Abstract
Oxidative stress and inflammation are increased with advancing age. Evidence suggests that oxidative stress and inflammation both lead to impaired vascular function. There is also evidence to suggest that inflammation may cause an increase in radical production leading to enhanced oxidative stress. In addition, oxidative stress may cause an increase in inflammation; however, the interactions between these factors are not fully understood. In this review, we propose the vascular health triad, which draws associations and interactions between oxidative stress and inflammation seen in ageing, and the consequences for vascular function. We review evidence suggesting that exercise may ameliorate the age-related decline in vascular function, through reductions in both oxidative stress and inflammation.
Keywords: Reactive oxygen and nitrogen species, Nitric oxide, Free radical, Inflammatory response, Older age, Endothelium
Introduction
Increased oxidative stress (Dhalla et al. 2000) and increased inflammation (Ross 1999) are risk factors for cardiovascular disease. In addition, vascular dysfunction is an early marker of poor cardiovascular health (Cohn 1999), which may occur as a result of a number of factors that alter as we age. Here, we propose a vascular health triad, which relates oxidative stress and inflammatory processes with vascular dysfunction. It is well characterised that oxidative stress (Harman 1956), inflammation (Singh and Newman 2011) and vascular function (Pierce et al. 2009) are all altered in ageing. The aim of this review was to describe the current understanding of the associations and interactions between oxidative stress, inflammation and vascular dysfunction, with a particular emphasis on ageing. Potential mechanisms of interaction will be discussed including nitric oxide (NO) bioavailability, low density lipoprotein oxidation, upregulation of pro-inflammatory proteins and nuclear factor-[kappa] B (NF-κB) as a potential integrative transcription factor. Finally, exercise is discussed as potential intervention to perturb the interactions between these processes.
Oxidative stress
Reactive oxygen and nitrogen species (RONS) are highly reactive free radical species, characterised by the presence of one or more unpaired electrons in their outer shells. RONS are natural derivatives of cellular oxidation processes, key in the regulation of normal biological processes such as cellular respiration and signalling. The reduction of molecular oxygen to form superoxide (O·−2) is central in directly or indirectly forming many other RONS such as peroxynitrite (ONOO·−), lipid peroxyl radicals and the highly toxic hydroxyl radicals. Antioxidants are a counterbalance to the reactivity of RONS, comprising enzymatic (superoxide dismutase (SOD), glutathione peroxidise, glutathione reductase and catalase) and non-enzymatic (glutathione (GSH) and vitamins C and E) sources. Oxidative stress is a state that may arise when a substantial pro-oxidant shift in the balance between RONS and antioxidants occurs. Subsequent radical-induced modification to cellular biomolecules such as proteins, lipid and DNA is characterised by adduct formation (Harman 1956). Some antioxidants, such as glutathione, function by directly interacting with RONS, mimicking their downstream targets and reducing oxidative stress. In situations where antioxidant defences sequester RONS, the net effect is the promotion of adaptive physiological responses, such as increased expression of protective enzymes and stress proteins (Ji 2001) that help to maintain redox balance and preserve free cellular thiols (Jacob and Ba 2011).
Due to the transient nature of RONS, it is common practice to measure the formation of adducts, which may act as “footprints” of radical-mediated reactions. Care must be taken in interpretation of results; employing this methodology as markers of oxidative stress are not necessarily measures of radical production. Common biomarkers of oxidative stress include protein carbonyls, malondialdehyde and isoprostanes, 3-nitrotyrosine (3NT) and 8-hydroxydeoxyguanosine that measure protein oxidation, lipid peroxidation, protein nitration and DNA oxidation, respectively. Similarly, antioxidant measures include SOD, catalase, glutathione (GSSG/GSH ratio) and total antioxidant capacity (Harman 1956).
