Abstract
Ovariectomy (OVX)-induced bone loss has been linked to increased bone turnover and higher bone matrix collagen degradation as the result of osteoclast activation. However, the role of degraded collagen matrix in the fate of resident bone-forming cells is unclear. In this report, we show that OVX-induced bone loss is associated with profound decreases in collagen 1 and Sirt1. This was accompanied by increases in expression and activity of the senescence marker collagenase and expression of p16/p21 in bone. Feeding a diet supplemented with blueberries (BB) to pre-pubertal rats throughout development or only prior to puberty [postnatal day 21 (PND21) to PND34] prevents OVX-induced effects on expression of these molecules at PND68. In order to provide more evidence and gain a better understanding on the association between bone collagen matrix and resident bone cell fate, in vitro studies on the cellular senescence pathway using primary calvarial cells and three cell lines (ST2 cells, OB6, and MLO-Y4) were conducted. We found that senescence was inhibited by collagen in a dose–response manner. Treatment of cells with serum from OVX rats accelerated osteoblastic cell senescence pathways, but serum from BB-fed OVX rats had no effect. In the presence of low collagen or treatment with OVX rat serum, ST2 cells exhibited higher potential to differentiate into adipocytes. Finally, we demonstrated that bone cell senescence is associated with decreased Sirt1 expression and activated p53, p16, and p21. These results suggest that (1) a significant prevention of OVX-induced bone cell senescence from adult rats can occur after only 14 days consumption of a BB-containing diet immediately prior to puberty, and (2) the molecular mechanisms underlying this effect involves, at least in part, prevention of collagen degradation.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9412-z) contains supplementary material, which is available to authorized users.
Keywords: Blueberry, Diet, Bone loss, Osteoblast, Senescence
Introduction
Bone loss triggered by declining serum sex steroids in both postmenopausal humans and in ovariectomized (OVX) animal models has been extensively investigated (Manolagas 2010). It has been suggested that postmenopausal osteoporotic bone loss largely results from stimulation of bone resorption via increased osteoclast (OC) formation or increased OC lifespan coupled with insufficient osteoblastic bone formation (Riggs et al. 2002). Bone resorption leads to degradation of extracellular matrix (ECM) which alters the environment for stem cell binding and differentiation. When cell adhesion receptors interact with ECM, intracellular signaling cascades are activated (Ward et al. 2007). Collagen type 1 (Col1) is the most abundant ECM protein in connective tissues and is also a major constituent of bone matrix. It has been shown that Col1 has the ability to support osteogenic differentiation of mesenchymal stromal cells (MSC) via integrin receptors and focal adhesion kinase (Salasznyk et al. 2004). Col1 may also play an important regulatory role in cell senescence (Choi et al. 2011). MSCs are marrow-derived and possess multilineage differentiation potential. They have characteristics of self-renewal and are believed to egress and circulate away from their niche (Katayama et al. 2006) in response to the needs and changes of their surrounding environment. How ECM signals MSC to determine whether they should enter senescence or differentiate into a range of tissue-specific cell types remains unknown. Moreover, links between OVX and MSC senescence, and whether MSC fate can be programmed early in life by bioactive factors found in diet has yet to be examined. Cellular senescence is a process of cell aging involving an irreversible growth arrest of mitotic cells and is different than programmed cell death (apoptosis). Impairment of tissue function is expected where senescent cells have accumulated (Satyanarayana et al. 2003).
Overexpression of collagenase activity or increased senescence associated beta-galactosidase activity has been used to evaluate cellular senescence in both cultured cells and in vivo (West et al. 1989; Dimri et al. 1995). More recently, biomarkers such as p53/p21cip1 and/or p16 overexpression, senescence-associated heterochromatin foci, and telomere dysfunction-induced foci have also been used to detect senescent cells (Krizhanovsky et al. 2008; Narita et al. 2006; Smogorzewska and de Lange 2004). Although the p53 and p16 pathways act in parallel to promote cellular senescence, their relative contribution to the process can be cell type-dependent (Campisi and d'Adda di Fagagna 2007). Moreover, cellular senescence can be triggered by telomere-dependent mechanisms involving shortening (Harley et al. 1990) and telomere-independent mechanisms involving p16 expression (Rheinwald et al. 2002). Cellular senescence has been widely investigated as a potential mechanism of tumor suppression; however, studies linking senescence to non-cancer tissue pathology, particularly to pathophysiology of skeletal changes, are currently lacking.
Blueberries (BB) have recently been shown to promote osteoblastic bone formation without affecting normal growth in rapidly growing rodents, and this bone-promoting effect appears to be due to stimulation of osteoblastic differentiation caused by phenolic acid metabolites derived from BB polyphenols (Chen et al. 2010). Consumption of a BB-containing diet during early life appears to delay bone-forming cells from entering into the aging process, and this may contribute to prevention of bone loss in adults (Zhang et al. 2011). The antisenscence properties of blueberry feeding appear similar to those ascribed to the polyphenol resveratrol in in vitro studies. However, the serum concentrations of resveratrol in animals consuming BB are negligible because of poor bioavailability. Resveratrol is well known to activate Sirt1, a member of the sirtuin family of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases (Vetterli et al. 2011).
Sirt1 is a candidate anti-aging gene. A previous report suggested that Sirt1 may repress p16 through deacetylation (Li and Tollefsbol 2011). There are bioactive compounds associated with feeding a BB diet, such as phenolic acids, in the circulation of animals (Chen et al. 2010). These compounds may be particularly effective in preventing bone-forming cells from entering into senescent programming. In the studies presented in this report, we examined the association between OVX-induced bone matrix degradation and osteoblastic cell senescence. We also determined whether a diet containing BB fed for a very short period of time during early development (between PND 21 and 34) or fed throughout development was able to prevent OVX-induced osteoblastic cell senescence later in life. We present evidence to suggest that BB prevents OVX-induced bone loss by preserving bone matrix and by inhibiting Col1 and Sirt1/p16 signaling in osteoblasts and their precursors.
