Abstract
It is well known that immune response decreases with aging. Salidroside (SDS), an antioxidant component isolated from the traditional Chinese medicine roseroot Rhodiola rosea, has been demonstrated to possess potent anti-aging and health-promoting activities. However, the mechanism underlying these activities is poorly understood. In this study, we clearly demonstrated that (1) dietary intake of SDS induced a considerable increase in total T cells (CD3+) and T helper cells (CD4+) in aged (21 months old) Wistar male rats; (2) SDS supplementation significantly increased the DTH response, a T cell-mediated immune response, in aged rats; and (3) SDS supplementation remarkably promoted the production of total anti-KLH IgG, anti-KLH IgG1, and anti-KLH IgG2α in aged rats without disturbing immune homeostasis. These indicate that SDS is able to counteract immunosenescence, thereby resulting in rejuvenation. Practically, SDS may be used to help the elderly to generate an improved response to vaccine with stronger humoral and cell-mediated immune responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-012-9394-x) contains supplementary material, which is available to authorized users.
Keywords: Rats, Salidroside, Ageing, Anti-ageing, Immune response
Introduction
Aging affects the immune system, which is manifested by age-dependent defects in both humoral and cell-mediated immune responses (Kovacs et al. 2009; Solana et al. 2006). A major alteration commonly observed in elderly subjects and old mice is the deficiencies in T cell functions exemplified by decreased T cell memory and exhaustion of the naïve T cell population with involution of the thymus (Chakravarti and Abraham 1999; Weng 2006). Among in vivo parameters of T cell-mediated immune response, the delayed-type hypersensitivity (DTH) reaction is depressed with advanced age. In particular, low or no DTH responses are often associated with increased morbidity and mortality (Cohn et al. 1983; Meakins et al. 1977; Wayne et al. 1990). Although the B cell compartment of the immune system is only influenced to a lesser extent by senescence, antigen-specific responses to immunization are altered with aging (Kumar and Burns 2008). As a result, elderly subjects are less able to mount an immune response after challenges with pathogens than are young adults and more susceptible than young to microbial infections, reactivation of latent viruses, autoimmune diseases, and neoplasia (Gomez et al. 2008; McGlauchlen and Vogel 2003) that contribute to morbidity and mortality.
It is well known that nutritional status exerts profound effects on immunity (Scrimshaw and SanGiovanni 1997). Nutritional interventions with essential nutrients or functional foods are thus increasingly become an effective approach to improve the immune system (Bogden and Louria 1999). For example, dietary supplementation with nutrients with antioxidant properties, such as β-carotene, vitamin E, and lycopene, has been shown to improve immune function in aged mice and humans (Kemp et al. 2002; Meydani et al. 1997; Vidal et al. 2010). Salidroside (SDS), a phenylpropanoid glycoside, is a potent antioxidant component isolated from the roseroot Rhodiola rosea (hong jing tian in Chinese), which grows in high altitude and other cold regions of the Northern Hemisphere and has long been used in traditional Chinese medicine. Accumulating data have indicated the hepatoprotective and neuroprotective role of SDS against different stresses both in vitro and in vivo, due to its antioxidant property (Guan et al. 2011; Kanupriya et al. 2005; Mao et al. 2010; Wu et al. 2009; Yu et al. 2008; Zhang et al. 2007; Zhu et al. 2011; Ma et al. 2009). SDS has also been found to possess anti-hypoxia, anti-inflammatory, anti-viral, anti-cancer, and anti-fatigue activities (Kelly 2001; Skopinska-Rozewska et al. 2008; Wang et al. 2009; Ye et al. 1993; Yu et al. 2008). More recently, it has been demonstrated that SDS is capable of protecting d-galactose-induced mouse model against aging (Mao et al. 2010) and a formulation of R. rosea extracts (SHR-5) containing SDS capable of increasing both mean and maximum life span of fruit fly (Schriner et al. 2009). Although SDS has been highly valued for many years for its health-promoting, anti-aging, and immune-boosting properties, scientific evidence supporting these benefits remains scarce. Moreover, the mechanism underlying the observed immune-boosting effects is poorly understood. This study was therefore undertaken to explore the effect of dietary intake of SDS on parameters of adaptive immunity in young, middle-aged, and aged rats.
Materials and methods
Animals and treatments
All experimental animals were treated in accordance with the guidelines of the Laboratory Animal Administration Law of China, with the permit number SD2007695 approved by the Ethics Committee of the Laboratory Animal Administration of Shandong province. Specific pathogen-free male Wistar rats (Rattus norvegicus, 8 weeks of age) were purchased from Shandong Lukang Pharmaceutical Co. Ltd (no. 20080002), and housed in an environmentally controlled atmosphere (temperature 22°C and relative humidity 56%) with a 12 h light/dark cycle. They were given free access to water and diet (see below) and provided with shredded wood flour bedding for social activity. Rats were initially housed five per cage, and then they were housed two per cage at age of 3 months. As Wistar male rats have a life span of approximately 24–30 months, thus 12-month-old and 21-month-old rats were used as models of middle-aged and aged rats, respectively.