Inflammation
Inflammation is a bodily defence mechanism that is initiated in response to tissue damage or infection, mediated by signalling cytokine molecules and characterised by the influx of migrating leukocytes (Medzhitov 2008). Acute inflammation is a transient, adaptive response leading to transcriptional activation of multiple anti-inflammatory cytokines, whereas chronic inflammation is characterised by an “overdrive” of inflammatory mediators, progressing to increased levels of low-grade systemic inflammation (Stevens et al. 2005). Increased levels of low-grade systemic inflammation are implicated in many disease states, notably dementia (Engelhart et al. 2004), rheumatoid arthritis (Lee and Weinblatt 2001) and cardiovascular disease (Tracy 1998). Indeed C-reactive protein (CRP), a marker of vascular inflammation, is present within most atherosclerotic plaques and is implicated in the pathogenesis, progression and complications of cardiovascular disease (Devaraj et al. 2009).
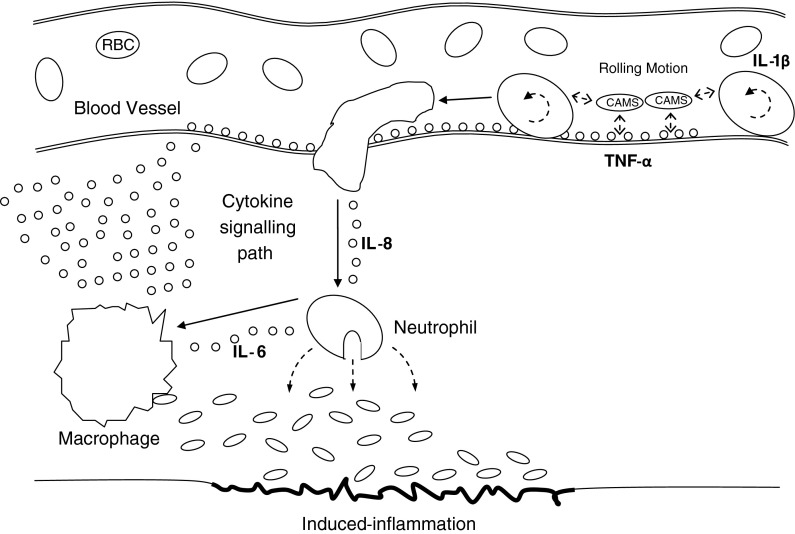
The inflammatory response (Fig. 1) is initiated by a rapid infiltration of leukocytes to the site of infection, which then engulf the invading pathogen. During this response, neutrophils weakly adhere to the blood vessel wall via cellular adhesion molecules (CAMS), initiating a rolling motion (Mayrovitz et al. 1977). The release of neutrophils to the site of infection is then mediated by a chemotactic gradient across the endothelial wall. Interleukin-1β (IL-1β) has been identified as the chemokine involved in this initial inflammatory process, alongside interleukin-8 (IL-8), which directly recruits neutrophils to the site of tissue damage (Utgaard et al. 1998). Another cytokine, interleukin-6 (IL-6), then recruits monocytes into blood vessels, instigating the differentiation of monocytes into pathogen-disposing macrophages (Kaplanski et al. 2003). Additionally, tumour necrosis factor-alpha (TNF-α) is involved in the recruitment of CAMS to the endothelial wall (Willerson and Ridker 2004; Bruunsgaard 2005). Inflammation can be quantified by monitoring total or subpopulations of white blood cells and/or measures of inflammatory signalling cytokines, such as IL-1β, IL-6, IL-8 and TNF-α.
Fig. 1.
Inflammatory response. Typical inflammatory response to a stimulus (infection, physical or mental stress). Blood leukocytes travel to the site of tissue damage through the actions of cytokine mediators (IL-1β, IL-8, IL-6 and TNF-α), disposing of foreign bodies
Vascular dysfunction
The healthy human vasculature displays multiple anti-inflammatory and anti-atherogenic processes, in order to protect it against disease. Vascular dysfunction is the impairment of blood vessel function, ensuing from numerous factors that disrupt the balance of vasoactive substances within the endothelium. The single cell endothelium lining blood vessels, once thought to be an inert barrier, are responsible for the release of multiple vasoactive substances, in particular NO (Rubanyi 1993). NO is a vasodilator that can cause direct relaxation of smooth muscle, augmenting blood flow and substrate delivery to tissues. NO also has multiple anti-atherogenic properties that prevent leukocyte and platelet adhesion to the vessel wall (Kubes et al. 1991). The balance of vasodilatatory factors, such as NO, with vasoconstrictive factors, such as endothelin-1 (ET-1), influences the health of the vasculature. Reduced bioavailability of NO and/or expression of endothelial nitric oxide synthase (eNOS) significantly reduces endothelium-dependent dilation (EDD), resulting in numerous processes that over time can progressively impair vascular function and initiate cardiovascular complications (Sandoo et al. 2010).
