Abstract
Ageing is accompanied with a decline in respiratory function. It is hypothesised that this may be attenuated by high physical activity levels. We performed spirometry in master athletes (71 women; 84 men; 35–86 years) and sedentary people (39 women; 45 men; 24–82 years), and calculated the predicted lung age (PLA). The negative associations of age with forced expiratory volume in 1 s (FEV1; 34 mL·year−1) and other ventilatory parameters were similar in controls and master athletes. FEV1pred was 9 % higher (P < 0.005) and PLA 15 % lower (P = 0.013) in athletes than controls. There were no significant differences between endurance and power athletes and sedentary people in maximal inspiratory and expiratory pressure. Neither age-graded performance nor weekly training hours were significantly related to lung age. Life-long exercise does not appear to attenuate the age-related decrease in ventilatory function. The better respiratory function in master athletes than age-matched sedentary people might be due to self-selection and attrition bias.
Keywords: FEV1, Lung age, Mouth pressure, Physical activity, Peak expiratory flow, Physical exercise
Introduction
During ageing, there is a progressive decline in respiratory function, due to factors such as a loss of respiratory muscle strength (Hautmann et al. 2000; Dempsey et al. 1990), increased stiffness of the chest wall, reduced elastic recoil of the lung and diffusion capacity, which ultimately may result in hypoxaemia even at rest (Hankinson et al. 1999; Klocke 1977). Although high physical activity levels may attenuate the age-related decline in respiratory function (Pelkonen et al. 2003; Freitas et al. 2010; Amara et al. 2001; Nystad et al. 2006), it is particularly in the elite endurance athlete that exercise performance is limited by hypoxaemia due to constraints of the respiratory system (Dempsey et al. 1990; Dempsey et al. 2008). One explanation for this apparent contradiction could be that the plasticity of the respiratory system is limited (Dempsey et al. 1990) due to, e.g. the size of the thorax and inability to increase blood volume in the lung despite increases in flow during training sessions. Nevertheless, we hypothesise that the exceptional demands placed on the respiratory system by the master athlete may attenuate the normally occurring age-related deterioration of the respiratory system.
Over the last decade we have done a series of studies in master athletes (Michaelis et al. 2008; Korhonen et al. 2006; Rittweger et al. 2009; Rittweger et al. 2004; Wilks et al. 2009; Ireland et al. 2011), i.e. people who train for and compete in their sports beyond a certain age, e.g. the age of 35 in track and field athletics (Reaburn and Dascombe 2008). Their outstanding performances are evidenced by the world record data (Rittweger et al. 2009). Since master athletes (a) train as vigorously as they possibly can, and (b) withdraw from competitions when they become sick or otherwise impaired, they constitute a unique population to study age-related changes without (or with very little) contribution of two usual confounders of old age, namely sedentarism and co-morbidity (Rittweger et al. 2004). The aim of the present study was therefore to compare in a cross-sectional study respiratory function in track and field master athletes and sedentary control people. Since the oxygen demand, and hence ventilation, is elevated over prolonged periods in exercising endurance athletes we hypothesised that the endurance runners would have better ventilatory function than age-matched power master athletes and a more sedentary population. While others have previously reported ventilatory function in master athletes, they did not include a control group, or only studied endurance athletes (McClaran et al. 1995; Pollock et al. 1974).
Materials and methods
Participants
We recruited 71 female and 84 male athletes at the World Master Athletics Championships in 2009 (Lahti, Finland) and the European Veterans Athletics Championships in 2010 (Nyíregyháza, Hungary). Master athletes are typically defined as ‘athletes older than 35 years of age and systematically train for, and compete in, organized forms of sport specifically designed for older adults’ (Reaburn and Dascombe 2008). Three athletes had an Asian background, two an African and the others were white Caucasians. Control participants (men n = 45; women n = 39) were recruited and measured at the Manchester Metropolitan University. Current smokers were excluded from the control group. One female endurance and one male power athlete reported they smoked, but less than a cigarette per day. Some of the athletes and control participants have been smoking in the past but their ventilatory function was in the normal range for the respective groups and their data were included in the analysis. Data were excluded if the forced expiratory volume in 1 s (FEV1) was below the 70 % predicted value and/or the FEV1/vital capacity (FEV1/FVC) was below 70 % (one case; www.goldcopd.com). The study was approved by the ethical committees of the Manchester Metropolitan University, University of Jyväskylä (Finland) and the Semmelweis Institute (Budapest, Hungary). All participants had given written informed consent before inclusion.
