Abstract
Frailty is a multidimensional geriatric syndrome characterised by a state of increased vulnerability to disease. Its causes are unclear, limiting opportunities for intervention. Age-related changes to the immune-endocrine axis are implicated. This study investigated the associations between the immune-endocrine axis and frailty as well as mortality 10 years later among men and women aged 65 to 70 years. We studied 254 participants of the Hertfordshire Ageing Study at baseline and 10-year follow-up. At baseline, they completed a health questionnaire and had collection of blood samples for immune-endocrine analysis. At follow-up, Fried frailty was characterised and mortality ascertained. Higher baseline levels of differential white cell counts (WCC), lower levels of dehydroepiandosterone sulphate (DHEAS) and higher cortisol:DHEAS ratio were all significantly associated with increased odds of frailty at 10-year follow-up. Baseline WCC and cortisol:DHEAS clearly discriminated between individuals who went on to be frail at follow-up. We present the first evidence that immune-endocrine biomarkers are associated with the likelihood of frailty as well as mortality over a 10-year period. This augments our understanding of the aetiology of frailty, and suggests that a screening programme at ages 60–70 years could help to identify individuals who are at high risk of becoming frail and who would benefit from early, targeted intervention, for example with DHEA supplementation or anti-inflammatory strategies. Progress towards the prevention of frailty would bring major health and socio-economic benefits at the individual and the population level.
Keywords: Frailty, Aging, Immunosenescence, Inflam-aging, White Blood Cells, DHEAS, Screening
Introduction
Frailty is a multidimensional geriatric syndrome (Bauer and Sieber 2008); it may be described as a state of increased vulnerability which results from decreased physiological reserves, multisystem dysregulation and limited capacity to maintain homeostasis (van Abellan et al. 2008). Frailty is of increasing global importance, impacting on all forms of adult health care as well as socio-economic policy. A person who is frail is less likely to be living independently at home, and people who are frail have an increased likelihood of morbidity and mortality (Cawthon et al. 2007; Fried et al. 2001). Frailty also has a predictive validity for adverse health outcomes including falls, disability and receipt of hospital care (Fried et al. 2001; Avila-Funes et al. 2008). A major health-care challenge of the 21st century is to identify people at risk of becoming frail and intervene early.
The Fried criteria (Fried et al. 2001), the most widely implemented objective approach to the classification of frailty, define frailty on the basis of weight loss, weakness, exhaustion, slowness and low activity. Prevalence estimates were recently reported as 8.5% for women and 4.1% for men among community-dwelling people aged 64 to 74 years (Syddall et al. 2010a). The numbers of frail older people will increase as population age, particularly as the numbers at the oldest ages are increasing the fastest.
Age-related changes in the immune-endocrine axis are implicated in a wide range of disease processes in older people, causing significant morbidity and mortality (De et al. 2006). For example, white blood cells are positively associated with cardiovascular disease, cancer and all-cause mortality (Grimm et al. 1985). Levels of dehydroepiandosterone sulphate (DHEAS) decline with age, and these too are associated with increased morbidity including cardiovascular disease (Thijs et al. 2003), osteoporosis (Zofkova and Hill 2008) and all-cause mortality (Phillips et al. 2010). Relationships between low thyroid function and lifespan in older people have also been noted, and raised serum-free T4 is associated with increased mortality in the oldest old (Parle et al. 2001).
These changes are also implicated in the frailty syndrome. Cross-sectional associations have been demonstrated with C-reactive protein (CRP), interleukin (IL)-6, total white blood cell counts (WCC), neutrophils, monocytes, insulin-like growth factor-1 and DHEAS (Walston et al. 2002; Voznesensky et al. 2009; Leng et al. 2004; Leng et al. 2007). However, causality cannot be determined in cross-sectional studies, and to date, only a small number of studies have examined longitudinal associations between inflammation and frailty (Puts et al. 2005; Curnow et al. 2005). Few other biomarkers associated with the onset of frailty have yet been identified. The objective of the current study was to investigate the associations between biomarkers of the immune-endocrine axis and frailty as well as all-cause mortality 10 years later among community-dwelling men and women aged 65 to 70 years.
Methods
The Hertfordshire Ageing Study (HAS) has been described previously (Syddall et al. 2010b). In brief, 717 men and women who were born in Hertfordshire, UK between 1920 and 1930 attended a home interview and clinic in 1994/5 where a wide range of markers of ageing were characterised. In 2003/5, a 10-year follow-up was conducted; 359 men and women participated in a home interview of whom 254 attended a clinic at which Fried frailty was assessed (Syddall et al. 2010a). All-cause mortality was ascertained between the two phases.