Materials and methods
Animals and diets
Time-impregnated female Sprague–Dawley rats (n = 6; Harlan Industries, Indianapolis, IN, USA) gestational day 4 were individually housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility at the Arkansas Children's Hospital Research Institute with constant humidity and lights on from 0600 to 1800 hours at 22°C. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences (UAMS). Pregnant rats were fed AIN-93G diets (Reeves et al. 1993). Litters from these dams were culled to five male and five female pups. At postnatal day 21 (PND21), two sets of female pups were randomly assigned (ten per group) to AIN-93G diets with or without blueberry (BB) supplementation until PND34. After PND34, all rats were fed control AIN-93G diet the rest of the experiment; at PND60, animals received ovariectomy surgery, and the experiment continued for another 3 weeks. These two groups were referred to as OVX and short-term BB diet OVX (STBB OVX). At PND21, another two sets of female rats were randomly assigned (n = 10) to AIN-93G control diet with or without BB diet throughout the experiment; at PND60, control diet animals received sham-operated surgery, and BB diet animals received OVX surgery, and the experiment continued for another 3 weeks. These two groups were referred to as sham and long-term BB diet OVX (LTBB OVX). Freeze-dried whole BB (Vaccinium angustifolium) powder (Hi-Actives Wild Blueberry) was kindly provided by VDF/FutureCeuticals, Momence, IL, USA. AIN-93G incorporated with 10 % freeze-dried BB powder (10 % BB) were made by Harlan Teklad (Madison, WI, USA). To eliminate caloric density as a confounding variable, all diets were formulated to be isocaloric and isonitrogenous. The diets contained the National Research Council nutrient recommendations and the same calcium and phosphorus levels (Chen et al. 2006). Animals were weighed every other day.
Bone histomorphometry and collagen type 1 immune staining
At sacrifice, the right rear tibia was removed and frozen in liquid nitrogen. The right tibial bones were then fixed, and sequential dehydration was carried out using different concentrations of alcohol. Undecalcified proximal tibial bone samples were embedded in plastic methyl methacrylate and sectioned at 4.5 μ thickness. Individual sections were Masson stained by standard histology special procedures. Histomorphometric analysis was performed with a digitizing morphometry system, which consists of an epifluorescent microscope (model BH-2, Olympus), a color video camera, and a digitizing pad (Numonics 2206) coupled to a computer (Sony) and a morphometry program OsteoMetrics (OsteoMetrics, Inc.). Standard immune staining using collagen type 1 antibody (Sigma-Aldrich, St. Louis, MO, USA) against endogenous collagen type 1 expression in bone was carried out using an unstained sliced tibial section from the above procedures.
Cell cultures
Neonatal rat calvarial osteoblastic cells were isolated from control rats at 3 days of age by sequential collagenase digestion using a method described previously (Chen et al. 2009). Rat neonatal calvarial osteoblastic cells, mouse origin bone marrow MSC line ST2, OB6 mature osteoblastic cells, and osteocytic cell MLO-Y4 cells (kindly provided by Endocrinology laboratory at UAMS at Little Rock, AR, USA) were cultured in α-MEM supplemented with 10 % fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT, USA). Cells were seeded in 6-well cell culture or 24-well plates at appropriate density of cells per well. Cell cultures were maintained in the presence of osteogenic medium (OB medium): minimal essential medium (Invitrogen, Carlsbad, CA, USA) with 10 % FBS and 1 mM ascorbyl-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), 4 Mm l-glutamine, and 100 U/ml each of penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO, USA) collagen type 1-coated plates were created according to a method provided by the manufacturers (Sigma-Aldrich, St. Louis, MO, USA); cells were treated with 2 % rat serum for indicated times with different experiments. MLO-Y4 cells are normally cultured on 15 μg/cm2 of Col1-coated plates. Cell cultures were stopped at indicated time points and were fixed for staining. RNA and protein were isolated for mRNA and protein expression assays.
Measurement of serum C1CP and collagenase
Rat serum ELISA for carboxy-terminal peptide fragment of type 1 collagen (CICP) and collagenase levels were performed using enzyme immunoassay kits from TSZ ELISA (TSZ Scientific, USA). The serum collagen degradation and bone resorption marker RatLaps ELISA kit was purchased from Nordic Bioscience Diagnostics A/S (Herlev, Denmark). According to the manufacturer's recommendation, 50 μl of serum from each sample was used, and the absorbance at 450 nm with subtraction at 650 nm was measured. Collagenase activity was measured using a red fluorescence probe-based kit from Abcam (ab112147, Abcam, Cambridge, MA, USA), following the assay protocol provided by the manufacturer.
Western blot and real-time reverse transcription-polymerase chain reaction
After removing of epiphysis and aspiration of bone marrow cells, right tibial bone tissue proteins and in vitro cultured cell proteins for Western immunoblot analysis were extracted using cell lysis buffer (RIPA Buffer, Boston Bioproducts, USA) as described previously (Chen et al. 2009). Western blot analyses were performed using standard protocols. The following primary antibodies were used: mouse monoclonal collagen type 1 (Sigma-Aldrich, St. Louis, MO, USA), goat polyclonal collagenase (Santa Cruz, CA, USA), mouse monoclonal beta-actin (Sigma-Aldrich, St. Louis, MO, USA), rabbit polyclonal SIRT1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal acetylated p53 (Cell Signaling, Danvers, MA, USA), rabbit polyclonal total p53 (Cell Signaling, Danvers, MA, USA), and rabbit polyclonal PPARγ (Abcam, Cambridge, MA, USA). Secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Blots were developed using chemiluminescence (PIERCE Biotechnology, USA) according to the manufacturer's recommendations. Quantification of the intensity of the bands in the autoradiograms was performed using a VersaDoc™ imaging system (Bio-Rad, Hercules, CA, USA). After aspiration of bone marrow cells, right femur total RNA and in vitro cultured cell RNA were extracted using TRI Reagent (MRC Inc., Cincinnati, OH, USA) according to the manufacturer's recommendations followed by DNase digestion and column cleanup using QIAGEN mini columns (QIAGEN, USA). Reverse transcription was carried out using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Real-time RT-PCR was carried out using SYBR Green and an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Primers for all genes used in this report were designed using Primer Express software 2.0.0 (Applied Biosystems, USA) and listed in Supplemental Table 1.