Middle- and long-distance runners (800 m–marathon), race-walkers and multi-event athletes (pentathlon, decathlon) were classified as endurance athletes, while sprinters and hurdlers (100–400 m), jumpers (long and triple jump, pole vault) and throwers (hammer, javelin, discuss, shot-put) as power athletes. The following groups were formed: Female Endurance (FE), Female Power (FP), Male Endurance (ME), Male Power (MP) athletes, and Female (FC) and Male (MC) control.
In master athletes, the self-rated best discipline, number of training sessions per week and the hours per session were assessed by interview. The age-graded performance (AGP) for the main event of the master athletes was calculated using the WMA Age-grading Calculator:
Spirometry
All experiments were performed using the same Micro Medical Spiro USB Spirometer and analysed with Spida 5 software (Cardinal Health, UK). The calibration of the spirometer was checked on a daily basis according to the ATS/ERS criteria (Miller et al. 2005). All experiments were performed in a seated position. Participants wore a noseclip and performed a maximal inspiration immediately followed by a forced maximal expiration. This procedure was repeated until the criteria set out by the ATS and ERS were met, i.e. a plateau in the expiration, expiration >6 s, no coughs and between-test variation for Forced Vital Capacity (FVC in liters) and FEV1 <0.15 L (Miller et al. 2005). Whenever participants did not fulfil the criteria, manoeuvres were repeated and checked for a quick start, early peak flow and sufficient expiration (Miller et al. 2005). The presented parameters are: FEV1 (in liters), FVC, Peak Expiratory Flow (PEF, in liters per second) and FEV1/FVC (in percent). The predicted values for FEV1 (FEV1pred) and PEF (PEFpred) were calculated according to equations that include age and height as provided by The Third National Health and Nutrition Examination Survey taking into account ethnic background (Hankinson et al. 1999). That population-based study examined respiratory function in a USA population of 7,429 healthy live-long non-smokers between 8 and 80 years of age; people suffering from wheezing, coughs, asthma, chronic obstructive pulmonary disease and lung cancer were excluded. Using the ‘normal values’ reported by Hankinson et al. (1999) in 988 healthy non-smokers between 20 and 84 years of age the following equations to calculate predicted lung age (PLA) using the manoeuvre with the highest FVC + FEV1 were derived by (Morris and Temple 1985):
 |
Where PLA is the predicted lung age in years and H is height in cm. Since FEV1 increases during maturation and reaches a peak between 20–25 years of age (Hankinson et al. 1999), the minimum lung age is set at 24 by the Spida software, while the maximum lung age is set at 80 years.
In a small number of athletes (MP: 5; ME: 4; FP: 4; FE 3) the diffusion capacity for carbon monoxide of the lungs (DL,CO) could also be determined at the Lahti City Hospital. Thereto, the participant was asked to exhale to residual volume and then quickly inhale till total lung capacity form the gas source with 0.3 % CO. After a 10-s breath-hold the participant was asked to exhale as fast as possible. Slots for DL,CO testing were only available in the evening hours and no such possibility existed in Nyíregyháza. These data are presented as %DL,COpred (Macintyre et al. 2005).
In the master athletes from Lahti and seven control participants, the maximal inspiratory (MIP) and expiratory (MEP) pressure were determined using a portable mouth pressure device (MicroRPM, Cardinal Healthcare, UK) as described previously (Hautmann et al. 2000). Thereto, participants were asked to inspire or expire as forcefully as possible after total expiration or inspiration respectively into the portable MicroRPM. To determine the maximal sniff nasal inspiratory pressure (SNIP) participants placed a probe in one of their nostrils while the other nostril was closed and the participant inspired as fast and as forceful as possible via the nose. For all manoeuvres, attempts were repeated, with a 30s interval between each attempt to prevent the development of respiratory muscle fatigue, until a maximum value was reached. Data are reported in kilopascals.
Statistics
Statistical analyses were carried out using SPSS 16.0. Differences between groups were compared with a two-way ANOVA with sex (men vs. women) and sport (three levels: control, power and endurance athletes) as factors and age as co-variate. A Kolmogorov–Smirnov test showed that all respiratory parameters had a normal distribution. If a significant sport effect was found a Bonferroni corrected post-hoc test was performed to detect the differences. Relationships between parameters are given as Pearson correlation coefficients. Differences and correlations were considered significant at P < 0.05. Values are reported as means ± SD.