At the 1994/5 baseline HAS home interview, a trained research nurse ascertained smoking habit, alcohol intake, self-reported walking speed, medications, and current or most recent full-time occupation and husband’s occupation for ever-married women. At the 1994/5 baseline HAS clinic, height and weight were measured. Grip strength was measured using a Jamar handgrip dynamometer. Venous blood was collected for laboratory-automated haemoglobin, WCC, erythrocyte sedimentation rate (ESR), albumin, thyroid-stimulating hormone (TSH), free T4 (T4), testosterone and sex hormone-binding globulin (SHBG) and serum stored at −80°C.
Serological analysis
The serum-free cytokines IL-1β, IL-6, IL-10 and TNF-α were simultaneously measured using commercially available multiplex luminometry, whilst C-reactive protein was measured using singleplex luminometry (Invitrogen, UK). Detection of serum cortisol and DHEAS was completed using commercially available enzyme-linked immunosorbent assay kits (IBL International, Germany).
Frailty was assessed at the 2003/5 follow-up. The Fried criteria define frailty as the presence of three or more of the following: unintentional weight loss (greater than 10 lbs over the past year), weakness, self-reported exhaustion, slow walking speed and low physical activity (Cawthon et al. 2007). These were operationalised as previously in the Hertfordshire Cohort Study (Syddall et al. 2010b) and were as follows: weakness was defined as a maximum grip strength of ≤ 30 kg for men and ≤ 20 kg for women (Lauretani et al. 2003), exhaustion was identified if the participant felt that everything they did was an effort for either moderate amounts or most of the time in the past week, slow walking speed was defined as a 3-m walk time in the slowest fifth of the HAS sex-specific distribution (≥4.04 s for men and ≥4.54 s for women) and low physical activity was identified if the participant had a short-form 36 (SF-36) physical functioning score in the bottom fifth of the HAS sex-specific distribution (<45 for men and <33 for women).
Intra- and inter-observer studies were carried out during the fieldwork. The HAS had ethical approval from the Hertfordshire and Bedfordshire Local Research Ethics Committee, and all participants gave written informed consent.
Statistical methods
Positively skewed biomarkers were loge transformed to normal distributions as necessary. Weight and height were positively correlated (men r = 0.53, p < 0.001; women r = 0.31, p < 0.001); to avoid multicolinearity problems, a standardised residual of weight adjusted for height was derived. Follow-up time was calculated as time elapsed in decimal years between the HAS 1994/5 baseline and 2003/5 follow-up clinics. Pearson’s correlation coefficients and principal components analysis were used to explore the inter-relationships between the immune-endocrine biomarkers. Cox’s proportional hazards models and logistic regression models were used to analyse the associations between the 1994/5 baseline panel of biomarkers and all-cause mortality and between data collection points and Fried frailty at the 2003/5 follow-up, respectively. Analyses were conducted with and without adjustment for the potential confounding effects of gender, follow-up duration (for logistic models for frailty only), and 1994/5 baseline age, height, weight for height, smoking status, alcohol consumption, social class in adulthood, walking speed and number of systems medicated. A 5% significance level was principally used to identify statistically significant associations, but a Bonferroni correction was also applied to enable identification of significant associations after allowance for multiple comparisons. Receiver operating characteristic (ROC) curves were used to explore the ability of the 1994/5 baseline biomarkers to discriminate between frailty status at follow-up in 2003/5. Data were principally analysed for men and women combined, but analyses were repeated for men and women separately. All analyses were conducted using Stata 11 (Stata Statistical Software, StataCorp 2009).
Results
In the 1994/5 baseline clinic, 411 men and 306 women participated; 81 men (19.7%) and 40 women (13.1%) had died by the time of the 2003/5 follow-up (hazard ratio (HR) for men compared with women 1.51, 95% confidence interval (95%CI) 1.03, 2.21, p = 0.03; total person years of follow-up 5,971).
Table 1 shows the baseline summary characteristics of the 153 men and 101 women who participated in the 1994/5 baseline clinic and who were also assessed for Fried frailty status at 2003/5 follow-up. The average age at the 1994/5 baseline clinic was 66.9 years for men and 67.3 years for women. The median follow-up time was 10.4 years for men and 9.9 years for women. At the 2003/5 follow-up, the prevalence of Fried frailty was 5.2% for men and 11.9% for women (p = 0.05 for gender difference). Immune biomarkers were strongly inter-correlated: the pairwise correlation coefficients between WCC and each of neutrophils, monocytes and lymphocytes ranged from 0.58 to 0.91, all p < 0.0001. In addition, neutrophils and monocytes were strongly correlated with one another (r = 0.58, p < 0.0001), and CRP was correlated with each of WCC, neutrophils, monocytes and ESR (correlation coefficients ranged from 0.33 to 0.37, all p < 0.0001). However, the endocrine markers cortisol and DHEAS were independent of one another (r = 0.01, p = 0.90) and of all the other biomarkers considered in Table 1 (correlations ranged from 0.02 to 0.15, p ≥ 0.03 for all) with the exception of the ratio of cortisol to DHEAS of which they are a component. A principal components analysis for the biomarkers in Table 1 confirmed these patterns of association: the first principal component accounted for 19% of the variation in the data and was a weighted average of WCC, neutrophils, monocytes, lymphocytes and CRP; the second component accounted for 12% of the variation in the data and was a contrast between cortisol and DHEAS.