Senescence-associated beta-galactosidase activity, staining, and oil Red-O staining
Senescence-associated beta-galactosidase activity assay was performed by beta-galactosidase enzyme assay kit (Promega, USA). The absorbance was measured at 420 nm according to the manufacturer's instruction. Fixed cell beta-galactosidase staining was also performed according to a method published previously (Dimri et al. 1995). Senescent cells were identified as blue-stained cells by standard light microscopy. Oil Red-O staining was performed on rosiglitazone (Cayman Chemical, Ann Arbor, MI, USA) and rat serum-treated cells as described previously (Chen et al. 2009).
Data and statistical analyses
Data were expressed as means ± SEM. One-way analysis of variance (ANOVA) was utilized followed by Student–Newman–Keuls post hoc analyses for multiple pairwise comparisons between treatment groups. Values were considered statistically significant at p < 0.05.
Results
Pre-pubertal consumption of a BB-containing diet prevents ovariectomy (OVX)-induced senescence pathway activation and adult bone loss
We previously demonstrated that OVX triggers senescence in osteoblastic cells (Zhang et al. 2011); however, the underlying mechanisms remain unrevealed. We hypothesize that OVX-induced bone collagen matrix degradation leads to bone loss, while at the same time, it may facilitate the entrance of resident bone-forming cells into the senescence pathway, and BB-containing diets may prevent the processes. Histomorphometric analysis (Fig. 1a) revealed that bone volume (percentage of trabecular bone volume per total volume) in rats after 3 weeks of OVX was lower compared to sham-operated rats (OVX, 11.3 ± 1.1 % TBV/TV versus sham, 13.9 ± 1.6 % TBV/TV; n = 9, p < 0.05; Fig. 1b). OVX bone volume in both BB groups (LTBB-OVX, 17.3 ± 1.1 % TBV/TV; n = 9 and STBB-OVX, 16.8 ± 1.7 % TBV/TV; n = 9) was increased (p < 0.05) versus the OVX group (Fig. 1b). Other bone density measures, such as total bone mineral density in these groups, were similar to those we have previously published (Zhang et al. 2011; Fig. 1c). Tibial bone sections from rats with different treatments were then immune-stained with collagen type 1 (Col1) antibody, and results showed that the Col1 network was profoundly deteriorated in OVX animals compared to those of sham rats (Fig. 1f). Animals receiving BB diet, either throughout development or only during early life, were protected from OVX-induced decreases in expression of Col1 (Fig. 1f). Interestingly, highly deprived Col1 areas in bone marrow from OVX rats were accompanied by increased adiposity (Fig. 1f). This was confirmed by measurement of fat volume/BV in tibial sections; %fat V/TV was ∼2-fold higher in OVX rats compared to their sham control (Fig. 1g). Using protein isolated from bone after aspiration of bone marrow, western blot analysis confirmed decreased Col1 protein expression in bone from OVX rats (Fig. 1d). On the other hand, collagenase expression in bone (a marker of senescence) and its activity were increased in OVX rats compared to the other three groups (Fig. 1d, e), indicating bone-forming cells may become senescent after OVX, and the protective effect of BB diets against bone loss may involve prevention of bone cell senescence. We therefore examined expression of several other cell senescence-associated specific biomarkers in the bone. We found that Sirt1 was significantly downregulated in bone from OVX rats compared to its expression in bone from sham animals (Fig. 2a). Sirt1 expression reverted to control levels in STBB-OVX rats, while the level of expression from LTBB-OVX rats was reversed to levels above those found in the sham group (Fig. 2a). Downregulated Sirt1 in bone in OVX rats was associated with increased acetylated p53 (Fig. 2a), but not total or phosphorylated p53 protein (data not shown), and increased PPARγ expression (Fig. 2a). Real-time PCR analysis for Sirt1 mRNA expression was consistent with western blot results (Fig. 2b). Moreover, p16 and p21, biomarkers for cellular senescence, mRNA expression were the opposite of Sirt1 in bone from OVX rats, and both long-term and short-term BB diet blocked the effect of OVX on p16 and p21 expression (Fig. 2b). Evidence has been previously reported that senescent cells (for example fibroblasts) exhibit senescence-associated secretory phenotype showing a pattern of gene expression that involves upregulation of secreted proteases, including matrix metalloproteinases (MMPs) (Campisi and d'Adda di Fagagna 2007). Similarly, senescent bone-forming cells in bone from OVX rats upregulated MMPs (Fig. 2b listed MMP2, 9, 13, 19), which have bone and collagen resorptive activities. Both long-term and short-term BB diet blocked the effects of OVX on the expression of these MMPs in bone (Fig. 2b). Moreover, rat serum ELISA revealed that levels of carboxy-terminal peptide fragment of type 1 collagen (CICP) was significantly lower in OVX rats compared to sham rats (Fig. 2c). On the other hand, secreted collagenase level and collagen degradation marker RatLaps (C-telopeptide degradation products from type 1 collagen released during osteoclastic bone resorption) were significantly higher in OVX rats compared to sham animals (Fig. 2d, e). Consistently, both long-term and short-term BB diet inhibited the effect of OVX on both C1CP and collagenase secretion in rat serum (Fig. 2c–e). These analyses of serum C1CP, a marker for de novo synthesis of collagen type 1, and collagenase secretion were consistent with their protein expression and further confirmed the association between collagen degradation and bone cell senescence in OVX rats, and the protective effect of BB diet on the process.
Fig. 1.