Results
Participant characteristics
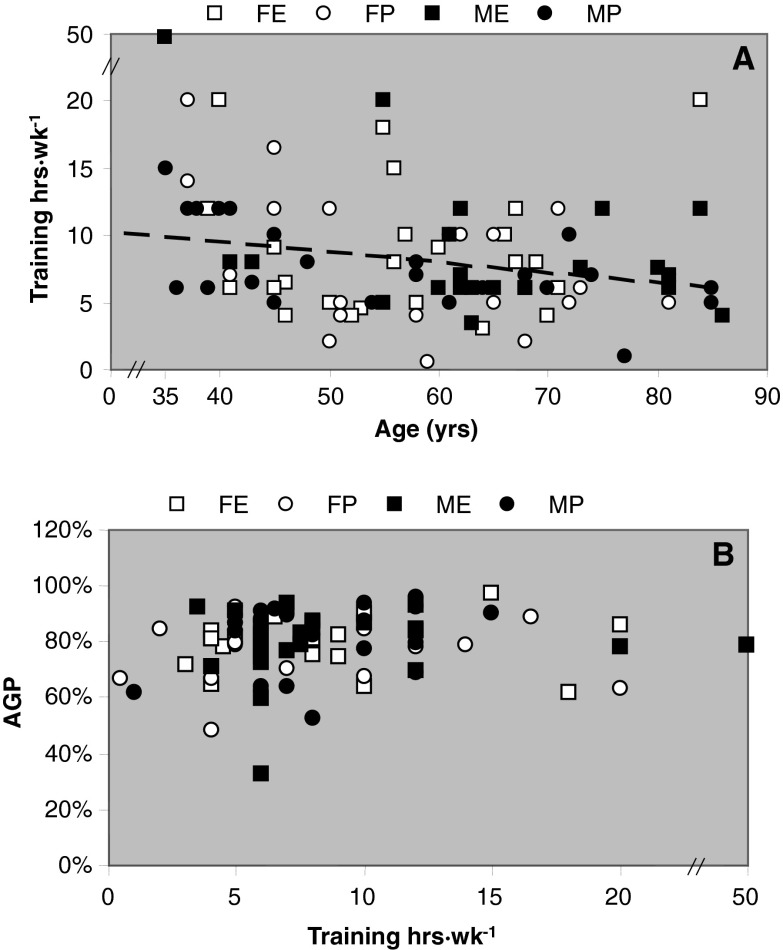
Height was negatively (R2 = 0.055; P < 0.001) and BMI was positively (R2 = 0.039; P = 0.002) related to age (data not shown). Body mass index (BMI) was lowest in endurance athletes (P < 0.001; Table 1). The weekly training hours were negatively related to age, indicating a decrement of 0.76 hours training per week per decade (Fig. 1; R2 = 0.063; P < 0.015; excluding an outlier who reported 50 h·wk−1 of training). The weekly training hours showed no significant correlation with the AGP of the athletes.
Table 1.
Participant characteristics
| FE n = 37 | FP n = 34 | FC n = 39 | ME n = 34 | MP n = 48 | MC n = 45 | |
|---|---|---|---|---|---|---|
| Age (years) | 56 ± 11 (39–84) | 56 ± 12 (36–81) | 63 ± 18 (24–82) | 62 ± 13 (35–86) | 57 ± 15 (35–85) | 59 ± 21 (24–81) |
| Body mass(kg) | 54.6 ± 6.3a (40.0–68.0) | 65.4 ± 15.8a,c (58.0–115.0) | 66.7 ± 13.9a,c (33.0–109.5) | 68.0 ± 9.0 (54.0–94.0) | 76.5 ± 11.6c (55.0–114.0) | 76.5 ± 15.9c (46.0–118.0) |
| Height (m) | 1.63 ± 0.07b (1.48–1.76) | 1.65 ± 0.08a (1.47–1.83) | 1.62 ± 0.07a,b (1.45–1.82) | 1.73 ± 0.07a,b (1.61–2.00) | 1.77 ± 0.08 (1.60–1.94) | 1.73 ± 0.08b (1.56–1.90) |
| BMI (kg·m−2) | 20.4 ± 1.5 (17.1–24.1) | 23.9 ± 4.6c (19.1–37.6) | 25.5 ± 5.1c (15.1–40.3) | 22.6 ± 2.4 (18.1–29.1) | 24.2 ± 2.5c (19.5–30.5) | 25.3 ± 4.3c (17.7–38.5) |
| AGP (%) | 81 ± 10 (59–98) | 76 ± 12 (49–93) | NA | 79 ± 12 (32–94) | 83 ± 11 (53–96) | NA |
| Training (h) | 8.7 ± 5.0 (3.0–20.0) | 7.9 ± 5.2 (0.5–20) | NA | 7.9 ± 3.7 (3.5–20.0)d | 7.7 ± 3.0 (1.0–15.0) | NA |
Mean ± SD (range)
FE female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control; BMI body mass index; AGP age-graded performance; NA not applicable
aDifferent from men at P < 0.001
bDifferent from power athletes P < 0.02
cDifferent from Endurance athletes
dExcluding a person who claimed to train 50 hours per week
Fig. 1.