Table 1.
Baseline (1994/5) characteristics of participants in the 2003/5 HAS follow-up
| Mean (SD) | Men (n = 153) | Women (n = 101) |
|---|---|---|
| Age (years) | 66.9 (2.2) | 67.3 (2.1) |
| Height (cm) | 172.6 (6.3) | 159.8 (5.1) |
| Weight (kg) | 80.4 (12.2) | 69.2 (10.6) |
| Current smokera | 19 (12.4) | 9 (8.9) |
| Moderate or higher weekly alcohol intakea, b | 40 (26.1) | 9 (8.9) |
| Non-manual social classa, c | 73 (48.3) | 43 (43.4) |
| Fairly brisk or fast walking speeda | 42 (27.5) | 26 (25.7) |
| Number of systems medicatedd | 1 (0,2) | 1 (0,2) |
| White cell count (×109/L)d | 5.5 (4.8, 6.6) | 5.5 (4.7, 6.5) |
| ESR (mm/hr)d | 6 (4, 10) | 14 (8, 20) |
| Neutrophils (×109/L)d | 3.4 (2.8, 4.0) | 3.2 (2.5, 4.0) |
| Monocytes (×109/L)d | 0.4 (0.3, 0.5) | 0.3 (0.3, 0.4) |
| Lymphocytes (×109/L)d | 1.6 (1.3, 2.0) | 1.7 (1.4, 2.1) |
| Albumin (g/L) | 42.7 (2.0) | 42.3 (2.0) |
| SHBG (nmol/L)d | 36.3 (27.9, 48.3) | 51.2 (34.2, 79.2) |
| Testosterone (nmol/L)d | 16.3 (11.1, 20.3) | No data |
| Haemoglobin (g/L) | 14.5 (1.0) | 13.5 (0.9) |
| TSH (mU/L)d | 1.7 (1.2, 2.3) | 2.1 (1.5, 3.3) |
| T4 (pmol/L)d | 14.2 (13.2, 15.6) | 13.7 (12.6, 15.3) |
| IL-1B (pg/mL)d | 11.3 (5.6, 17.7) | 18.3 (11.7, 26.2) |
| IL-6 (pg/mL)d | 1.2 (0.2, 2.0) | 0.9 (0.4, 2.0) |
| IL-10 (pg/mL)d | 2.3 (2.5, 4.7) | 1.9 (0.3, 2.1) |
| CRP (mg/L)d | 1.9 (0.9, 4.0) | 3.1 (1.1,5.6) |
| DHEAS (nmol/L)d | 2181 (1734, 2888) | 1495 (797, 2261) |
| Cortisol (nmol/L)d | 308 (235, 385) | 274 (210, 349) |
| Ratio of cortisol:DHEASd | 0.12 (0.09, 0.17) | 0.19 (0.10, 0.38) |
| Follow-up time (years)d | 10.4 (10.2, 10.5) | 9.9 (9.8, 10.1) |
| Fried frailtya, e | 8 (5.2) | 12 (11.9) |
aNumber and percentage
bDefined as weekly alcohol consumption of ≥11 units for men and ≥8 units for women
cDefined as classes I, II and IIINM of the 1990 OPCS coding of social class. Social class was identified on the basis of own current/most recent full-time occupation for men and never-married women and from husband’s occupation for ever-married women
dVariable was positively skewed; median and inter-quartile range presented
eAssessed at the 2003/5 follow-up
Table 2 presents the associations between the panel of biomarkers as measured for 717 men and women at the 1994/5 clinic and all-cause mortality between HAS follow-ups for men and women combined. In unadjusted analyses, a standard deviation (SD) increase in the levels of ESR (HR (95%CI) 1.33 (1.11, 1.58), p = 0.002), neutrophils (HR (95% CI) 1.33 (1.11,1.59), p = 0.002), monocytes (HR (95%CI) 1.19 (1.00, 1.43), p = 0.05) and IL-1β (HR (95%CI) 1.17 (1.00, 1.36), p = 0.04) were all associated with increased likelihood of mortality between data collection points. The associations between ESR and neutrophils and mortality were also significant after Bonferroni correction (p < 0.003). The results were unaltered by adjustment for gender and age at 1994/5 baseline (Table 2). However, the associations between monocytes, IL-1β and mortality were attenuated by also adjusting for height, weight for height, smoking, alcohol, social class, walking speed and number of systems medicated, all as ascertained in 1994/5 baseline.
Table 2.