NO is a transient molecule, due to its rapid oxidation in vivo (Beckman et al. 1990), and is therefore very difficult to directly monitor in blood or urine. A number of different measures have been used to try to assess NO availability. Traditional methods involve functional assessments of NO-related vasomotion in response to physiological and/or pharmacological stimuli. Additionally, circulatory markers of NO in plasma and urine are commonly used as indicators of endothelial function and damage in vivo (Moshage et al. 1995). The metabolites of NO, nitrite (NO−2) and nitrate (NO−3) have recently been assessed to monitor NO bioavailability in exercising humans (Lauer et al. 2008). Quantification of these metabolites (NOx) is achieved using a luminescent assay-based technique (Moshage et al. 1995).
The aged human vasculature
The ageing human vasculature is associated with a gradual decline in EDD (Seals et al. 2011) which may manifest over time as increased large artery stiffness (reduced compliance) and increased intima-media wall thickness (Tanaka et al. 2000). For an overview of the assessments of endothelial function and lifestyle and biological factors that affect the vasculature with ageing, the reader is referred to a recent review by Seals et al. 2011. As the current review is related to oxidative stress and inflammation, the following sections will focus on their interactions as well as their associations with vascular dysfunction. Many studies attempting to elucidate the mechanisms behind this decline have employed animal models. However, recent evidence has demonstrated for the first time in humans that an age-related reduction in endothelial function was a result of decreased NO (Rodriguez-Manas et al. 2009). Rodriguez-Manas et al. (2009) showed enhanced levels of O·−2 and ONOO·− alongside a reduction in endothelial dilations to NO-dependent bradykinin. Increases in oxidative stress and vascular wall inflammation are thought to be pivotal in the mechanisms that reduce availability of NO, indeed, theories have suggested for some time that low-grade systemic inflammation (Chung et al. 2001) and enhanced oxidative stress (Harman 2006) could be the root of many age-related vascular pathologies. Enhanced inflammation and oxidative stress can cause migration of lymphocytes, lipids and fibrous elements into the vessel wall, resulting in a disrupted homeostatic equilibrium within the vascular tree. These physiological alterations to blood vessels, alongside reduced availability of NO can decrease substrate delivery to tissues, promoting further pro-inflammatory and oxidative transcription.
Oxidative stress and vascular dysfunction with age
The deleterious effects of oxidative stress have been implicated in ageing for more than 50 years. Since Harman proposed the “free radical theory of ageing” in 1956, free radicals have been thought to progressively disrupt cellular processes as we age (Harman 1956). Despite lifestyle modifications (i.e. diet, physical activity and medical care), Harman suggested that an inborn ageing process was attributable to the increased accumulation of radical-induced adducts on cellular constituents, resulting in progressive structural and functional changes. Ageing is also associated with a decrease in circulating antioxidants (Pinzani et al. 1997). Higher levels of oxidative stress in ageing have been associated with reduced vascular function (Donato et al. 2007), and of particular interest is the impact of oxidative stress on plasma low-density lipoproteins (LDL). Brinkley et al. (2009b) showed that enhanced modification of the protein and lipid components of LDL is paralleled with an impairment of vascular function and a stiffening of the arteries.