a Training hours per week and b relationship between age-graded performance (AGP) and training hours of master athletes at different ages. FE Female endurance; FP female power; ME male endurance; MP male power. Linear regression line: training h·wk−1 = −0.0759 × age (years) + 12.523; R 2 = 0.0625; P = 0.014
Spirometry
The average ventilatory parameters predicted for age in the control and athletic groups are shown in Table 2. In Fig. 2 it can be seen that the FEV1 was higher in men than women (P < 0.001) and that it was negatively related to age (men R2 = 0.47; women R2 = 0.61); this decrease amounted to about 34 mL·year−1 in all groups, irrespective of being an athlete or not. The FEV1 of both endurance and power athletes was, however, higher than that of control participants (P < 0.05). Also FEV1pred was 9 % points higher in the athletes than in control participants (P < 0.005), with no difference between power and endurance athletes (P = 0.116, Table 2).
Table 2.
Ventilatory characteristics in athletes and controls
| FE n = 35 | FP n = 28 | FC n = 38 | ME n = 30 | MP n = 42 | MC n = 45 | |
|---|---|---|---|---|---|---|
| FEV1PRED (%) | 105.4 ± 13.4a | 101.1 ± 12.6a | 94.1 ± 11.4 | 107.6 ± 9.8a | 102.6 ± 12.5a | 96.5 ± 14.4 |
| FEV1/FVC | 73.3 ± 7.3a | 76.0 ±5.5a | 80.8 ± 6.1 | 74.6 ± 8.5a | 74.5 ± 7.8a | 78.8 ± 6.1 |
| PEFPRED | 104.9 ± 20.4a | 104.8 ± 13.7a | 88.2 ± 20.6 | 106.3 ± 16.2a | 107.8 ± 17.3a | 96.6 ± 20.1 |
| PLA/age | 0.87 ± 0.24b | 0.93 ± 0.29b | 1.09 ± 0.27 | 0.86 ± 0.18b | 0.94 ± 0.25b | 1.03 ± 0.27 |
Mean ± SD; for further description of statistics, see text
FE female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control; BMI body mass index; FEV 1pred predicted forced expiratory volume in 1 s; FVC/FEV 1 forced vital capacity divided by FEV1; PEF pred predicted peak expiratory flow; PLA predicted lung age
aDifferent from control participants at P < 0.005
bDifferent from control participants at P < 0.05
Fig. 2.
Individual data for forced expiratory volume in 1 s (FEV 1); solid line men: FEV1 = −0.034*age (yrs) + 5.54; R 2 = 0.47; P < 0.001; broken line women: FEV1 = −0.033 × age (years) + 4.56; R 2 = 0.61; P < 0.001. FE Female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control participants. For further description of statistics, see text
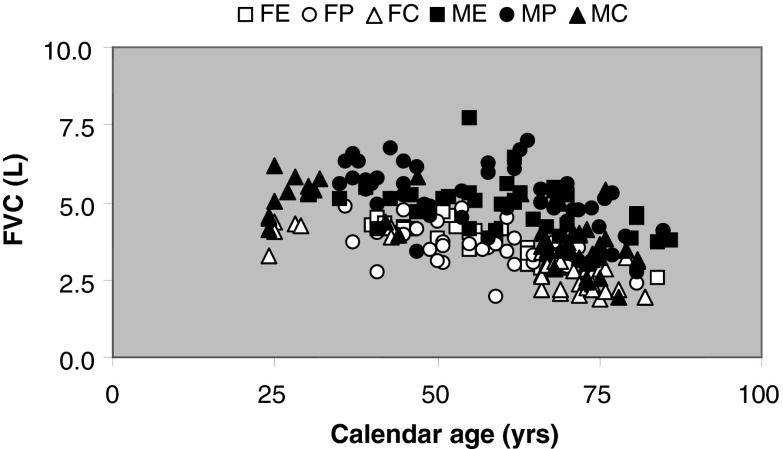
FVC was negatively associated with age in all groups (slope of linear regression, 36 mL·year−1; R2 = 0.24; P < 0.001; Fig. 3). Men had a larger FVC than women (P < 0.001) and the FVC of control participants was smaller than that of athletes (Fig. 3). The FEV1/FVC was approximately 5 % higher in control participants than athletes (P < 0.001; Table 2). PEF was higher in men than women and negatively associated with age (P < 0.001; men R2 = 0.23; women R2 = 0.38; Fig. 4). PEF and PEFpred were higher in the athletes than controls (P < 0.001; Fig. 4 and Table 2). Athletes had on average a 15 % lower PLA than age-matched controls (P < 0.05; Table 2), corresponding with the higher FEV1 in athletes. Excluding people with a PLA of 24 and 80 years gave essentially the same result, though the difference between power athletes and controls disappeared (P = 0.232).