Associations between 1994/5 biomarkers and all-cause mortality between HAS follow-ups for men and women combined
| Biomarker | Number | HRa | 95%CI | p | HRb | 95%CI | p | HRc | 95%CI | p | HRd | 95%CI | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White cell count (×109/L) | 697 | 1.18 | (0.98, 1.42) | 0.081 | 1.17 | (0.98, 1.41) | 0.089 | 1.15 | (0.96, 1.39) | 0.133 | 1.15 | (0.95, 1.38) | 0.155 |
| ESR (mm/hr) | 708 | 1.33 | (1.11, 1.58) | 0.002 | 1.31 | (1.10, 1.56) | 0.002 | 1.22 | (1.01, 1.48) | 0.037 | 1.22 | (1.01, 1.47) | 0.041 |
| Neutrophils (×109/L) | 708 | 1.33 | (1.11, 1.59) | 0.002 | 1.31 | (1.09, 1.57) | 0.003 | 1.21 | (1.00, 1.47) | 0.046 | 1.21 | (1.00, 1.46) | 0.054 |
| Monocytes (×109/L) | 708 | 1.19 | (1.00, 1.43) | 0.054 | 1.19 | (0.99, 1.42) | 0.064 | 1.13 | (0.93, 1.36) | 0.214 | 1.12 | (0.93, 1.35) | 0.235 |
| Lymphocytes (×109/L) | 708 | 1.10 | (0.91, 1.32) | 0.319 | 1.11 | (0.93, 1.33) | 0.261 | 1.06 | (0.88, 1.28) | 0.560 | 1.07 | (0.88, 1.29) | 0.490 |
| Albumin (g/L) | 708 | 0.93 | (0.77, 1.11) | 0.401 | 0.92 | (0.77, 1.11) | 0.386 | 0.94 | (0.78, 1.12) | 0.480 | 0.94 | (0.79, 1.12) | 0.488 |
| SHBG (nmol/L) | 689 | 0.93 | (0.77, 1.11) | 0.411 | 0.93 | (0.78, 1.11) | 0.433 | 0.95 | (0.79, 1.14) | 0.587 | 0.98 | (0.82, 1.18) | 0.856 |
| Testosterone (nmol/L)e | 711 | 1.18 | (0.98, 1.42) | 0.075 | 1.16 | (0.97, 1.40) | 0.108 | 1.15 | (0.94, 1.40) | 0.172 | 1.14 | (0.94, 1.39) | 0.193 |
| Haemoglobin (g/L) | 408 | 0.98 | (0.78, 1.24) | 0.894 | 0.98 | (0.78, 1.24) | 0.888 | 0.99 | (0.76, 1.28) | 0.937 | 1.00 | (0.77, 1.30) | 0.989 |
| TSH (mU/L) | 708 | 0.94 | (0.78, 1.13) | 0.521 | 0.96 | (0.80, 1.15) | 0.655 | 0.96 | (0.80, 1.15) | 0.643 | 0.97 | (0.81, 1.17) | 0.783 |
| T4 (pmol/L) | 696 | 0.95 | (0.79, 1.14) | 0.609 | 0.96 | (0.80, 1.15) | 0.664 | 1.00 | (0.83,1.20) | 0.979 | 1.00 | (0.83, 1.20) | 0.987 |
| IL-1B (pg/mL) | 705 | 1.17 | (1.00, 1.36) | 0.044 | 1.18 | (1.00, 1.38) | 0.045 | 1.16 | (0.98, 1.37) | 0.085 | 1.14 | (0.96, 1.35) | 0.149 |
| IL-6 (pg/mL) | 504 | 0.97 | (0.78, 1.20) | 0.760 | 0.98 | (0.79, 1.22) | 0.857 | 0.96 | (0.77, 1.19) | 0.703 | 0.96 | (0.77,1.19) | 0.706 |
| IL-10 (pg/mL) | 439 | 1.09 | (0.87, 1.37) | 0.431 | 1.09 | (0.87, 1.37) | 0.459 | 1.02 | (0.81, 1.29) | 0.846 | 1.02 | (0.81, 1.28) | 0.882 |
| CRP (pg/mL) | 537 | 0.95 | (0.78, 1.17) | 0.655 | 0.95 | (0.78, 1.17) | 0.647 | 0.98 | (0.80, 1.20) | 0.834 | 0.98 | (0.80, 1.21) | 0.875 |