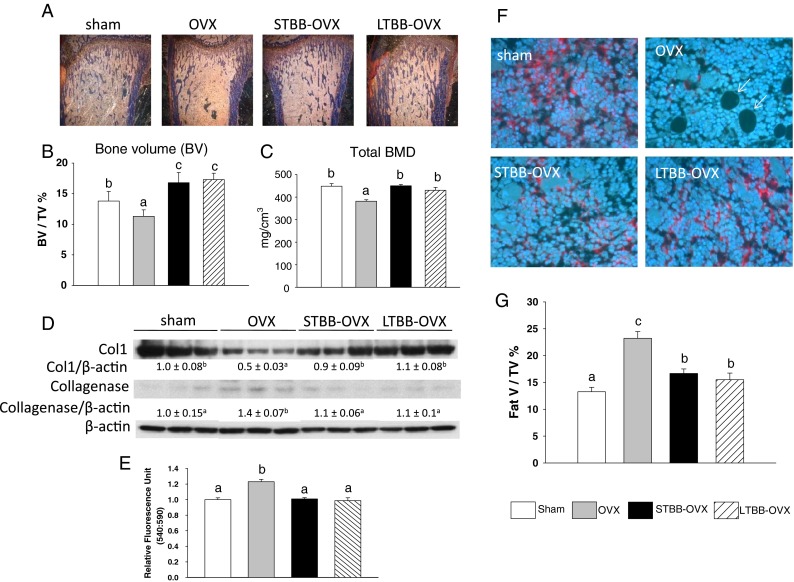
Long-term continuous and short-term early-in-life BB diet prevents OVX-induced bone loss and collagen degradation in female rats. A Static histomorphometric representative Masson staining images from rat tibias. Blue color stained is bone spicules, yellow/tan color stained is bone marrow. B Histomorphometric parameter trabecular bone volume (BV)/total tissue volume (TV), number of rats per group, n = 9. C Tibial total bone density data from peripheral quantitative CT scan (Zhang et al. 2011), number of rats per group, n = 9. D Western blot analysis of collagen type 1 and collagenase in bone from four different diet groups, β-actin for the protein loading control. Eight to nine samples in each group were randomly pooled into three samples per group and were presented. Numbers represent means ± SEM of the ratios of intensity of the bands of each target proteins over β-actin protein for each treatment. E Collagenase activity in rat serum, n = 9. F Immune staining of bone sections from four different diet groups using antibody against type 1 collagen; red color indicates immune-stained collagen type 1 fibers, and white arrows indicate adipocytes occupied vacuoles. G Histomorphometric quantitation of relative volume occupied by fat (by tracing adipocyte-like cells in marrow area) was assessed in H&E stained section. OVX ovariectomy, LTBB-OVX long-term blueberry supplemented diet throughout experiment and ovariectomy, STBB-OVX short-term blueberry diet for 14 days from weaning PND21 to PND34, then switch to control diet and ovariectomy
Fig. 2.
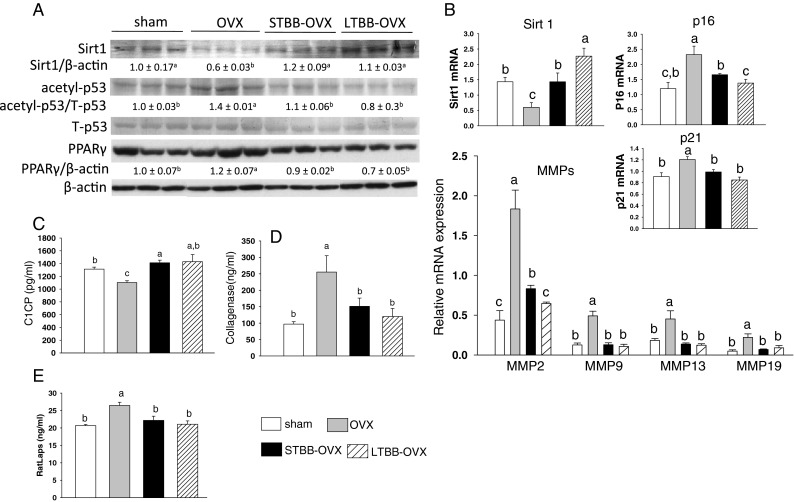
Dietary BB prevents OVX-activated senescence pathway in bone. A Western blot analysis of Sirt1, acetylated p53, total p53, and PPARγ in bone from four different diet groups, β-actin for the protein loading control. Eight to nine samples in each group were randomly pooled into three samples per group and were presented. Numbers represent means ± SEM of the ratios of intensity of the bands of each target proteins over control protein for each treatment. B Real-time PCR analysis for mRNA of Sirt1, p16, p21, and MMP2, 9, 13, 19 in bone with different diets. Data are expressed as mean ± SEM (n = 8 per group). ELISA for serum C1CP (C), collagenase (D), and collagen degradation maker RatLaps (E). Means with different letters differ significantly from each other at p < 0.05, a > b > c. OVX ovariectomy, LTBB-OVX long-term blueberry supplemented diet throughout experiment and ovariectomy, STBB-OVX short-term blueberry diet for 14 days from weaning PND21 to PND34, then switch to control diet and ovariectomy
Collagen type 1 matrix prevents osteoblastic cell senescence pathways
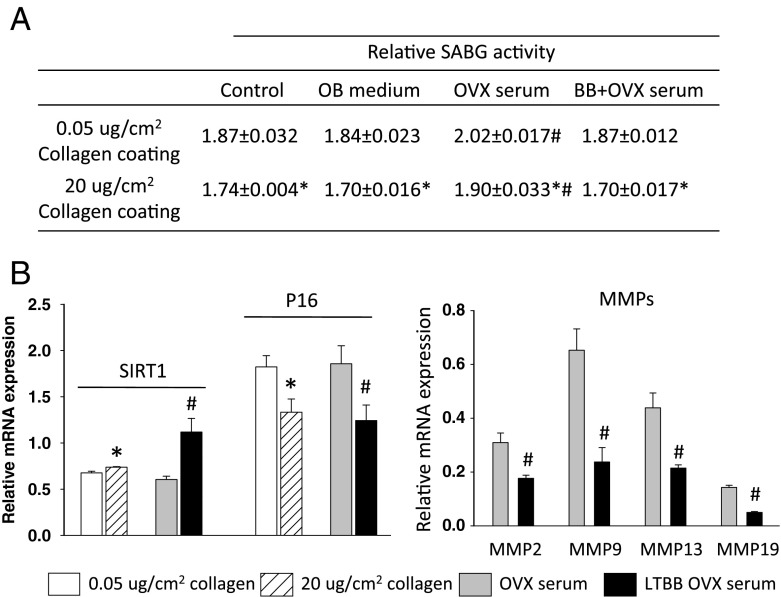
We examined whether the phenotype of osteoblastic cells is influenced by its microenvironment, in particular by changes in the Col1 bone matrix associated with OVX and consumption of BB diets. Isolated neonatal calvarial cells, osteoblastic cell line OB6 cells, and osteocytic cell line MLO-Y4 cells were cultured in different concentrations of Col1-coated plates for 3 days. Cells were also treated with or without 2.5 % serum from either OVX rats or LTBB-OVX rats for 3 days. We first measured cell protein and senescence-associated beta-galactosidase (SABG) activity. We found that SABG activity in calvarial cells cultured in high collagen concentration (20 μg/cm2 of Col1) coated wells was significantly lower than those cells cultured in low collagen concentration (0.05 μg/cm2 of Col1) coated wells (Fig. 3a, OB6 data presented in Supplemental Table 2). SABG activity was found to be lowest in cells grown in high concentration collagen-coated wells + treatment with LTBB-OVX serum (Fig. 3a). The highest SABG activity was found in cells treated with OVX serum and cultured in low concentration collagen-coated wells (Fig. 