi.6.1.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol Aging. 1998;19:561–568. doi: 10.1016/S0197-4580(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Brewer GJ (2010) Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol 45:173–179 [DOI] [PMC free article] [PubMed]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Maldonado MA, Bokov AF, Majumder S, Oddo S. CBP gene transfer increases BDNF levels and ameliorates learning and memory deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:22687–22692. doi: 10.1073/pnas.1012851108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptively active complex of app with fe65 and histone acetyltransferase tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Chen ES, Ernst C, Turecki G. The epigenetic effects of antidepressant treatment on human prefrontal cortex BDNF expression. Int J Neuropsychopharmacol. 2011;14:427–429. doi: 10.1017/S1461145710001422. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Mol Brain Res. 1997;49:71–81. doi: 10.1016/S0169-328X(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Cyr AR, Domann FE. The redox basis of epigenetic modifications: from mechanisms to unctional consequences. Antioxid Redox Signal. 2011;15:551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclot F, Meffre J, Jacquet C, Gongora C, Maurice T. Mice knock out for the histone acetyltransferase p300/CREB binding protein-associated factor develop a resistance to amyloid toxicity. Neuroscience. 2010;167:850–863. doi: 10.1016/j.neuroscience.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Mungenast A, Tsai LH. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sc. 2010;31:605–617. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Fischle W, Emiliani S, Hendzel MJ, Nagase T, Nomura N, Voelter W, Verdin E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J Biol Che. 1999;274:11713–11720. doi: 10.1074/jbc.274.17.11713. [DOI] [PubMed] [Google Scholar]
- Fleming JL, Phiel CJ, Toland AE (2011) The role for oxidative stress in aberrant DNA methylation in Alzheimer's disease. Curr Alzheimer Res. doi:10.2174/1567212216050622050 [DOI] [PubMed]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara D, Fukuchi M, Miyashita T, Tabuchi A, Takasaki I, Naruse Y, Mori N, Kondo T, Tsuda M. Remote control of activity-dependent BDNF gene promoter-I transcription mediated by REST/NRSF. Biochem Biophys Res Commun. 2009;384:506–511. doi: 10.1016/j.bbrc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76:347–354. doi: 10.1016/S0169-328X(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Huysseune S, Kienlen-Campard P, Hébert S, Tasiuax B, Leroy K, Devuyst O, Brion J, De Strooper B, Octave J. Epigentic control of aquaporin 1 expression by the amyloid precursor protein. FASEB J. 2009;23:4158–4167. doi: 10.1096/fj.09-140012. [DOI] [PubMed] [Google Scholar]
- Ishimaru N, Fukuchi M, Hirai A, Chiba Y, Tamura T, Takahashi N, Tabuchi A, Tsuda M, Shiraishi M. Differential epigenetic regulation of BDNF and NT-3 genes by trichostatin A and 5-aza-2'-deoxycytidine in Neuro-2a cells. Biochem Biophys Res Commun. 2010;394:173–177. doi: 10.1016/j.bbrc.2010.02.139. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Whelan CM, Berezovska O, Hyman BT. The gamma secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J Biol Chem. 2002;277:28530–28536. doi: 10.1074/jbc.M203372200. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Kochinke K, Oortveld MA, Marks H, Kramer D, de Jong EK, Asztalos Z, Westwood JT, Stunnenberg HG, Sokolowski MB, Keleman K, Zhou H, van Bokhoven H, Schenck A. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011;9:e1000569. doi: 10.1371/journal.pbio.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Tamura R, Hareyama N, Imai S, Narita M, Torigoe K, Niikura K, Takeshima H, Ando T, Igarashi K, Kanno J, Ushijima T, Suzuki T, Narita M. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21:127–132. doi: 10.1002/hipo.20775. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. 2007;1122:130–143. doi: 10.1196/annals.1403.009. [DOI] [PubMed] [Google Scholar]