| DHEAS (nmol/L) | 673 | 1.18 | (0.97, 1.43) | 0.091 | 1.19 | (0.98, 1.44) | 0.083 | 1.12 | (0.92, 1.37) | 0.262 | 1.10 | (0.90, 1.35) | 0.354 |
| Cortisol (nmol/L) | 692 | 1.08 | (0.90, 1.31) | 0.409 | 1.08 | (0.89, 1.31) | 0.426 | 1.09 | (0.90, 1.32) | 0.395 | 1.08 | (0.89, 1.30) | 0.430 |
| Ratio of cortisol:DHEAS | 689 | 1.04 | (0.86, 1.26) | 0.668 | 1.04 | (0.86, 1.25) | 0.710 | 1.02 | (0.84, 1.24) | 0.814 | 1.00 | (0.82, 1.21) | 0.978 |
HR hazard ratio, CI confidence interval, p p value
aUnadjusted hazard ratio for mortality per standard deviation (SD) increase in biomarker
bHazard ratio adjusted for gender and baseline age
cHazard ratio adjusted as in footnote b and also for baseline height, weight for height, smoking, alcohol, social class and waking speed
dHazard ratio adjusted as in footnote c and also adjusted for baseline number of systems medicated as a marker of comorbidity
eBased on data for men only
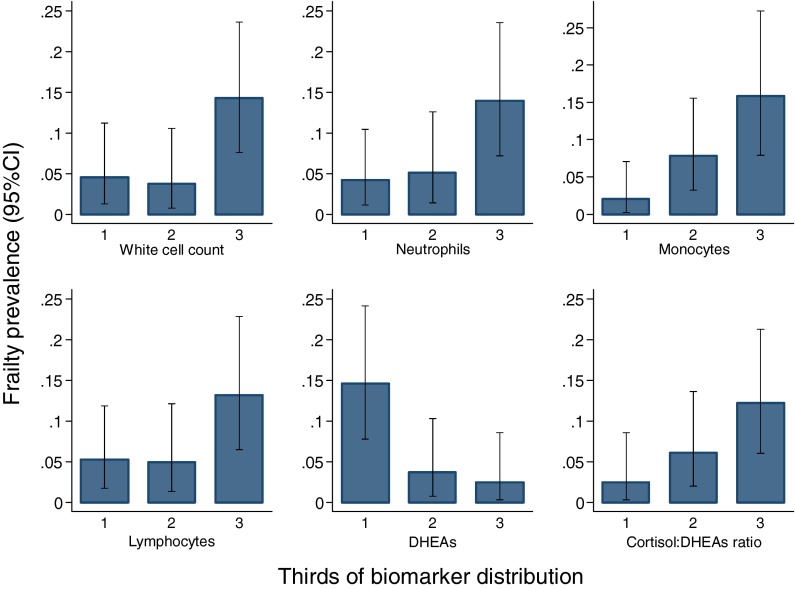
Table 3 presents the associations between the panel of biomarkers as measured at the 1994/5 baseline and Fried frailty status at 2003/5 follow-up for men and women combined. In unadjusted analyses, an SD increase in the levels of WCC (odds ratio OR (95%CI) 2.51 (1.49, 4.24), p = 0.001), ESR (OR (95%CI) 2.23 (1.28, 3.88), p = 0.005), neutrophils (OR (95%CI) 2.17 (1.29, 3.65), p = 0.004), monocytes (OR (95%CI) 3.22 (1.80, 5.74), p < 0.001), lymphocytes (OR (95%CI) 2.00 (1.23, 3.25), p = 0.005), T4 (OR (95%CI) 1.86 (1.11, 3.13), p = 0.02) and ratio of cortisol to DHEAS (OR (95%CI) 1.78 (1.17, 2.72), p = 0.007), and an SD decrease in the levels of DHEAS (OR (95%CI) 2.04 (1.30,3.23), p = 0.002) were all associated with increased odds of frailty at 2003/5 follow-up. The associations between WCC, monocytes and DHEAS and frailty were also significant after Bonferroni correction (p < 0.003). The results were unaltered by adjustment for gender, age and duration of follow-up (Table 3). However, the associations between ESR (p = 0.05) and T4 (p = 0.07) were attenuated by also adjusting for height, weight for height, smoking, alcohol, social class and walking speed. The associations between WCC, monocytes, lymphocytes, DHEAS and ratio of cortisol to DHEAS with frailty were little altered by additionally adjusting for number of systems medicated at the 1994/5 baseline as a marker of co-morbidity. The prevalence of frailty according to thirds of the distribution of white cell count, neutrophils, monocytes, lymphocytes, DHEAS and ratio of cortisol to DHEAS is shown in Fig. 1.
Table 3.