3a), and the magnitude of effect of OVX serum treatment on SABG was greater than the effect of collagen alone. RNA from calvarial cells cultured in low and high collagen-coated wells and low collagen-coated wells treated with OVX or LTBB-OVX serum for 3 days was extracted for real-time PCR analysis (in triplicate). Results revealed that the expression of Sirt1 in cells cultured in high collagen-coated wells was significantly higher, whereas p16 expression was significantly lower than those in cells cultured in low concentration collagen-coated wells (Fig. 3b). Similarly, cells treated with OVX serum clearly expressed much less Sirt1, but higher p16 compared to their expression in cells treated with LTBB-OVX serum (Fig. 3b). The protective effects of a high concentration of collagen and of serum derived from animals fed BB diets on osteoblastic senescence were further confirmed by collagenase expression (Fig. 3b) and SABG activity staining (Fig. 3c). Interestingly, collagen-supported cells showed increased differentiation potential indicated by the higher levels of osteoblastic cell differentiation markers, Runx2 and ALP, in these cells compared to cells with less collagen support (Fig. 3d). Osteocytes, another osteoblastic cell type in bone, originating from mature osteoblasts and which are entirely trapped by bone matrix, function to monitor bone remodeling. We therefore examined if osteocytes behave in similar ways to osteoblasts in their phenotypic changes in response to altered collagen concentration and OVX/OVX BB rat serum. Similar osteoblastic cell cultures were performed on osteocytic MLO-Y4 cells. As was expected, SABG activity in osteocytes cultured in high concentration collagen and cells treated with BB diet serum showed significantly lower activity compared to cells cultured in low concentration collagen and treated with OVX serum (Fig. 4a). Real-time PCR analysis revealed that Sirt1 expression in cells cultured on high concentrations of collagen was also significantly higher than in MLO-Y4 cells cultured on low concentrations of collagen (Fig. 4b). All other changes on cellular senescence markers were similar to what we found in osteoblasts (Fig. 4b), indicating that OVX-induced collagen degradation promotes osteocyte senescence and BB diet prevents it.
Fig. 3.
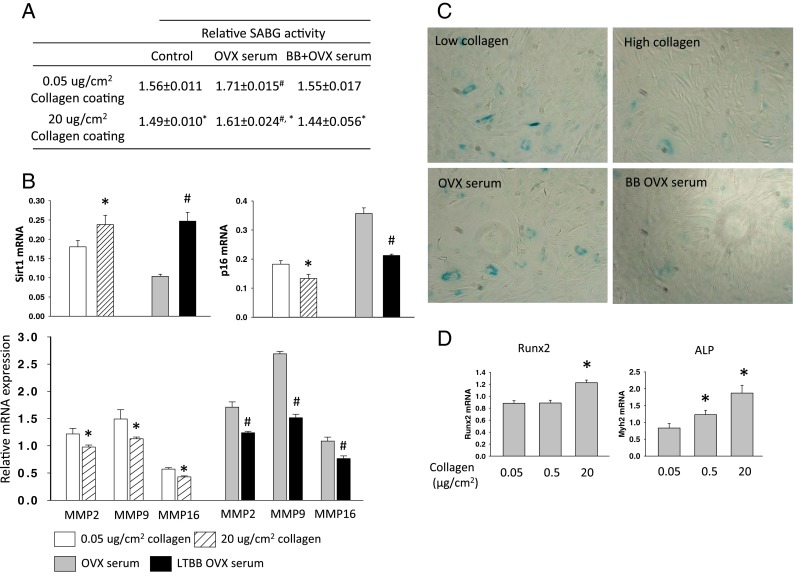
High collagen coating and BB diet rat serum delay primary calvarial osteoblastic cell entering senescence pathway. A Senescence-associated beta-galactosidase activity analyses in total proteins isolated from calvarial cells cultured in different concentration of collagen-coated wells and treated with OVX or LTBB-OVX rat serum for 3 days. #p < 0.05: versus control, *p < 0.05: 0.05 versus 20 μg/cm2 collagen coating, by t test. Data are expressed as mean ± SEM with triplicates. B Real-time PCR analysis for Sirt1, p16, and MMPs mRNA expressions in total RNA isolated from osteogenic calvarial cells after cells cultured in different concentrations of collagen-coated wells and treated cells cultured in low collagen-coated plate with OVX or LTBB OVX rat serum for 3 days. *p < 0.05: significant difference between 0.05 and 20 μg/cm2 collagen coating. #p < 0.05: significant difference between OVX rat serum and LTBB OVX rat serum treatment by t test. Data are expressed as mean ± SEM with triplicates. C Senescence-associated beta-galactosidase (SABG) activity staining in calvarial cells cultured in different concentrations of collagen-coated wells and treated with OVX or LTBB OVX rat serum for 3 days. Blue-colored cells are SABG-positive senescent cells. D Real-time PCR analysis for Runx2 and ALP (alkaline phosphatase) mRNA expression in calvarial cells cultured in different concentrations of collagen-coated wells for 3 days. Data are expressed as mean ± SEM (triplicates). *p < 0.05 compare to 0.05 collagen coating by t test
Fig. 4.