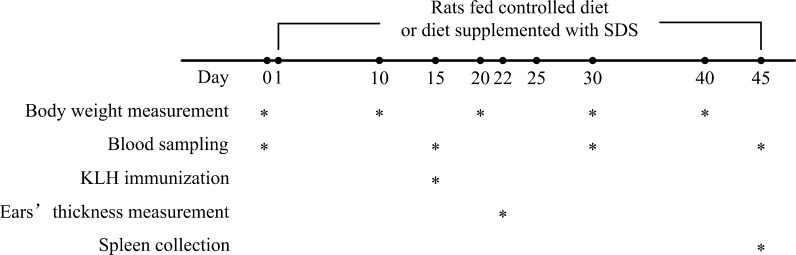
Both young (2 months old, n = 20) and middle-aged (12 months old, n = 20) as well as aged (21 months old, n = 20) rats were divided at random into two groups of ten animals: (1) Rats of control groups (n = 10 rats per age group) were fed with the controlled diet (complete semisynthetic columniformed diet containing 18% crude proteins and 5% cellulose following the Chinese Association For Laboratory Animal Sciences) and (2) Rats of experimental groups (SDS group, n = 10 rats per age group) were fed with the controlled diet supplemented with SDS at a dose of 24 mg/kg of body weight continuously for 45 days (Fig. 1). The dose of SDS used in this study was according to the observations by Du et al. (2009). SDS was dissolved in distilled water (24 mg/mL). An aliquot portion of SDS solution (correlated with each rat body weight) was taken and injected into a small piece of the controlled diet. The food with injected SDS was given to the rat, making sure it ate the food completely. Drinking bottles with freshly mineral water were replaced daily. Throughout the 45-day study period, rats were allowed free access to diet and drinking water. Body weights were measured and recorded every 10 days (Fig. 1). At the end of the study, rats were killed under anesthesia.
Fig. 1.
A schematic diagram of the experiment. The asterisk indicates the time points at which the different trials were carried out. Rats of control and experimental groups were fed with controlled diet and diet supplemented with SDS, respectively. Body weights were measured on 0, 10, 20, 30, and 40 days of the trial. Blood was sampled on 0, 15, 30, and 45 days of the trial. DTH response was assayed on day 22 of the trial (i.e., 7 days after KLH immunization). Spleen was collected at 45 day of the trial (i.e., sacrifice day)
Chemicals
SDS (purity, ≥98%) was purchased from Yuancheng Gongchuang Technology Co. Ltd (Wuhan, China). Keyhole limpet hemocyanin (KLH), fetal calf serum (FCS), concanavalin A (ConA), and lipopolysaccharide (LPS) were all from Sigma-Aldrich (St. Louis, MO, USA). Imject Alum was from Thermo Scientific (Rockford, IL, USA). Biotin-conjugated goat anti-rat IgG, and mouse anti-rat IgG1 and IgG2α were from Abcam (Cambridge, UK). Phycoerythrin (PE)-conjugated anti-rat CD3 antibody (T cells; clone eBioG4.18) was from eBioscience (San Diego, CA, USA), and Fluorescein isothiocyanate (FITC) conjugated anti-rat CD4 (T helper cells; clone W3/25), PE-conjugated anti-rat CD8 (T cytotoxic cells; clone G28) and FITC-conjugated anti-rat CD44 (memory T cells; clone OX-49) antibodies were from Biolegend (San Diego, CA, USA). CellTiter 96 AQueous One Solution Reagent was from Promega (Madison, WI, USA). 3,3',5,5'-Tetramethyl benzidine (TMB) peroxidase substrate was from Sangon (Shanghai, China). Penicillin and streptomycin were from Klontech (Jinan, China). HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) was from Solarbio (Beijing, China) and 96-well plates from Corning Incorporated (NY, USA).
Immunization protocol
Rats were immunized as described by Vidal et al. (2010). The T cell-dependent humoral response (antigen-specific antibody production) was measured after immunization of rats with KLH, an innocuous protein isolate that generates a strong T cell-mediated antibody response and has been extensively used in animals. All rats were immunized on day 15 of the study with a subcutaneous injection of 250 μg of KLH emulsified in 1% Imject Alum. Blood samples were collected on days 0, 15, 30, and 45 for serum or plasma preparation (Fig. 1).
DTH response assay
The DTH response was used as an in vivo measure of cellular immunity. The response was calculated as the difference in ear thickness of the rats before and after challenge with a recall antigen. DTH response assay was performed according to the method of Vidal et al. (2010). In brief, on day 22 of the study (i.e., 7 days after KLH immunization), the thickness of the ears were measured with a micrometer, and immediately thereafter, the DTH response was elicited by intradermal injection of KLH (20 μL of 1 μg/mL in phosphate-buffered saline (PBS)) into the right ear. For control, vehicle (20 μL of PBS) alone was injected into the left ear. At 12 and 24 h after challenge, the thickness of the control ear (PBS) and the challenged ear (KLH) were measured. The DTH responses were expressed as the magnitude of ear swelling, using the following formula: △ in ear thickness of challenged ear (KLH) − △ in ear thickness of control ear (PBS), where △ in ear thickness = ear thickness postchallenge − ear thickness prechallenge.