Fig. 3.
Forced vital capacity (FVC) in FE female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control participants
Fig. 4.
Peak expiratory flow (PEF) in FE female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control participants. For description of statistics, see text
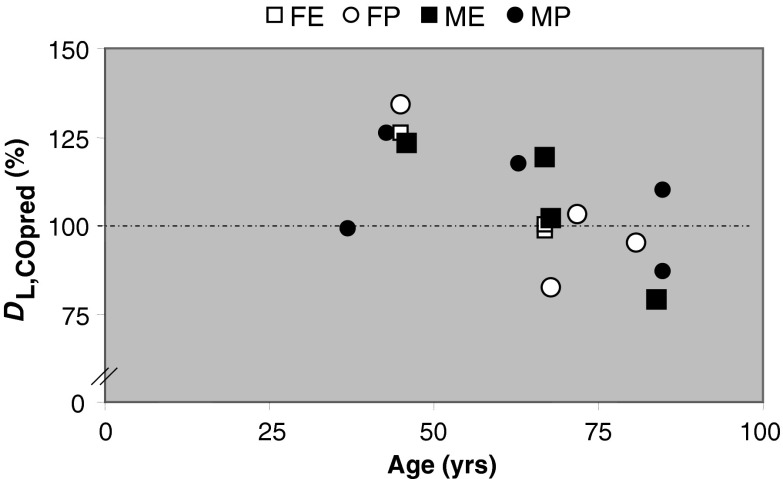
Figure 5 suggests that master athletes, independent of their specialty, might have a better diffusion capacity than expected. Although it may appear from the figure that this advantage could diminish after the age of 50, this remains speculative because of the small sample size.
Fig. 5.
Diffusion capacity as a percentage of the predicted capacity (DL,CO pred) in FE female endurance; FP female power; ME male endurance; MP male power athletes. Broken line 100 % line
Inspiratory and expiratory pressures
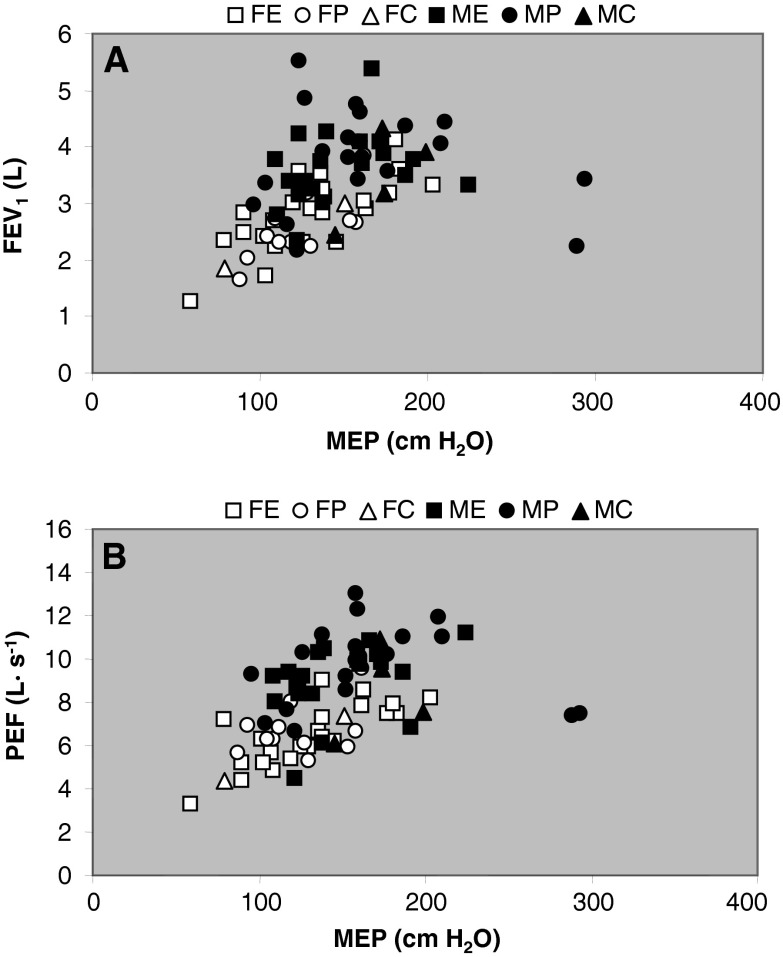
The MEP (Fig. 6a), MIP (Fig. 6b) and SNIP (Fig. 6c) were all higher in men than women and negatively related with age (0.048 < R2 < 0.17; P < 0.05). There was no significant difference in any of these parameters between endurance and power athletes or control participants. The FEV1 (Fig. 7a; R2 = 0.18; P < 0.001) and PEF (Fig. 7b; R2 = 0.22; P < 0.001) correlated with MEP.