High collagen coating and BB diet rat serum delay osteocytic cell entering senescence pathway. A Senescence-associated beta-galactosidase activity analyses in total proteins isolated from MLO-Y4 osteocytic cells cultured in different concentrations of collagen-coated wells and treated with OVX or LTBB-OVX rat serum for 3 days. #p < 0.05: versus vehicle control, *p < 0.05: 0.05 versus 20 μg/cm2 collagen coating, by t test. Data are expressed as mean ± SEM with triplicates. OB medium osteogenic medium as defined in “Materials and methods” section. B Real-time PCR analysis for Sirt1, p16, and MMPs mRNA expressions in total RNA isolated from MLO-Y4 cells cultured in different concentrations of collagen-coated wells and treated cells in low coated plate with OVX or LTBB-OVX rat serum for 3 days. *p < 0.05: significant difference between 0.05 and 20 μg/cm2 collagen coating. #p < 0.05: significant difference between OVX rat serum and LTBB-OVX rat serum treatment by t test. Data are expressed as mean ± SEM with triplicates
Effects of BB on mesenchymal stromal cell senescence and adipogenic transition
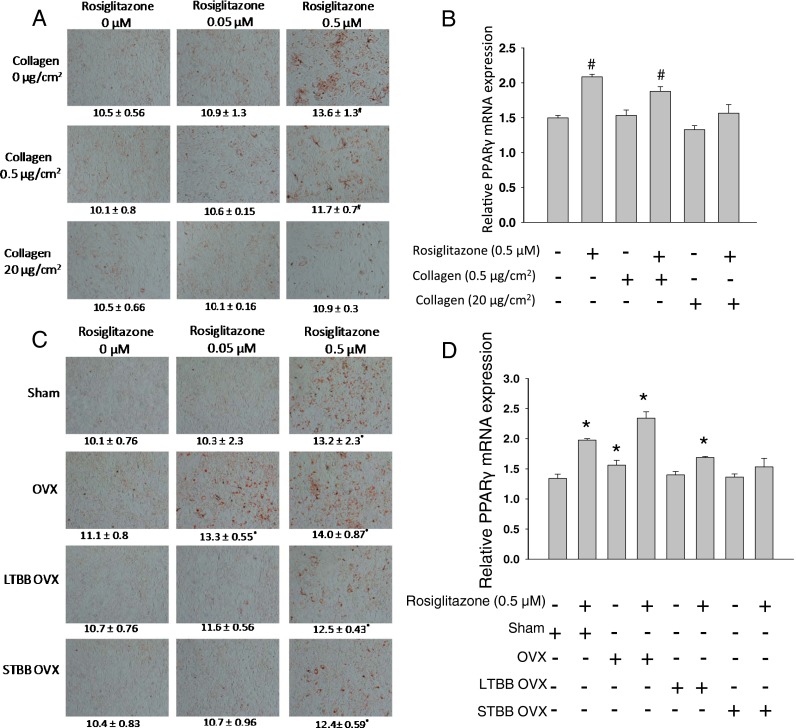
Previous data demonstrated that stem cells, such as MSCs, differentiate into tissue-specific cell types that are sensitive to environmental conditions (Engler et al. 2006). Collagen matrix may also provide these cells a niche to maintain their phenotype and support self-renewal. We hypothesized that unbalanced bone remodeling after OVX in females leads to significant loss of collagen matrix and that this loss of collagen matrix may also affect stromal cell senescence. To test this, we cultured ST2 cells, bone marrow-derived stromal mesenchymal cells, in different Col1 concentration coated plates treated with or without OVX or LTBB-OVX rat serum and measured the MSC senescence pathway. We found clear evidence indicating that increased Col1 inhibited the MSCs senescence pathway and increased MSCs differentiation into osteoblasts (Fig. 5). LTBB-OVX serum inhibited OVX serum-induced stimulation of the MSC senescence pathway and prevented inhibition of MSCs differentiation into osteoblasts (Fig. 5). Similar to data presented in Fig. 4, suppression of MSC senescence pathway was evaluated by increased SABG activity, p16/p21, MMPs expression, and decreased Sirt1 expression (Fig. 5a, b). Interestingly, using an ELISA analysis, we found decreased CICP and increased collagenase in culture medium of ST2 cells treated with OVX rat serum (data not shown). Inhibition of MSC differentiation into osteoblasts was determined by decreased expression of osteoblast differentiation markers, ALP and Runx2, and myosin 4 and 7 (Myh4, 7) (Fig. 5c, d); their involvement in both osteoblast differentiation and senescence has previously been described in our lab (Zhang et al. 2011). Finally, we tried to determine whether MSCs grown on less collagen support or treatment with OVX rat serum favors differentiation into other cell types, such as adipocytes, over osteoblasts under appropriate stimuli. To do this, we either cultured ST2 cells on different concentrations of Col1 or treated cells grown on low collagen with either sham, OVX, STBB-OVX, or LTBB-OVX rat serum for 3 days; cells were then treated with different concentrations of rosiglitazone for an additional 5 days to stimulate adipogenesis. Oil Red-O staining demonstrated that ST2 cells grown on high collagen had less oil Red-O-positive adipocyte-like cells than those grown without or on low collagen (Fig. 6a). Real-time PCR analysis for PPARγ expression further supported the idea that ST2 cells have less potential to differentiate toward adipocyte-like cells under rosiglitazone stimulation in high collagen compared to cells without collagen (Fig. 6b). Similarly, ST2 cells pre-treated with OVX serum had a higher intensity of oil Red-O-positive adipocyte-like cells than those cells pre-treated with either LTBB-OVX or STBB-OVX serum (Fig. 6c). Real-time PCR analysis for PPARγ expression confirmed the higher potential of ST2 cells to differentiate toward adipocyte-like cells under rosiglitazone stimulation in OVX serum pre-treated cells and the reduced potential of cells exposed to sera from OVX BB-fed rats to differentiate toward adipocyte-like cells (Fig. 6d). These data may explain why we observed an increase in adiposity that accompanied a reduced collagen network in OVX rats in vivo (Fig. 1b).
Fig. 5.