Associations between 1994/5 biomarkers and Fried frailty status at 2003/5 follow-up for men and women combined
| Biomarker | Number | ORa | 95%CI | p | ORb | 95%CI | p | ORc | 95%CI | p | ORd | 95%CI | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White cell count (×109/L) | 252 | 2.51 | (1.49, 4.24) | 0.001 | 2.85 | (1.60, 5.07) | <0.001 | 2.36 | (1.24, 4.51) | 0.009 | 2.22 | (1.14, 4.33) | 0.019 |
| ESR (mm/hr) | 246 | 2.23 | (1.28, 3.88) | 0.005 | 2.36 | (1.29, 4.30) | 0.005 | 2.04 | (0.99, 4.21) | 0.054 | 1.77 | (0.85, 3.65) | 0.125 |
| Neutrophils (×109/L) | 252 | 2.17 | (1.29, 3.65) | 0.004 | 2.42 | (1.37, 4.25) | 0.002 | 1.92 | (1.05, 3.52) | 0.035 | 1.83 | (0.96, 3.52) | 0.068 |
| Monocytes (×109/L) | 252 | 3.22 | (1.80, 5.74) | 0.000 | 4.11 | (2.11, 7.99) | 0.000 | 4.23 | (1.94, 9.22) | 0.000 | 3.88 | (1.73, 8.69) | 0.001 |
| Lymphocytes (×109/L) | 252 | 2.00 | (1.23, 3.25) | 0.005 | 2.07 | (1.26, 3.40) | 0.004 | 2.00 | (1.14, 3.53) | 0.016 | 2.09 | (1.15, 3.79) | 0.016 |
| Albumin (g/L) | 251 | 0.70 | (0.44, 1.10) | 0.123 | 0.66 | (0.41, 1.06) | 0.085 | 0.59 | (0.34, 1.02) | 0.058 | 0.48 | (0.26, 0.91) | 0.024 |
| SHBG (nmol/L) | 252 | 0.98 | (0.63, 1.52) | 0.936 | 0.90 | (0.57, 1.40) | 0.631 | 0.89 | (0.51, 1.55) | 0.678 | 0.88 | (0.48, 1.61) | 0.688 |
| Testosterone (nmol/L)e | 151 | 1.54 | (0.70, 3.42) | 0.285 | 1.06 | (0.44, 2.56) | 0.892 | 4.06 | (0.81, 20.39) | 0.089 | 5.42 | (0.95, 30.87) | 0.057 |
| Haemoglobin (g/L) | 252 | 0.84 | (0.52, 1.37) | 0.495 | 0.84 | (0.50, 1.42) | 0.522 | 0.69 | (0.39, 1.22) | 0.203 | 0.82 | (0.46, 1.48) | 0.515 |
| TSH (mU/L) | 247 | 0.75 | (0.48, 1.17) | 0.202 | 0.72 | (0.45, 1.16) | 0.178 | 0.73 | (0.42, 1.26) | 0.254 | 0.68 | (0.39, 1.20) | 0.186 |
| T4 (pmol/L) | 250 | 1.86 | (1.11, 3.13) | 0.019 | 2.08 | (1.16, 3.72) | 0.014 | 1.78 | (0.95, 3.32) | 0.070 | 1.41 | (0.74, 2.67) | 0.296 |
| IL-1B (pg/mL) | 180 | 0.92 | (0.51, 1.63) | 0.764 | 0.94 | (0.51, 1.72) | 0.839 | 0.81 | (0.43, 1.54) | 0.525 | 0.76 | (0.40, 1.44) | 0.400 |
| IL-6 (pg/mL) | 151 | 1.75 | (0.87, 3.54) | 0.116 | 1.93 | (0.90, 4.16) | 0.092 | 1.65 | (0.73, 3.76) | 0.231 | 1.64 | (0.71, 3.76) | 0.243 |
| IL-10 (pg/mL) | 194 | 0.58 | (0.29, 1.14) | 0.116 | 0.55 | (0.26, 1.18) | 0.124 | 0.47 | (0.17, 1.29) | 0.142 | 0.63 | (0.22, 1.83) | 0.400 |
| CRP (pg/mL) | 243 | 1.49 | (0.88, 2.51) | 0.138 | 1.59 | (0.88, 2.86) | 0.123 | 1.18 | (0.58, 2.42) | 0.649 | 1.04 | (0.49, 2.18) | 0.926 |
| DHEAS (nmol/L) | 246 | 0.49 | (0.31, 0.77) | 0.002 | 0.45 | (0.27, 0.74) | 0.002 | 0.42 | (0.23, 0.74) | 0.003 | 0.50 | (0.27, 0.91) | 0.023 |
| Cortisol (nmol/L) | 246 | 1.04 | (0.63, 1.74) | 0.869 | 1.08 | (0.63, 1.84) | 0.789 | 1.07 | (0.59, 1.94) | 0.824 | 1.14 | (0.60, 2.16) | 0.699 |
| Ratio of cortisol:DHEAS | 246 | 1.78 | (1.17, 2.72) | 0.007 | 2.03 | (1.25, 3.30) | 0.004 | 2.02 | (1.20, 3.41) | 0.008 | 1.79 | (1.03, 3.10) | 0.037 |
OR odds ratio, CI confidence interval, p p value
aUnadjusted odds ratio for frailty per standard deviation (SD) increase in biomarker
bOdds ratio adjusted for gender, baseline age and follow-up duration
cOdds ratio adjusted as in footnote b and also for baseline height, weight for height, smoking, alcohol, social class and waking speed
dOdds ratio adjusted as in footnote c and also adjusted for baseline number of systems medicated as a marker of comorbidity
eBased on data for men only
Fig. 1.