Fig. 6.
a Maximal expiratory (MEP), b inspiratory (MIP) mouth pressures and c sniff nasal inspiratory (SNIP) pressure in FE female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control
Fig. 7.
Correlation between maximal inspiratory mouth pressure (MEP) with a forced expiratory volume in 1 s (FEV 1) and b peak expiratory flow (PEF) in FE: Female endurance; FP female power; FC female control; ME male endurance; MP male power; MC male control
Respiratory function and training status
FEV1pred (R2 = 0.025; P < 0.02) and PEFpred (R2 = 0.026; P < 0.02) were negatively associated with BMI. PLA did not depict any relationship with AGP (R2 = 0.032; P = 0.076) or training hours per week (R2 = 0.026; P = 0.176).
Discussion
This cross-sectional study has compared ventilatory function of Master athletes with a more sedentary reference population and with established cohort data from the literature (Hankinson et al. 1999). The main findings were that Master athletes have greater FVC, FEV1 and FEV1pred values, but lower FEV1/FVC and predicted lung age than the more sedentary population. No group differences were found in ventilatory pressures (MEP, MIP or SNIP). Overall, with the exception of FEV1/FVC, it is concluded that ventilation, i.e. the convective gas transport in the respiratory system, is better in Master athletes than non-athletes.
Most of the athletes adhered to a high volume of training, similar to those reported before (Ireland et al. 2011; Korhonen et al. 2006). This and the similar age-graded performance to that seen in previous work (Wilks et al. 2009) indicates that our sample of Master athletes were a ‘typical’ selection, even if no systematic study exists regarding exercise habits in these people. However, because of self-selection (after all it is the Master athletes themselves who decide to participate in their sports) it cannot be determined here (and in fact also not easily in other studies) whether the observed benefits are a cause or an effect of the athletic engagement and intense training. What will be discussed in the following, however, is whether long-term engagement in sports, such as master athletics, could mitigate the well-established decline in respiration, keeping in mind the limitations of a cross-sectional design.
Is the better ventilatory function in master athletes due to higher exercise levels?
Not only cross-sectional (Amara et al. 2001; Nystad et al. 2006) but also longitudinal (Pelkonen et al. 2003) studies have reported that high physical activity levels attenuate the age-related decline in respiratory function. Our data appear to support the benefit of regular exercise for ventilatory function as also the ventilatory function of master athletes was better than that of age-matched non-athletes, to such an extent that the lungs of both power and endurance master athletes were on average 15 % ‘younger’ than non-master athletes. Part of a potential beneficial effect of high physical activity levels on ventilatory function might be attributable to a higher respiratory muscle strength (Freitas et al. 2010; Hautmann et al. 2000), but our preliminary data showed no significant differences in respiratory muscle strength between athletes and controls. In fact, the main benefit of respiratory muscle training for improved endurance exercise performance is not so much derived from an increased strength as from an increased endurance of the respiratory muscles (Spengler and Boutellier 2000), which has not been tested here, but must be suspected to contribute to their running performance. In any case, the similar respiratory muscle strength across athletes and their sedentary counterparts suggests that factors independent of the respiratory muscles, such as lung recoil and chest compliance, contribute to the better ventilatory function in the athletes. These factors are essentially non-trainable, and if so the inherently better ventilatory function may induce people to engage in sports. In other words, the better ventilatory function of the master athletes might be a reflection of self-selection bias to participate in sports.
Respiratory function: a determinant of aerobic performance at old age?
Endurance athletes have a higher and prolonged demand for gas exchange, and thus ventilation during exercise than power athletes and non-athletes. This is reflected by the observation that endurance athletes might develop hypoxaemia (Dempsey et al. 2008; Prefaut et al. 1994). Therefore, we hypothesised that ventilatory function would be better maintained during ageing in endurance athletes. In line with our expectation athletes indeed had better respiratory function than age-matched controls, but in contrast to our expectation there was no difference between endurance and power athletes. As in the control population, also in the master endurance athletes the older individuals had a lower ventilatory function. This might explain why master endurance athletes might develop hypoxaemia even earlier and with larger magnitudes during exercise than young endurance athletes (McClaran et al. 1995; Prefaut et al. 1994). It has been suggested that hypoxaemia may be caused by initial hypoventilation followed by increased interstitial pulmonary oedema (Prefaut et al. 1994). Overall, the data presented here and the rapid decline in ventilatory function without a decline in maximal heart rate over a 6-year period (McClaran et al. 1995) suggest that the age-related deterioration of respiratory function outruns the function of other determinants of aerobic capacity. It thus might be that limits in ventilatory function become more important in determining endurance performance at old age.