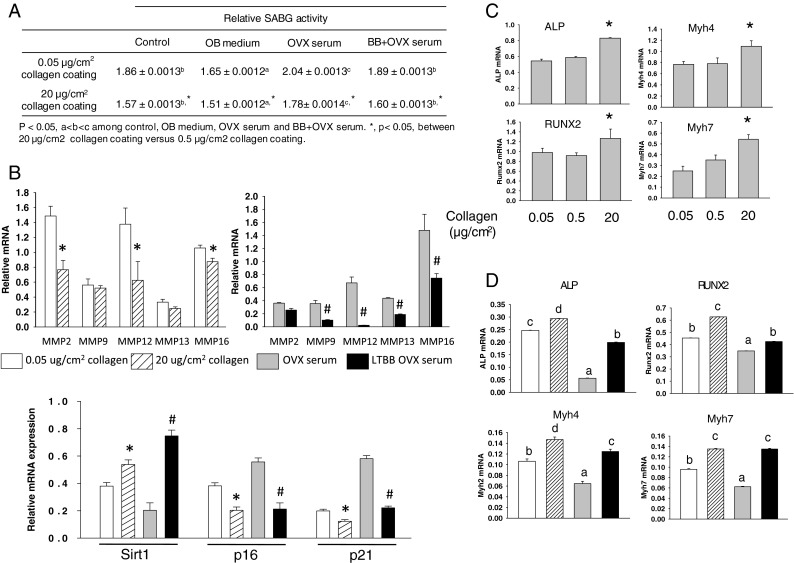
High collagen coating and BB diet rat serum delay mesenchymal stromal cells entering senescence pathway but stimulate their differentiation into osteoblasts. A Senescence-associated beta-galactosidase activity analyses in total proteins isolated from ST2 cells. B Real-time PCR analysis for MMPs, Sirt1, p16, and p21 mRNA expressions in total RNA isolated from ST2 cells cultured in different concentrations of collagen-coated wells and treated cells in low collagen-coated plate with OVX or LTBB-OVX rat serum for 3 days. *p < 0.05: significant difference between 0.05 and 20 μg/cm2 collagen coating. #p < 0.05: significant difference between OVX rat serum and LTBB-OVX rat serum treatment by t test. Data are expressed as mean ± SEM with triplicates. C Real-time PCR analysis for ALP (alkaline phosphatase), Runx2, and myosin 4 and 7 mRNA expression in ST2 cells cultured in different concentrations of collagen-coated wells for 3 days. Data are expressed as mean ± SEM (triplicates). *p < 0.05 compare to 0.05 collagen coating by t test. D OVX rat serum downregulated myosin 4, 7, ALP, and Runx2 gene expressions and protection of their effect by serum from BB diet OVX rats. Data are expressed as mean ± SEM (triplicates). Means with different letters differ significantly from each other at p < 0.05, a < b < c < d
Fig. 6.
Protection of high concentration collagen coating from rosiglitazone-induced adipogenesis in ST2 cells. A ST2 cells were cultured in different collagen concentration coated wells for 3 days, and then they were treated with different concentrations of rosiglitazone for an additional 5 days. Pictures (×10) were taken from oil Red-O stained cells and represented picture from each treatment of triplicates. B Real-time PCR analysis for mRNA expressions of PPARγ in ST2 cells from duplicated experiment of A. C ST2 cells were cultured and treated with 2 % of either OVX or BB OVX rat serum for 3 days, and then they were treated with different concentrations of rosiglitazone for an additional 5 days. Pictures (×10) were taken from oil Red-O stained cells and represented picture from each treatment of triplicates. D Real-time PCR analysis for mRNA expressions of PPARγ in ST2 cells from duplicated experiment of c. Numbers under each represented picture are means ± SEM of the intensity of oil Red-O stained cells from triplicates and read by a plate reader. #p < 0.05, compare to non-collagen coating; *p < 0.05, compare to sham rat serum. Data are expressed as mean ± SEM (triplicates)
Discussion
We have presented results supporting a novel mechanistic paradigm linking OVX-induced degradation of bone matrix collagen to activation of the osteoblastic cell senescence pathway. As previously described (Zhang et al. 2011), treatment of animals with BB-supplemented diets either for a short term prior to puberty or continuously from weaning through adulthood prevented osteoblastic cells from entering OVX-triggered senescence. As a common source of osteoblasts and adipocytes, naïve bone marrow stromal cells may be more sensitive than mature osteoblasts or osteocytes to the changes in their environmental conditions causing premature senescence. Moreover, our data suggested that coincident with premature senescence, stromal cells became susceptible to adipogenic stimuli and differentiated into adipocyte-like cells. These data extend our previous results and suggest that OVX-induced bone loss in rats may involve osteoblastic cell senescence, and this process is prevented by dietary supplementation with BB. It is well known that bone loss in adulthood occurs after sex steroids start to decline. This loss of bone is believed to be due to increased differentiation, activation, and prolonged life span of osteoclasts. However, increased osteoblast apoptosis may also play a pathological role (Kousteni et al. 2002). Experimental findings during the previous several decades still cannot fully explain the pathophysiology of sex steroid deficiency-induced bone loss. In a previous report, we demonstrated that in addition to preventing OVX-induced bone loss, BB treatment of OVX rats actually increased BV/TV and osteoblasts number compared to sham-operated controls (Zhang et al. 2011). In our current report, we have demonstrated that OVX-induced bone loss occurs with osteoblastic cell senescence and degradation of bone matrix collagen. OVX not only promotes osteoblastic cell senescence but also suppresses the differentiation potential of osteoblast precursors.
Collagen type 1 is structurally composed of a three-component chain complex and is known to deteriorate as osteoporosis develops (Bailey and Knott 1999). To replace the degraded collagen, bone compensates by remodeling to prevent the decrease of bone tensile strength (Wall et al. 1979). Collagen provides a niche for stem cells in bone marrow. Decreased collagen in bone may force cell fate decisions, driving stromal cells into terminal differentiation into adipocytes. This is consistent with observations of accumulated bone marrow adiposity after OVX in the current report and after ovarian dysfunction in humans and rodents (Ecklund et al. 2010; Rosen and Bouxsein 2006). Indeed, stromal cell differentiation towards tissue-specific functional cells is extremely sensitive to environmental conditions (McBeath et al. 2004; Kilian et al. 2010), such as tissue elasticity (Engler et al. 2006). Three-dimensional matrix cell cultures could be used to confirm our in vivo data and test our hypothesis. However, by using culture plates coated with different levels of collagen, we were able to determine that dietary BB is capable of maintaining osteoblast differentiation potential and prevent cells entering senescence. This appears to occur as the result of preserving cytostructural protein expression (Zhang et al. 2011). Without appropriate collagen bone matrix support, mature osteogenic cells rapidly enter senescence, and initiation of adipogenic transition of senescent MSCs occurs. Conversely, evidence has also been reported that osteoblastic transition occurs in senescent vascular smooth muscle cells where collagen matrix is excessively laid down (Nakano-Kurimoto et al. 2009). We have not directly tested whether differentiated senescent osteoblasts are capable of re-differentiation into other cell types. However, the matrix-related signals (i.e., calcium signaling and nicotinamide adenine dinucleotide phosphate oxidase activation; Prosser et al. 2011) involved in tissue senescence require further investigation.