Blood sampling and hematologic index/biochemical parameter assays
Blood samples were collected from the tail vein on days 0, 15, and 30 of the study. At sacrifice (day 45), blood was collected from living heart. Blood samples were divided into two parts: One part of the blood was directly used for hematologic index assay and the other part, used to prepare serum by centrifugation at 10,000×g at 4°C for 15 min. The hematologic indexes of the blood, including red blood cells (RBC), white blood cells (WBC), hemoglobin (HGB), and platelets (PLT), were analyzed by an automatic hematologic analyzer (Sysmex XS-800i). The biochemical parameters of the serum, including alanine transaminase (ALT) and aspartate transferase (AST), were immediately analyzed using an automatic biochemistry analyzer (Hitachi P7600). The remaining serum was aliquoted and stored at −80°C until used.
KLH-specific IgG concentration assay
The levels of KLH-specific IgG, IgG1, and IgG2α in the blood were measured by enzyme-linked immunosorbent assay according to the method of Vidal et al. (2010), with a slight modification. Briefly, microtiter plates were coated with KLH (50 μL/well at 5 μg/mL for total IgG and IgG1, and 10 μg/mL for IgG2α), incubated at 37°C for 2 h and blocked with 5% FCS for 1 h at 37°C. Serum samples were then added into the wells after dilution (1/2,000 for total IgG, and 1/100 for IgG1 and IgG2α), and the plates were incubated at 37°C for 1 h. Bound antibodies were detected following incubation with a biotin-conjugated goat anti-rat IgG (1/200,000), or mouse anti-rat IgG1 (1/20,000) and IgG2α (1/20,000) at 37°C for 1 h in a water bath. Plates were read at 450 nm after the addition of the TMB peroxidase substrate. Anti-KLH IgG, IgG1, and IgG2α were expressed as median of normalized optical density at 450 nm.
Assay for cellular composition of splenocytes
The percentages of major T and B cell subsets in spleen were assayed using fluorescence-activated cell sorting analysis. Single-cell suspensions of splenocytes (1 × 106 cells/sample) were stained for cell surface antigen expression with following antibodies: PE-conjugated anti-rat CD3, FITC-conjugated anti-rat CD4, PE-conjugated anti-rat CD8, and FITC-conjugated anti-rat CD44. Unlabeled cells were used as a negative control. Cells were analyzed on a Beckman EPICS XL-MCL ADC flow cytometry equipped with SystemII™ analysis software.
Assay for proliferation of splenocytes
Splenocytes were suspended in complete RPMI-1640 medium containing 100 U/mL penicillin, 100 mg/mL streptomycin, 10 mM HEPES, and 10% heat-inactivated FCS. Cell viability was tested by Trypan blue exclusion. Splenocytes (1 × 105 cells/well) were seeded in 96-well plates and cultured in the presence or absence of the T cell mitogen ConA at 10 μg/mL, or the B cell mitogen LPS at 5 μg/mL or the antigen KLH at 100 μg/mL at 37°C for 64 h in an atmosphere of 5% CO2 and 95% humidity. Subsequently, 20 μL of CellTiter 96 AQueous One Solution Reagent was added into each well, incubated at 37°C for 4 h in a humidified atmosphere with 5% CO2 and then the absorbance at 490 nm were measured under a spectrophotometer (Tecan, Austria).
Statistical analysis
Statistical analysis was performed using Student’s t test in the software SPSS 13.0. Analysis was done separately for the young rat study, the middle-aged rat study, and the aged rat study as these studies were carried out independently. Data were expressed as the median ± standard error of the median (SE of median). Probability values of less than 0.05 were considered as significant.
Results
Effects of SDS on general condition and physiology of rats
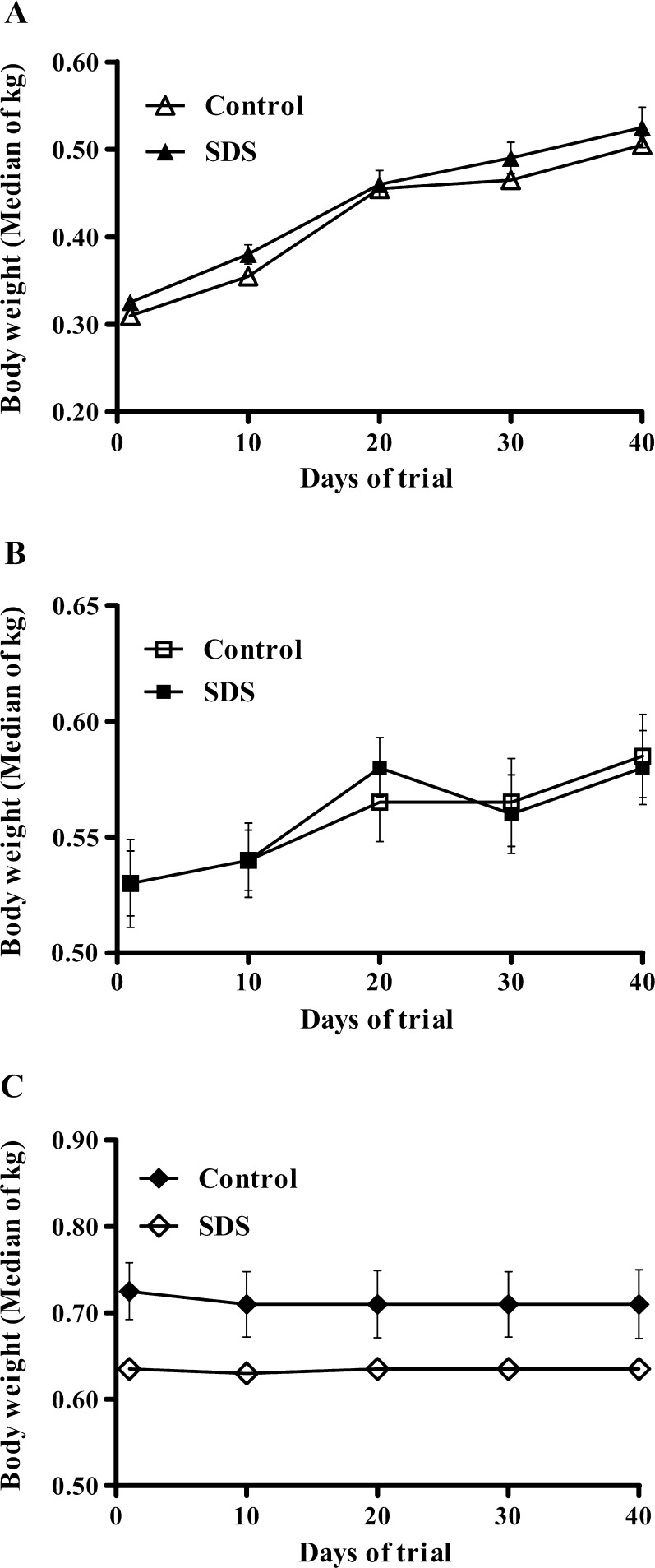