Prevalence of Fried frailty at 2003/5 follow-up for men and women combined according to biomarker distribution at 1994/5 baseline
ROC curves were used to explore the ability of white cell count (an immune marker) and the ratio of cortisol to DHEAS (an endocrine marker) at the 1994/5 baseline to discriminate between frail and non-frail individuals at the 2003/5 follow-up. The ROC areas under the curve were 0.70 (95%CI 0.55, 0.84) for WCC and 0.72 (95%CI 0.59, 0.86) for ratio of cortisol to DHEAS; both areas under the curve are suggestive of fair-good discrimination between frail and non-frail individuals. The optimal categorisations for discrimination between frail and non-frail individuals were ≥6 × 109/L versus <6 × 109/L for WCC (frailty prevalence 12.1% versus 4.6%, respectively) and ≥0.172 versus <0.172 for ratio of cortisol to DHEAS (frailty prevalence 12.6% versus 3.8%, respectively). Further discrimination was achieved by combining the categorisations of WCC and ratio of cortisol to DHEAS; the prevalence of frailty at 2003/5 follow-up among individuals who had a white cell count <6 × 109/L and a ratio of cortisol to DHEAS <0.172 at 1994/5 baseline was only 2.0% in contrast with a prevalence of 8.0% among individuals who had only one of WCC ≥6 × 109/L or ratio ≥0.172 and a frailty prevalence of 17.1% among individuals with WCC ≥6x109/L and ratio of cortisol to DHEAS ≥0.172. Results were similar for men and women analysed separately (data not shown), but the strength of statistical significance was lower for all associations owing to a limited power from smaller sample sizes.
Discussion
We have shown that higher baseline levels of total WCC, neutrophils, monocytes, lymphocytes, ESR and free T4, and lower levels of DHEAS as well as higher ratios of cortisol to DHEAS were all associated with an increased likelihood of being frail at 10-year follow-up in a cohort of community-dwelling older people. Higher baseline levels of ESR, neutrophils, monocytes and IL-1β were associated with increased likelihood of all-cause mortality across the 10 years.
We have also demonstrated that a single baseline marker of the immune axis (WCC), combined with a baseline marker of the endocrine axis (cortisol:DHEAS), clearly discriminated between individuals who went on to be frail at 10-year follow-up. If validated in other populations, our results suggest that a screening programme at ages 60–70 years could help to identify individuals who are at high risk of becoming frail and who would benefit from early, targeted intervention. This might include comprehensive geriatric assessment and medical, nutritional and exercise treatments (Ko 2011; Liu and Fielding 2011). Pharmacological interventions are also being trialled (Walston et al. 2002; Kenny et al. 2010). Furthermore, these biomarkers are simple, inexpensive and routinely used in clinical practice; they may be combined with other markers (e.g. grip strength, sarcopenia) to increase accuracy and generate a functional predictive tool of clinical value. This has implications in terms of health-care planning and policymaking and high potential of significant social, economic and well-being benefits at both individual and global population level.
These findings complement and add to cross-sectional studies by Leng et al. (2009) who showed that high neutrophil and monocytes counts were associated with frailty in disabled older women and also Voznesensky et al. (2009) who demonstrated associations with DHEAS in community-dwelling older people. This is the first study to show longitudinal associations with frailty across both the immune and endocrine axis, suggesting that the age-associated changes to the cellular immune system and hypothalamic-pituitary-adrenal (HPA) axis are central to the rate of development of age-associated diseases and, as a consequence, to frailty and mortality.
White blood cells are important co-ordinators of inflammation; the age-associated decline of the immune system, immunesenescence, is well documented (Hunt et al. 2010). These changes contribute to inflamm-ageing, a progressive increase in pro-inflammatory status with age (Franceschi et al. 2000) and potentially an important mechanism explaining the association of white cells with prevalent frailty and mortality. Associations between specific pro-inflammatory cytokines or CRP and frailty have not been shown in this study; this may reflect the complexities of inflammation which involves an inflammatory milieu of multiple proteins rather than a specific inflammatory protein.
Both cortisol and the DHEAS are outputs of the HPA axis; cortisol has mainly immunosuppressive actions, while DHEAS is immune enhancing (Hunt et al. 2010). With ageing, there is an increasing imbalance between the two hormones, due to reducing DHEAS levels from age 30 years onwards (adrenopause), and relatively stable cortisol levels (Phillips et al. 2007). Raised levels of thyroid hormone are thought to further modulate the HPA axis through central changes in sensitivity to corticotrophin-releasing hormone (Lizcano and Rodriguez 2011). These changes are believed to contribute to the process of immunesenescence (Buford and Willoughby 2008). Furthermore, cortisol and DHEAS have direct effects on frailty via interaction with anabolic and catabolic pathways within myocytes. Importantly, this ratio can be manipulated via pharmacological supplementation with DHEA to raise serum DHEAS levels. A recent randomised controlled trail by Kenny and colleagues demonstrated that DHEA supplementation improved sarcopenia in already frail women involved in gentle exercise when compared to placebo (Kenny et al. 2010).