Possible contributors to the age-related decline in ventilation
Although one might still argue that the better ventilatory function in master athletes than the control group is explicable by their higher levels of physical activity, we would like to draw the attention to the similar slopes of the regression lines of FEV1 vs. age in our control population and master athletes (−34 mL·year−1). It thus seems as though athletes may start ‘higher’ when they are young, but would then be ‘losing’ ventilatory function at the same rate as the average population. This suggests that the effects of age are inert to exercise, neither mitigating the age-related decline nor enhancing ventilatory function. In fact, a longitudinal study has even reported that FEV1 over 5 years increased in those with low, but decreased in those with high cardiorespiratory fitness at the first visit (Cheng et al. 2003). Moreover, a 12-week endurance training programme did not induce any changes in ventilatory function except for an increase in PEF in men but not women (Hulke and Phatak 2011). In another study former athletes had a higher FEV1 and FVC than currently active 60–67-year-old athletes (Saltin and Grimby 1968) and young endurance athletes had similar ventilatory function as young sedentary people (Hagberg et al. 1988). These observations and the absence of significant correlations between training hours per week or age-graded performance with ventilatory function in our master athletes further supports the idea that regular physical activity has limited, if any, impact on ventilatory function, e.g. due to structural limitations of the respiratory system (Dempsey et al. 1990; Harms 2006), or that it may even be detrimental.
If exercise is indeed detrimental to ventilatory function the downward slope of FEV1 over age should have been steeper in athletes, which was not observed. However, it is almost certain that our cross-sectional data are affected by attrition bias in the master athletes, as those athletes with particularly poor ventilatory function are likely to end their sporting career, thus generating a ‘fitter’ group average by their absence. This interpretation is indeed supported by a longitudinal study on endurance master athletes where it was found that the decline in respiratory function, such as PEF and FEV1, over a 6-year period was in fact larger than expected from cross-sectional studies (McClaran et al. 1995).
Study limitations
One limitation is that the master athletes came from all over the globe while control participants were primarily from Manchester, UK. Nevertheless, the age-related differences in ventilatory function followed similar patterns in each population and were similar to those observed in a 5-year follow-up study (Rossi et al. 2011). This suggests that the different origin of the study populations did not significantly interfere with our data. In our cross-sectional study, part of the age-related differences could in theory be due to changes in the environment in the last century, such as improvement in health care and nutrition, but this would also apply to the master athletes. The deterioration in ventilatory function in the control population is well established. Therefore, we contend that the age-related differences we observed in master athletes similarly reflect a pattern of intra-individual changes with age in ventilatory function in athletes. The few longitudinal data that are available appear to support our conclusions.
Conclusions
In conclusion, ventilatory function in master athletes was superior to that in age-matched controls in this study. The absence of any group difference in maximal ventilatory pressure generation suggests that non-muscular, and thus non-trainable factors are responsible for the better ventilatory function in the athletes. The better ventilatory function is most likely a reflection of self-selection and attrition bias in the master athletes. The results also support the notion in the literature that a decrease in ventilatory function may become a more important limiting factor of aerobic capacity with increasing age. However, longitudinal studies in master athletes and training studies are required to further elucidate this important aspect.
Acknowledgments
We appreciate the financial support from Stratec Company (Pforzheim, Germany) to perform oxygen diffusion measurements. We appreciate the support by Kurt Kaschke, Dieter Massin, Winston Thomas and Bridget Cushen as representatives from WMA, EVAA and BMAF. We are grateful to the participants—without their contribution this study would not have been possible.