A key mechanism implicated in the altered oxidation status of LDL, and thus vascular dysfunction, is the interaction between NO and O·−2. Endothelium-derived NO has a high tendency to react with oxidants, in particularly O·−2. During cellular stresses, this can lead to the formation of the potent-free radical ONOO·− (Pryor and Squadrito 1995). O·−2 and ONOO·− production have both been shown to increase with age and elevated levels of these radicals have been proposed to enhance adduct formation and thus contribute to the age-related decline in vascular function (Rodriguez-Manas et al. 2009). ONOO·− formation directly removes NO from the vascular endothelium and can therefore reduce the vasodilatory capacity of the vessel. ONOO·− can also interact with the tyrosine residues of proteins (e.g. protein component of LDL), which results in the formation of 3NT, a compound recognised as a stable marker of protein nitration (Hensley et al. 1997). ONOO·−-modified LDL interaction with invading macrophages is directly linked with the progression of atherosclerosis (Leeuwenburgh et al. 1997). Large quantities of modified LDL can be loaded into macrophages via enhanced uptake by “scavenger receptors” (Goldstein et al. 1979). This unregulated accumulation of modified LDL can lead to the formation of atherosclerotic lesions within the vasculature, directly narrowing blood vessels and reducing blood flow capacity. Enhanced O·−2 and ONOO·− formation within aged vasculature can therefore functionally and morphologically impair vascular function, by reducing NO bioavailability to the endothelium and hence reducing the ability to dilate and by physically narrowing blood vessels due to plaque formation.
Inflammation and vascular dysfunction with age
Inflammatory proteins (Il-1β, TNF-α, IL-6 and CRP) are continuously upregulated during the ageing process, inducing higher systemic concentrations of inflammatory mediators (Roubenoff et al. 1998). Numerous factors are associated with increased inflammation as we age, notably enhanced sarcopenia and decreased sex hormones (Chung et al. 2009). Increased visceral adiposity is associated with enhanced release of IL-6 and TNF-α (Trayhurn and Wood 2005), via the redistribution of macrophages to the excess adipose tissue (Singh and Newman 2011). Correlations between IL-6 and TNF-α with sarcopenia in elderly subjects also link inflammation with reduced muscle mass in ageing (Visser et al. 2002). Additionally, elevated concentrations of IL-6 seen with age have been correlated with reduced sex hormones oestrogen and androgen (Ray et al. 1997). It is worth noting that dehydroepiandrosterone, an abundant precursor for testosterone and known to have antioxidant properties (Aldred and Griffiths 2004), is also decreased with age (Labrie et al. 1997). This is an example of a number of indirect associations between oxidative stress and the inflammatory process in ageing.
The observed increase in inflammatory mediators seen with age can initiate numerous processes that lead to impaired vascular function and promotion of cardiovascular and metabolic complications (Kim et al. 2006). CRP and TNF-α can directly disrupt the balance of vasoactive factors within the vasculature by downregulating the expression of eNOS (Singh et al. 2007; Zhang et al. 2009) and by activating ET-1 (Verma et al. 2002). This shift has also been linked to an enhanced expression of CAMS within the vasculature (Pasceri et al. 2000). Overexpression of CAMS during low-grade inflammation can result in invading leukocytes, present during inflammation, adhering to the endothelial wall (Willerson and Ridker 2004). Over time, inflammation-induced leukocyte adhesion can progressively alter the structure of the vessel wall (Sluiter et al. 1993), reducing blood flow capacity as described earlier. Similar to the oxidative stress/vascular function interaction, these effects are both direct and indirect mechanisms of reducing NO bioavailability to the endothelium and thus impairing vascular function. Hence, it appears that both oxidative stress and inflammatory processes can induce a marked reduction of endothelium-derived NO.
NO bioavailability with age
In addition to reduced NO availability as described above, previous research has also suggested that oxidative stress and inflammatory processes seen in ageing may disrupt the chain of NO synthesis and function. Nitric oxide synthase (NOS) is the key regulatory enzyme in the synthesis of NO, comprising endothelial (eNOS), inducible (iNOS) and neuronal (nNOS) isoforms, regulated under specific conditions. Notably, eNOS is responsible for producing relatively small amounts of transient NO, involved in dilating the endothelial layer (Moncada and Higgs 1993), whereas during periods of elevated inflammation, iNOS produces much larger amounts of NO in a sustained response to kill invading microorganisms (Vane et al. 1994). NOS utilises the substrate l-arginine and cofactor tetrahydrobiopterin (BH4) to form NO with molecular oxygen (Lundberg et al. 2008). Recent evidence has demonstrated decreased availability of l-arginine (Bode-Böger et al. 2003) and BH4 (Eskurza et al. 2005) in sedentary older adults. Additionally, evidence has directly linked these reductions in l-arginine availability to eNOS, with elevated levels of oxidised LDL (Wang et al. 2011).