Our present report confirms our previous data demonstrating that OVX leads to osteoblastic cell senescence (Zhang et al. 2011). Osteoblastic cell senescence was also observed in another condition of impaired estrogen signaling, chronic high-dose alcohol intake (Chen et al. 2009). Taken together, these results suggest that we have elucidated the mechanisms involved in estrogen deficiency-induced senescence. Moreover, we demonstrate a persistent effect of BB diet to prevent senescence of osteoblasts and their precursors. Our in vivo and in vitro data showed increases in collagenase activity and expression in circulation, in bone, and in osteoblastic cells cultured on lower collagen concentration coated plates or after treatment of OVX rat serum. Along with increased senescence associated beta-galactosidase activity, we believe that these data support an association between senescent bone-forming cells and OVX-induced bone pathology. Determining the exact and precise mechanism of dietary BB's protective effect, particularly the persistent effects of BB supplementation in early development, against bone cell senescence has not been elucidated at this time. There could be several circulating bioactive BB diet-associated compounds involved. We have previously hypothesized that aside from known dietary components required for bone development and health (such as adequate protein, calories, calcium, phosphorus, and vitamin D), certain other dietary factors or phytochemicals may increase bone development and bone mass which ultimately reduces the risk or degree of osteoporotic bone loss later in life. Bioactive compounds in serum associated with a BB-containing diet are still under investigation in our laboratory. For example, BB polyphenol-derived phenolic acids may contribute to the prevention of osteoblastic cell senescence. We speculate that short-term consumption of a BB diet in early development may epigenetically modify osteoblastic cell senescence-associated genes leading to persistent effects on bone in adult life. This hypothesis and whether BB diet produces similar bone effects in humans will be examined in future studies.
Sirt1 is one of the mammalian sirtuin homologs and a protein deacetylase that plays a central role in metabolic homeostasis and has also been shown as an energy sensor to mediate effects of calorie restriction-induced lifespan extension (Hu et al. 2011). The beneficial effects of Sirt1 activation could be tissue or organ-dependent. In agreement with this, both our in vivo and in vitro evidence suggest that BB diet was capable of blocking or reversing OVX-induced deactivation of Sirt1. As a result, OVX-induced p53 acetylation and p16 activation were alleviated by BB diet leading to reduction of osteoblastic cell senescence. This was consistent with previous evidence showing that p16-controlled cellular lifespan extension may be through Sirt1-mediated epigenetic mechanisms (Li and Tollefsbol 2011). This may also explain our data showing that giving a BB diet for only a short term early in life prevented OVX-induced osteoblastic cell senescence in adults. A previous study reported that activation of Sirt1 suppressed PPARγ expression resulting in less adipogenic potential from MSCs (Picard et al. 2004). A recent study has shown that senescent human diploid fibroblasts could be restored by modulation of the ECM (Choi et al. 2011). These lines of evidence are consistent with our data demonstrating that BB diet was able to block OVX-induced osteoblast senescence by reactivation of Sirt1.
In conclusion, we suggest that the effect of OVX on osteoblastic cell senescence is associated with bone matrix degradation and that short-term feeding of a diet containing BB just prior to puberty can prevent these effects later in adult life. We have also identified molecular targets (i.e., Sirt1) in the senescence pathway whereby BB prevents senescence and differentiation to adipocytes. These insights may be fundamental to explain the pathophysiological mechanism of estrogen deficiency-induced bone loss.
Electronic supplementary materials
Real-time reverse-transcription polymerase chain reaction (RT-PCR) primer sequences (DOC 62 kb)
Senescence-associated beta-galactosidase (SABG) activity in OB6 cells (PPT 128 kb)
Acknowledgments
We would like to thank the following people for their technical assistance: Matt Ferguson, Trae Pittman, and Tammy Dallari. This study was supported by ARS CRIS #6251-51000-005-03S.
References
- Bailey AJ, Knott L. Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Exp Gerontol. 1999;34:337–351. doi: 10.1016/S0531-5565(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther. 2006;319:1182–1190. doi: 10.1124/jpet.106.109454. [DOI] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways while estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009;24:221–230. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–2411. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- Choi HR, Cho KA, Kang HT, Lee JB, Kaeberlein M, Suh Y, Chung IK, Park SC. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell. 2011;10:148–157. doi: 10.1111/j.1474-9726.2010.00654.x. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liu J, Wang J, Liu Q. The controversial links among calorie restriction, SIRT1, and resveratrol. Free Radic Biol Med. 2011;51:250–256. doi: 10.1016/j.freeradbiomed.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tollefsbol TO. p16(INK4a) suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanisms. PLoS One. 2011;6:e17421. doi: 10.1371/journal.pone.0017421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeleta tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, Takahashi T, Matoba S, Yamada H, Okigaki M, Matsubara H. Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiol Heart Circ Physiol. 2009;297:H1673–1684. doi: 10.1152/ajpheart.00455.2009. [DOI] [PubMed] [Google Scholar]
- Narita M, Narita M, Krizhanovsky V, Nuñez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechno-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricalà C, O'Toole KM. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana A, Wiemann SU, Buer J, Lauber J, Dittmar KE, Wüstefeld T, Blasco MA, Manns MP, Rudolph KL. Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J. 2003;22:4003–4013. doi: 10.1093/emboj/cdg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- Vetterli L, Brun T, Giovannoni L, Bosco D, Maechler P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J Biol Chem. 2011;286:6049–6060. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JC, Chatterji SK, Jeffery JW. Age-related changes in the density and tensile strength to human femoral cortical bone. Calcif Tissue Int. 1979;27:105–108. doi: 10.1007/BF02441170. [DOI] [PubMed] [Google Scholar]
- Ward DF, Jr, Salasznyk RM, Klees RF, Backiel J, Agius P, Bennett K, Boskey A, Plopper GE. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells Dev. 2007;16:467–480. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lazarenko OP, Blackburn ML, Shankar K, Badger TM, Ronis MJ, Chen JR. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One. 2011;6:e24486. doi: 10.1371/journal.pone.0024486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time reverse-transcription polymerase chain reaction (RT-PCR) primer sequences (DOC 62 kb)
Senescence-associated beta-galactosidase (SABG) activity in OB6 cells (PPT 128 kb)