References
- Amara CE, Koval JJ, Paterson DH, Cunningham DA. Lung function in older humans: the contribution of body composition, physical activity and smoking. Ann Human Biol. 2001;28(5):522–536. doi: 10.1080/03014460010029758. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Macera CA, Addy CL, Sy FS, Wieland D, Blair SN. Effects of physical activity on exercise tests and respiratory function. British J Sports Med. 2003;37(6):521–528. doi: 10.1136/bjsm.37.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey JA, Johnson BD, Saupe KW. Adaptations and limitations in the pulmonary system during exercise. Chest. 1990;97(3 Suppl):81S–87S. doi: 10.1378/chest.97.3_Supplement.81S-a. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, McKenzie DC, Haverkamp HC, Eldridge MW. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest. 2008;134(3):613–622. doi: 10.1378/chest.07-2730. [DOI] [PubMed] [Google Scholar]
- Freitas FS, Ibiapina CC, Alvim CG, Britto RR, Parreira VF. Relationship between cough strength and functional level in elderly. Rev Bras Fisiot (Sao Carlos (Sao Paulo, Brazil)) 2010;14(6):470–476. doi: 10.1590/S1413-35552010000600004. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Yerg JE, 2nd, Seals DR. Pulmonary function in young and older athletes and untrained men. J Appl Physiol. 1988;65(1):101–105. doi: 10.1152/jappl.1988.65.1.101. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol. 2006;151(2–3):124–131. doi: 10.1016/j.resp.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Hautmann H, Hefele S, Schotten K, Huber RM. Maximal inspiratory mouth pressures (PIMAX) in healthy subjects—what is the lower limit of normal? Respir Med. 2000;94(7):689–693. doi: 10.1053/rmed.2000.0802. [DOI] [PubMed] [Google Scholar]
- Hulke SM, Phatak MS. Effect of endurance training on lung function: a longitudinal study. Int J Biol Med Res. 2011;2(1):443–446. [Google Scholar]
- Ireland A, Korhonen M, Heinonen A, Suominen H, Baur C, Stevens S, Degens H, Rittweger J. Side-to-side differences in bone strength in master jumpers and sprinters. J Musculoskelet & Neuronal Interact. 2011;11(4):298–305. [PubMed] [Google Scholar]
- Klocke RA. Influence of aging on the lung. In: Finch CE, Hayflick L, editors. Handbook of the biology of aging. London: Van Nostrand Reinhold; 1977. pp. 432–444. [Google Scholar]
- Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101(3):906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol. 1995;78(5):1957–1968. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- Michaelis I, Kwiet A, Gast U, Boshof A, Antvorskov T, Jung T, Rittweger J, Felsenberg D. Decline of specific peak jumping power with age in master runners. J Musculoskelet Neuronal Interact. 2008;8(1):64–70. [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Morris JF, Temple W. Spirometric "lung age" estimation for motivating smoking cessation. Preventive Medicine. 1985;14(5):655–662. doi: 10.1016/0091-7435(85)90085-4. [DOI] [PubMed] [Google Scholar]
- Nystad W, Samuelsen SO, Nafstad P, Langhammer A. Association between level of physical activity and lung function among Norwegian men and women: the HUNT study. Int J Tuberc Lung Dis. 2006;10(12):1399–1405. [PubMed] [Google Scholar]
- Pelkonen M, Notkola IL, Lakka T, Tukiainen HO, Kivinen P, Nissinen A. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med. 2003;168(4):494–499. doi: 10.1164/rccm.200208-954OC. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Miller HS, Jr, Wilmore J. Physiological characteristics of champion American track athletes 40 to 75 years of age. J Geront. 1974;29(6):645–649. doi: 10.1093/geronj/29.6.645. [DOI] [PubMed] [Google Scholar]
- Prefaut C, Anselme F, Caillaud C, Masse-Biron J. Exercise-induced hypoxemia in older athletes. J Appl Physiol. 1994;76(1):120–126. doi: 10.1152/jappl.1994.76.1.120. [DOI] [PubMed] [Google Scholar]
- Reaburn P, Dascombe B. Endurance performance in masters athletes. Eur Rev Aging Phys Act. 2008;5(1):31–42. doi: 10.1007/s11556-008-0029-2. [DOI] [Google Scholar]
- Rittweger J, Kwiet A, Felsenberg D. Physical performance in aging elite athletes—challenging the limits of physiology. J Musculoskelet Neuronal Interact. 2004;4(2):159–160. [PubMed] [Google Scholar]
- Rittweger J, di Prampero PE, Maffulli N, Narici MV. Sprint and endurance power and ageing: an analysis of master athletic world records. Proc Biol Sci. 2009;276(1657):683–689. doi: 10.1098/rspb.2008.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AP, Watson NL, Newman AB, Harris TB, Kritchevsky SB, Bauer DC, Satterfield S, Goodpaster BH, Zamboni M (2011) Effects of body composition and adipose tissue distribution on respiratory function in elderly men and women: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed]
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38(6):1104–1115. doi: 10.1161/01.CIR.38.6.1104. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Boutellier U. Breathless legs? Consider training your respiration. News Physiol Sci. 2000;15:101–105. doi: 10.1152/physiologyonline.2000.15.2.101. [DOI] [PubMed] [Google Scholar]
- Wilks DC, Winwood K, Gilliver SF, Kwiet A, Chatfield M, Michaelis I, Sun LW, Ferretti JL, Sargeant AJ, Felsenberg D, Rittweger J. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone. 2009;45(1):91–97. doi: 10.1016/j.bone.2009.03.660. [DOI] [PMC free article] [PubMed] [Google Scholar]