Young, middle-aged, and aged male rats in all diet groups were healthy throughout the experiment. As shown in Fig. 2, body weights were not affected by dietary SDS supplementation. Median body weight values of both young and middle-aged rats were not different among the diet groups at the beginning of the study and increased from 0.33 ± 0.01 to 0.53 ± 0.02 kg in young rats, and from 0.53 ± 0.01 to 0.58 ± 0.01 kg in middle-aged rats (Fig. 2a, b). Median body weight values of aged rats were 0.64 ± 0.01 kg at the start of the study (day 0), which remained no change till the end of the trial (Fig. 2c). Besides, hematologic index and biochemical parameter assays revealed that median hematologic indices (Electronic supplementary material S1) and ALT/AST activities (Electronic supplementary material S2) of young, middle-aged, and aged rats both remained similar among the diet groups, suggesting that general physiology of rats was not affected by dietary SDS supplementation. These data indicated that no significant differences in general condition and physiology were observed among the diet groups both in young and middle-aged as well as aged rats throughout the trial period.
Fig. 2.
Body weights of young (2 months old) rats (a), middle-aged (12 months old) rats (b), and aged (21 months old) rats (c) fed with controlled diet or diet supplemented with salidroside for 45 days. Values are medians of kilograms ± standard error (SE) of medians
Effects of SDS on cellular composition of splenocytes in vivo
As shown in Table 1, SDS supplementation had little effect on the rate of total T cytotoxic cells (CD8+) in spleen from young, middle-aged, and aged rats. Although total T cells (CD3+) and T helper cells (CD4+) in spleen from young rats were not affected by SDS supplementation, they were significantly increased in middle-aged and aged rats fed SDS-supplemented diet (Table 1). The percentage of total memory T cells (CD44+) in spleen from middle-aged rats was also markedly increased by SDS supplementation, but it was not influenced in young and aged rats (Table 1). Clearly, in vivo total T cells (CD3+) and T helper cells (CD4+) in aged rats were significantly increased by dietary SDS supplementation.
Table 1.
Flow cytometry assays of cellular composition of splenocytes from young, middle-aged, and aged rats fed controlled diet or diet supplemented with SDS
| Cells/Makersa,b | Young rats | p value | Middle-aged rats | p value | Aged rats | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | ||||
| CD3+ | 42.2 ± 1.5 | 43.3 ± 2.2 | NS | 37.4 ± 1.4 | 42.9 ± 1.3 | 0.003 | 37.2 ± 1.2 | 45.4 ± 2.3 | 0.009 |
| CD4+ | 20.6 ± 1.3 | 25.0 ± 1.1 | NS | 18.1 ± 0.8 | 22.1 ± 0.5 | 0.0003 | 17.6 ± 0.7 | 20.0 ± 1.3 | 0.024 |
| CD8+ | 21.5 ± 1.2 | 23.3 ± 2.3 | NS | 21.1 ± 2.1 | 23.0 ± 0.7 | NS | 22.1 ± 2.3 | 24.7 ± 2.6 | NS |
| CD44+ | 41.6 ± 1.4 | 42.3 ± 2.1 | NS | 35.6 ± 1.5 | 41.7 ± 1.4 | 0.006 | 36.2 ± 2.0 | 41.2 ± 2.5 | NS |
C control, NS not significant
aThe percentages of major T and B cell subsets in spleen were assayed as described in “Materials and methods”
bValues are medians ± standard error (SE) of medians
Effects of SDS on ex vivo proliferation of splenocytes
The ex vivo proliferative responses of rat splenocytes to SDS were shown in Table 2. Upon stimulation with ConA, a T cell mitogen, or with LPS, a B cell mitogen, the proliferative response of splenocytes from young rats fed SDS-supplemented diet was significantly increased compared with those fed the controlled diet. In contrast, dietary SDS supplementation exerted little effect on the proliferation of splenocytes from middle-aged and aged rats, upon stimulation with ConA or LPS. Young rats fed SDS-supplemented diet had a significantly higher proliferative response upon stimulation with the immunogen KLH than those fed the controlled diet, whereas such an effect was not observed in middle-aged and aged rats. No age-associated difference in ConA-, LPS-, and KLH-stimulated proliferation was found in splenocytes from middle-aged and aged rats.