Our study has some limitations. Firstly, we cannot completely exclude the effects of co-existing subclinical infections at the time of immune-endocrine analysis. However, participants were presumed fit and able to attend clinic appointments for data collection. Results were screened prior to analysis for patterns suggestive of acute infection or haematological malignancy, and four results were removed from the data set. Secondly, study participants were lost to follow-up between the 1994/5 baseline and 2003/5 follow-up clinics due to a variety of reasons (including mortality, loss to follow-up, refusal to participate), and we have previously shown that a healthy participant effect is, unsurprisingly, evident in HAS (20). In the current study, the 153 men who went on to have frailty classified at 2003/5 HAS follow-up were significantly (p < 0.05) younger, less likely to be current smokers, were of higher social class and had lower white cell count, ESR, neutrophil, monocytes, T4, IL-6 and CRP levels at the 1994/5 clinic than the 258 men who only participated in the 1994/5 baseline study. Selection effects were less evident for women; the 101 women who went on to have frailty classified at 2003/5 HAS follow-up were significantly (p < 0.05) less likely to be current smokers and had lower neutrophil levels at the 1994/5 clinic than the 205 women who only participated in the 1994/5 study. These selection effects have the potential to bias our results. However, our analyses were internal to the HAS sample; bias would only be introduced if the associations between biomarkers and frailty were systematically different among those who participated in our study and those who did not; this seems unlikely. Finally, there are numerous candidate biomarkers within the immune-endocrine axis that we did not analyse, and there is a scope to widen this biomarker battery. However, this may add unnecessary complexity, and we specifically chose readily available biomarkers to facilitate easy translation into the clinical environment.
In conclusion, we present the first evidence that immune-endocrine biomarkers can predict the likelihood of frailty as well as mortality over a 10-year period. This augments our understanding of the aetiology of frailty and suggests the potential for screening and early identification and intervention in those at high risk. Progress towards the prevention of frailty would bring major health and socio-economic benefits both at the individual and the population level. Further work is needed to replicate these findings in other studies and to elucidate the underlying mechanisms to develop the necessary evidence base for successful intervention strategies.
Acknowledgements
This work was funded by the Medical Research Council, the Biotechnology and Biological Sciences Research Council and the University of Southampton.
References
- Avila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le GM, Ritchie K, et al. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63(10):1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JM, Sieber CC. Sarcopenia and frailty: a clinician’s controversial point of view. Exp Gerontol. 2008;43(7):674–678. doi: 10.1016/j.exger.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Buford TW, Willoughby DS. Impact of DHEA(S) and cortisol on immune function in aging: a brief review. Appl Physiol Nutr Metab. 2008;33(3):429–433. doi: 10.1139/H08-013. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46(11):4251–4259. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- De MM, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Grimm RH, Jr, Neaton JD, Ludwig W. Prognostic importance of the white blood cell count for coronary, cancer, and all-cause mortality. JAMA. 1985;254(14):1932–1937. doi: 10.1001/jama.1985.03360140090031. [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Walsh BM, Voegeli D, Roberts HC. Inflammation in aging part 1: physiology and immunological mechanisms. Biol Res Nurs. 2010;11(3):245–252. doi: 10.1177/1099800409352237. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58(9):1707–1714. doi: 10.1111/j.1532-5415.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- Ko FC. The clinical care of frail, older adults. Clin Geriatr Med. 2011;27(1):89–100. doi: 10.1016/j.cger.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di IA, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Huang Y, Yeh SH, Leng SX, Xue QL, Tian J, Huang Y, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the Women’s Health and Aging Studies I. Exp Gerontol. 2009;44(8):511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27(1):101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano F, Rodriguez JS. Thyroid hormone therapy modulates hypothalamo-pituitary-adrenal axis. Endocr J. 2011;58(2):137–142. doi: 10.1507/endocrj.K10E-369. [DOI] [PubMed] [Google Scholar]
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358(9285):861–865. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Burns VE, Lord JM. Stress and exercise: getting the balance right for aging immunity. Exerc Sport Sci Rev. 2007;35(1):35–39. doi: 10.1097/jes.0b013e31802d7008. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam Experience Study. Eur J Endocrinol. 2010;163(2):285–292. doi: 10.1530/EJE-10-0299. [DOI] [PubMed] [Google Scholar]
- Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63(4):403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie SA. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39(2):197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syddall HE, Simmonds SJ, Martin HJ, Watson C, Dennison EM, Cooper C, et al. Cohort profile: The Hertfordshire Ageing Study (HAS) Int J Epidemiol. 2010;39(1):36–43. doi: 10.1093/ije/dyn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol. 2003;58(5):403–410. doi: 10.2143/AC.58.5.2005304. [DOI] [PubMed] [Google Scholar]
- van Abellan KG, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–406. doi: 10.1093/ageing/afp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Zofkova I, Hill M. Biochemical markers of bone remodeling correlate negatively with circulating TSH in postmenopausal women. Endocr Regul. 2008;42(4):121–127. [PubMed] [Google Scholar]