- Liu TF, Yoza BK, El GM, Vachharajani VT, McCall CE. NAD + −dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem. 2011;286:9856–9864. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Gupta S, Parrish RR, Grissom NM, Davis RL (2011) Epigenetic mechanisms: critical contributors to long-term memory formation. Neuroscientist 17:616–632 [DOI] [PubMed]
- Marushige K. Activation of chromatin by acetylation of histone side chains. Proc Natl Acad Sci U S A. 1976;73:3937–3941. doi: 10.1073/pnas.73.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One. 2009;4:e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Celerier A, Jacquet C, Copois V, Mechti N, Ozato K, Gongora C. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Covington HE, III, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography: broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Q. 1986;64:355–391. doi: 10.2307/3350025. [DOI] [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- Paula-Lima AC, Adasme T, Sanmartin C, Sebollela A, Hetz C, Carrasco MA, Ferreira ST, Hidalgo C. Amyloid β-Peptide oligomers stimulate RyR-mediated Ca(2+) release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic ppine remodeling produced by BDNF. Antioxid Redox Signal. 2011;14:1209–1223. doi: 10.1089/ars.2010.3287. [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, Mount HT, Mufson EJ, Salehi A, Fahnestock M. Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer's disease. J Neurosci. 2009;29:9321–9329. doi: 10.1523/JNEUROSCI.4736-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E, Sousa-Victor P, Ballestar E, Munoz-Canoves P. Epigenetic regulation of myogenesis. Epigenetics. 2009;4:541–550. doi: 10.4161/epi.4.8.10258. [DOI] [PubMed] [Google Scholar]
- Petricevic M, Denko CW, Messineo L. Temporal changes in histone acetylation. Mech Ageing Dev. 1978;8:241–248. doi: 10.1016/0047-6374(78)90023-4. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Pina B, Martinez P, Suau P. Differential acetylation of core histones in rat cerebral cortex neurons during development and aging. Eur J Biochem. 1988;174:311–315. doi: 10.1111/j.1432-1033.1988.tb14099.x. [DOI] [PubMed] [Google Scholar]
- Pirola L, Balcerczyk A, Okabe J, El-Osta A. Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol. 2010;6:665–675. doi: 10.1038/nrendo.2010.188. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol Cell. 2004;14:727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Sanosaka T, Namihira M, Nakashima K. Epigenetic mechanisms in sequential differentiation of neural stem cells. Epigenetics. 2009;4:89–92. doi: 10.4161/epi.4.2.8233. [DOI] [PubMed] [Google Scholar]
- Sarnow P, Rasched I, Knippers R. A histone H4-specific methyltransferase: properties, specificity and effects on nucleosomal histones. Biochim Biophys Acta. 1981;655:349–358. doi: 10.1016/0005-2787(81)90045-9. [DOI] [PubMed] [Google Scholar]
- Sieber MW, Guenther M, Kohl M, Witte OW, Claus RA, Frahm C. Inter-age variability of bona fide unvaried transcripts normalization of quantitative PCR data in ischemic stroke. Neurobiol Aging. 2010;31:654–664. doi: 10.1016/j.neurobiolaging.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Silhol M, Arancibia S, Perrin D, Maurice T, Alliot J, Tapia-Arancibia L. Effect of aging on brain-derived neurotrophic factor, proBDNF, and their receptors in the hippocampus of Lou/C rats. Rejuvenation Res. 2008;11:1031–1040. doi: 10.1089/rej.2008.0791. [DOI] [PubMed] [Google Scholar]
- Slagboom PE, de Leeuw WJ, Vijg J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990;53:243–257. doi: 10.1016/0047-6374(90)90042-E. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Marini AM, Lipsky RH. Effects of histone deacetylase inhibitor Trichostatin A on epigenetic changes and transcriptional activation of Bdnf promoter 1 by rat hippocampal neurons. Ann N Y Acad Sci. 2010;1199:186–193. doi: 10.1111/j.1749-6632.2009.05175.x. [DOI] [PubMed] [Google Scholar]
- Tuma RS. Targeted epigenetic therapies: the next frontier? J Natl Cancer Inst. 2010;102:1824–1825. doi: 10.1093/jnci/djq520. [DOI] [PubMed] [Google Scholar]
- Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139:139–150. doi: 10.1016/S0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, Shannon WC. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human life span. Gene Expr Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]