Table 2.
Proliferative responses of splenocytes from young, middle-aged, and aged rats fed controlled diet or diet supplemented with SDS
| Immunogensa,b | Young rats | p value | Middle-aged rats | p value | Aged rats | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | ||||
| ConA | |||||||||
| (T cell mitogen) | 0.46 ± 0.26 | 0.61 ± 0.02 | 0.0002 | 0.45 ± 0.02 | 0.49 ± 0.02 | NS | 0.45 ± 0.02 | 0.49 ± 0.03 | NS |
| LPS | |||||||||
| (B cell mitogen) | 0.50 ± 0.01 | 0.58 ± 0.02 | 0.006 | 0.48 ± 0.04 | 0.51 ± 0.02 | NS | 0.48 ± 0.03 | 0.51 ± 0.02 | NS |
| KLH | |||||||||
| (Antigen-specific) | 0.53 ± 0.01 | 0.60 ± 0.03 | 0.03 | 0.46 ± 0.04 | 0.52 ± 0.02 | NS | 0.46 ± 0.04 | 0.51 ± 0.02 | NS |
C control, NS not significant
aSplenocytes were cultured in the presence or absence of ConA (10 μg/mL), LPS (5 μg/mL), or KLH (100 μg/mL) for 64 h and pulsed with CellTiter 96 AQueous One Solution Reagent during the last 4 h. OD values were measured to determine the proliferative responses of cells
bValues are medians of OD values ± standard error (SE) of medians
Effects of SDS on antigen-specific humoral and cell-mediated immune responses
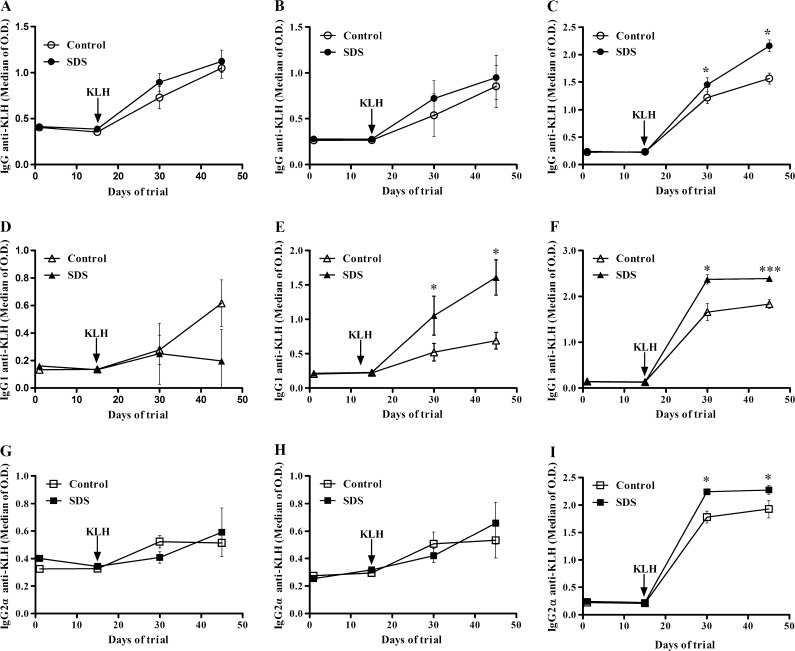
KLH-specific serum immunoglobulin G2α (IgG2α) and IgG1 levels, measured after immunization with KLH, were used as markers of T helper 1 (Th1)- and Th2-dependent humoral responses, respectively. SDS supplementation had no effect on total IgG levels in blood of young, middle-aged, and aged rats (Electronic supplementary material S3). As shown in Fig. 3, levels of total antigen-specific IgG (i.e., anti-KLH IgG) were significantly increased in aged rats fed SDS-supplemented diet (at day 30, 1.22 ± 0.11 for control group and 1.45 ± 0.13 for SDS group, p = 0.046; and at day 45, 1.57 ± 0.10 for control group and 2.16 ± 0.11 for SDS group, p = 0.018) (Fig. 3c) but not in young and middle-aged rats (Fig. 3a, b). SDS supplementation affected blood levels of KLH-specific IgG1 in middle-aged and aged rats and blood level of KLH-specific IgG2α in aged rats (Fig. 3e, f, and i). Anti-KLH IgG1 levels were significantly increased by dietary SDS supplementation in middle-aged rats (at day 30, 0.52 ± 0.13 for control group and 1.05 ± 0.28 for SDS group, p = 0.016; at day 45, 0.69 ± 0.12 for control group and 1.61 ± 0.26 for SDS group, p = 0.013) and in aged rats (at day 30, 1.66 ± 0.19 for control group and 2.37 ± 0.10 for SDS group, p = 0.015; at day 45, 1.83 ± 0.10 for control group and 2.39 ± 0.06 for SDS group, p = 0.0007), but level of anti-KLH IgG1 in serum was not affected in young rats (Fig. 3d). In aged rats, anti-KLH IgG2α level was increased by SDS supplementation, but such an effect was not observed in young and middle-aged rats (Fig. 3g, h).
Fig. 3.
Anti-keyhole limpet hemocyanin (KLH) IgG isotype (a, b, and c), anti-KLH IgG1 isotype (d, e, and f) and anti-KLH IgG2α isotype (g, h, and i) responses in young, middle-aged, and aged rats, respectively. The rats were fed controlled diet or diet supplemented with SDS. Antibody levels were determined as described in “Materials and methods”. Values are medians of measured optical density (OD) at 450 nm ± SE of medians. ***p < 0.001, **p < 0.01, and *p < 0.05, the differences of comparing SDS group to control group
The DTH response was used to evaluate effects on cellular Th1-dependent immunity. As shown in Table 3, SDS supplementation had a significant effect on the DTH response in aged rats at 24 and 48 h after KLH challenge and on the DTH response in middle-aged rats at 48 h after KLH challenge. No difference in the DTH response was found in young rats fed SDS diet compared with control group.
Table 3.
DTH responses of young, middle aged, and aged rats fed controlled diet or diet supplemented with SDS
| Time (h)a,b | Young rats | p value | Middle-aged rats | p value | Aged rats | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | C (n = 10) | SDS (n = 10) | ||||
| 24 | 122 ± 51 | 148 ± 31 | NS | 220 ± 46 | 234 ± 54 | NS | 51 ± 21 | 238 ± 66 | 0.037 |
| 48 | 151 ± 40 | 150 ± 46 | NS | 112 ± 25 | 236 ± 62 | NS | 75 ± 19 | 160 ± 22 | 0.017 |
C control, NS not significant
aTo elicit the delayed-type hypersensitivity (DTH) response, young, middle aged, and aged rats were immunized and challenged with keyhole limpet hemocyanin (KLH) as described in “Materials and methods”. DTH responses were measured as the difference in ear thickness and expressed in micrometers
bValues are medians ± standard error (SE) of medians
Discussion
SDS is a multifunctional bioactive component, which has been shown to possess anti-oxidant, anti-hypoxia, anti-inflammatory, anti-viral, anti-cancer, anti-fatigue, and anti-aging activities. This study explored the effects of SDS on ex vivo and in vivo parameters of adaptive immunity in young, middle-aged, and aged rats. Dietary supplementation with SDS was started 2 weeks before immunization of the rats with a T cell-dependent antigen, KLH, and continued until the end of the experiment, so that the diet had the opportunity to modulate cell-mediated immune responses during both the initiation and promotion of the immune response toward the antigen challenge. Our results clearly demonstrated that SDS has rejuvenation properties via counteracting immunosenescence, which is best characterized by poor T cell-mediated immune function and low protection after vaccination. First, SDS induced a considerable increase in total T cells (CD3+) and T helper cells (CD4+) in aged rats. Second, SDS supplementation significantly increased the DTH response, a T cell-mediated immune response, in aged rats. Third, SDS supplementation remarkably promoted the production of total anti-KLH IgG, anti-KLH IgG1, and anti-KLH IgG2α in aged rats without disturbing immune homeostasis.
Incompetence to proliferate in response to antigen is a typical feature of the decline in T cell function with aging (Haynes and Maue 2009). Here, we showed that both total T cells (CD3+) and T helper cells (CD4+) in aged rats were significantly increased by dietary SDS supplementation. Moreover, although a marked increase in proliferative response of splenocytes to the recall antigen KLH was only observed in young rats fed SDS-supplemented diet, splenocytes from young, middle-aged, and aged rats all proliferated in the presence of KLH. These data provide an ex vivo evidence showing that a T cell-mediated memory response took place. Dietary with SDS supplementation significantly stimulated splenocyte proliferation to T cell mitogen ConA and B cell mitogen LPS in young rats, although it had little effect on splenocyte proliferation in response to ConA and LPS in middle-aged and aged rats. This absence of splenocyte proliferation-stimulating effect by SDS supplementation in middle-aged and aged rats may be due to the mitogen concentrations used. Indeed, it has been shown that an increased proliferative response of splenocytes following supplementation with conjugated linoleic acid was observed only with suboptimal and supraoptimal concentrations of ConA in young mice and optimal concentration of ConA in aged mice (Hayek et al. 1999).
DTH response is an in vivo indicator of the ability to mount a T cell-mediated response and is mainly driven by memory CD4+ T cells which release interleukin-2 and interferon-γ to signal macrophages to an area after an encounter with the recall antigen. It has been widely used in nutrition immunology studies of elderly subjects and old mice (Bogden et al. 1994; Pallast et al. 1999; Wu et al. 1998; Vos et al. 2006). An age-associated difference in DTH response was noted in the presence of SDS. Dietary with SDS supplementation had little effect on the DTH response in young and middle-aged rats, but it significantly increased the DTH response in aged rats. These suggest that T cell-mediated immune responses are benefited by SDS supplementation. Clinically, this may have a critical implication, because DTH responses that decrease with age are inversely correlated with morbidity and mortality in elderly subjects (Linton and Dorshkind 2004; Scherer and Bircher 2010).
Humoral response to antigen challenge changes with aging (Frasca et al. 2005). For example, the primary antibody response to the KLH antigen has been reported to decline in elderly subjects (Smith et al. 2004). An age-associated difference in T cell-mediated antibody response to the KLH antigen was noted in the presence of SDS as well. Although SDS supplementation had little effect on the production of total anti-KLH IgG, anti-KLH IgG1 and anti-KLH IgG2α in young rats, it significantly promoted the production of all of them in aged rats. These observed changes in antibody responses in aged rats fed SDS-supplemented diet are indicative of improvements in antigen-driven responses because total antigen nonspecific IgG levels were not affected. These suggest that dietary with SDS supplementation is able to modulate immune responses without disrupting immune homeostasis. Importantly, dietary with SDS supplementation resulted in a significant increase in the KLH-specific IgG1 antibody response in middle-aged and aged rats as well as a significant increase in the KLH-specific IgG2α response in aged rats. It is known that the production of IgG2α is promoted by Th1 cytokines and the production of IgG1 antibody by Th2 cytokines (Snapper and Mond 1993). Thus, this observation suggests that dietary with SDS supplementation could be beneficial to both Th1- and Th2-type immune responses.
SDS supplementation stimulated in vivo proliferation of total CD3+ cells and CD4+ helper cells but not cytotoxic CD8+ cells in middle-aged and aged rats. Similarly, dietary with SDS supplementation enhanced ex vivo KLH-specific T cell-mediated immune responses but not mitogen (ConA and LPS)-stimulated responses in middle-aged and aged rats. These suggest that SDS supplementation may exert an effect on antigen processing and presentation and/or on antigen-presenting cells (APC) function. It is possible that SDS supplementation could influence APC function by promoting cell-surface expression of KLH-derived peptide-MHC class II molecule complexes and/or co-stimulatory molecules required for optimal T cell response. These aspects demand further study in the future.
In summary, this study highlights the anti-aging and immunostimulatory properties of SDS via enhancing in vivo humoral and cell-mediated immune responses in aged rats after antigen challenge. It also boosts the notion that dietary intake of SDS may help the elderly to generate an improved response to vaccine with stronger humoral and cell-mediated immune responses.
Electronic supplementary material
(DOC 47 kb)
(DOC 29 kb)
(DOC 31 kb)
Acknowledgments
The authors thank Ms. Jie Ma and Mr. Hongmiao Wang for their aids in collection of the blood and tissues of the animals. The authors also thank Dr. Gamal El-Sokkary for his valuable comments and suggestions on this paper. This work was supported in part by a grant (2012CB114404) of Ministry of Science and Technology (MOST) of China.
Footnotes
Linlin Lu and Jiangshui Yuan contributed equally to this work.
References
- Bogden JD, Louria DB. Aging and the immune system: the role of micronutrient nutrition. Nutrition. 1999;15(7–8):593–595. doi: 10.1016/S0899-9007(99)00093-3. [DOI] [PubMed] [Google Scholar]
- Bogden JD, Bendich A, Kemp FW, Bruening KS, Shurnick JH, Denny T, Baker H, Louria DB. Daily micronutrient supplements enhance delayed-hypersensitivity skin test responses in older people. Am J Clin Nutr. 1994;60(3):437–447. doi: 10.1093/ajcn/60.3.437. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Abraham GN. Aging and T-cell-mediated immunity. Mech Ageing Dev. 1999;108(3):183–206. doi: 10.1016/S0047-6374(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Cohn JR, Hohl CA, Buckley CE. The relationship between cutaneous cellular immune responsiveness and mortality in a nursing-home population. J Am Geriatr Soc. 1983;31(12):808–809. doi: 10.1111/j.1532-5415.1983.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Du B, Yan T, Ma Y, Wang Q, Yang W. Effects of salidroside on hematological system in rats and mice. Chinese J Experiment Trad Med Form. 2009;15:51–54. [Google Scholar]
- Frasca D, Riley RL, Blomberg BB. Humoral immune response and B-cell functions including immunoglobulin class switch are downregulated in aged mice and humans. Semin Immunol. 2005;17(5):378–384. doi: 10.1016/j.smim.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43(8):718–728. doi: 10.1016/j.exger.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Wang W, Lu J, Qian WH, Huang GR, Deng XM, Wang XL. Salidroside attenuates hydrogen peroxide-induced cell damage through a cAMP-dependent pathway. Molecules. 2011;16(4):3371–3379. doi: 10.3390/molecules16043371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek MG, Han SN, Wu D, Watkins BA, Meydani M, Dorsey JL, Smith DE, Meydani SN. Dietary conjugated linoleic acid influences the immune response of young and old C57BL/6NCrlBR mice. J Nutr. 1999;129(1):32–38. doi: 10.1093/jn/129.1.32. [DOI] [PubMed] [Google Scholar]
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21(4):414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanupriya D, Prasad D, Ram MS, Kumar R, Sawhney RC, Sharma SK, Ilavazhagan G, Kumar D, Banerjee PK. Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem. 2005;275(1–2):1–6. doi: 10.1007/s11010-005-7637-1. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Rhodiola rosea: a possible plant adaptogen. Alternative Med Rev. 2001;6(3):293–302. [PubMed] [Google Scholar]
- Kemp FW, DeCandia J, Li WJ, Bruening K, Baker H, Rigassio D, Bendich A, Bogden JD. Relationships between immunity and dietary and serum antioxidants, trace metals, B vitamins, and homocysteine in elderly men and women. Nutr Res. 2002;22(1–2):45–53. doi: 10.1016/S0271-5317(01)00373-6. [DOI] [Google Scholar]
- Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30(7):319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expet Rev Vaccine. 2008;7(4):467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Ma L, Cai DL, Li HX, Tong BD, Wang Y, Pei SP. Protective effects of salidroside on oxidative damage in fatigue mice. Zhong Xi Yi Jie He Xue Bao. 2009;7(3):237–241. doi: 10.3736/jcim20090308. [DOI] [PubMed] [Google Scholar]
- Mao GX, Deng HB, Yuan LG, Li DD, Li YY, Wang Z. Protective role of salidroside against aging in a mouse model induced by d-galactose. Biomed Environ Sci. 2010;23(2):161–166. doi: 10.1016/S0895-3988(10)60047-5. [DOI] [PubMed] [Google Scholar]
- McGlauchlen KS, Vogel LA. Ineffective humoral immunity in the elderly. Microb Infect. 2003;5(13):1279–1284. doi: 10.1016/j.micinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186(3):241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277(17):1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- Pallast EG, Schouten EG, de Waart FG, Fonk HC, Doekes G, von Blomberg BM, Kok FJ. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr. 1999;69(6):1273–1281. doi: 10.1093/ajcn/69.6.1273. [DOI] [PubMed] [Google Scholar]
- Scherer K, Bircher AJ. Danger signs in drug hypersensitivity. Med Clin North Am. 2010;94(4):681–689. doi: 10.1016/j.mcna.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Avanesian A, Liu YX, Luesch H, Jafari M. Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses. Free Radic Biol Med. 2009;47(5):577–584. doi: 10.1016/j.freeradbiomed.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66(2):S464–S477. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- Skopinska-Rozewska E, Malinowski M, Wasiutynski A, Sommer E, Furmanowa M, Mazurkiewicz M, Siwicki AK. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol J Vet Sci. 2008;11(2):97–104. [PubMed] [Google Scholar]
- Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97(2):491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24(5):491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Vidal K, Benyacoub J, Sanchez-Garcia J, Foata F, Segura-Roggero I, Serrant P, Moser M, Blum S. Intake of a milk-based wolfberry formulation enhances the immune response of young-adult and aged mice. Rejuv Res. 2010;13(1):47–53. doi: 10.1089/rej.2009.0895. [DOI] [PubMed] [Google Scholar]
- Vos AP, Haarman M, Buco A, Govers M, Knol J, Garssen J, Stahl B, Boehm G, M’Rabet L. A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Inter Immunopharmacol. 2006;6(8):1277–1286. doi: 10.1016/j.intimp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Ding Y, Zhou J, Sun X, Wang S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against Coxsackievirus B3. Phytomedicine. 2009;16(2–3):146–155. doi: 10.1016/j.phymed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;45(2):M45–M48. doi: 10.1093/geronj/45.2.M45. [DOI] [PubMed] [Google Scholar]
- Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24(5):495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Han SN, Bronson RT, Smith DE, Meydani SN. Dietary supplementation with mushroom-derived protein-bound glucan does not enhance immune function in young and old mice. J Nutr. 1998;128(2):193–197. doi: 10.1093/jn/128.2.193. [DOI] [PubMed] [Google Scholar]
- Wu TJ, Zhou HP, Jin ZX, Bi SH, Yang XL, Yi DH, Liu WY. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur J Pharmacol. 2009;613(1–3):93–99. doi: 10.1016/j.ejphar.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Ye YC, Chen QM, Jin KP, Zhou SX, Chai FL, Hai P. Effect of salidroside on cultured myocardial cells anoxia/reoxygenation injuries. Zhongguo Yao Li Xue Bao. 1993;14(5):424–426. [PubMed] [Google Scholar]
- Yu S, Liu M, Gu XS, Ding F. Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol. 2008;28(8):1067–1078. doi: 10.1007/s10571-008-9284-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564(1–3):18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Shi YP, Wu D, Ji YJ, Wang X, Chen HL, Wu SS, Huang DJ, Jiang W. Salidroside protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt dependent pathway. DNA Cell Biol. 2011;30(10):809–819. doi: 10.1089/dna.2010.1183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 47 kb)
(DOC 29 kb)
(DOC 31 kb)