Despite conflicting evidence from animal-based literature showing increased (van der Loo et al. 2000), decreased (Rippe et al. 2010) and unchanged (Yang et al. 2009) eNOS expression in ageing animals, recent evidence in humans has indicated that NO production is well preserved in older adults (Rodriguez-Manas et al. 2009). Rodriguez-Manas et al. (2009) showed similar levels of eNOS in all subjects across a wide age range (18–91 years); however, mRNA levels of iNOS were markedly increased in the microvascular tissue of older subjects, alongside an age-related decline in NO-mediated vasodilatation. Additionally, subjects showed increased production of O·−2, and also ONOO·− with age, indicating enhanced O·−2 scavenging of NO. Hence, a paradox appears whereby in ageing humans there is elevated NO production within the vasculature, associated with reduced NO bioavailability, due to free radical scavenging. Firstly, the production of NO via iNOS is a markedly different response to that of NO via eNOS. Beyond the low levels of NO needed to transiently dilate smooth muscle cells, the large amounts of NO produced via iNOS surpass the physiological amounts required to dilate the vessel in a long and sustained manner, acting toxic, as well as also being susceptible to oxidative reactions (Guzik et al. 2003). As shown by Rodriguez-Manas et al. 2009 and previously (van der Loo et al. 2000), there are higher levels of ONOO·− formation in older adults when higher levels of NO and O·−2 are coexistent. In addition to this, substrate and cofactor availability can indirectly exacerbate levels of oxidative stress further in ageing. In vitro evidence has demonstrated that under conditions of low substrate and/or cofactor availability that NOS may directly produce O·−2 (Xia 2007). Seeing as iNOS, not eNOS, is chronically upregulated in ageing (Rodriguez-Manas et al. 2009) it seems likely that iNOS is the NOS isoform depleting substrate and cofactor availability in ageing. However, with no direct evidence of this in older humans, it is possible that both isoforms present within vasculature may directly form O·−2. Indeed, in older rats, reduced BH4 availability has been associated with increased O−2· production by eNOS (Jacobson et al. 2007), an increased presence of nitrolysated proteins on eNOS and reduced endothelial-dependent dilation (Yang et al. 2009).
NO production by eNOS appears to be preserved with age, and the age-associated upregulation of iNOS may initially act to counteract conditions of high oxidative stress and inflammation, to aid NO-mediated dilation. However, a vicious cycle may then develop whereby production of NO from iNOS is scavenged by O·−2, forming ONOO·−, in the process abolishing negative feedback to iNOS to reduce the production of further NO. The resultant stimuli (i.e. oxidative stress and/or inflammation) to produce excessive amounts of NO may then: (a) reduce cofactor and substrate availability, thus increasing O·−2 formation by NOS (eNOS or iNOS), thus enhancing further radical scavenging or (b) produce excessive quantities of NO in a sustained manner that may act toxic to cells. All these factors, coupled alongside enhanced production of vasoconstrictive factors, such as ET-1 with age (Donato et al. 2009a), can act to disrupt endothelial homeostasis and impair vascular function. Further research is required to elucidate the precise mechanisms involved in the known age-dependent decline in NO availability that link impaired vascular function with increased oxidative stress and inflammation.
Oxidative stress and inflammation—interactions/similarities
The separate interactions between oxidative stress and inflammation with vascular dysfunction are well characterised and some of the key aspects are described within this review; however, the specific interaction between oxidative stress and inflammation has never been fully elucidated. Oxidative stress and pro-inflammatory processes are often deemed to be mutually dependent, with many studies suggesting that oxidative stress is a direct stimulus for inflammation and vice versa (Kim et al. 2006). Both are implicated in a number of diseased states, most notably rheumatoid arthritis (RA) (Seven et al. 2008), a chronic systemic disease characterised by persistent high-grade systemic inflammation. Seven et al. (2008) noted that the higher levels of systemic inflammation found in RA patients correlated with enhanced protein carbonyl and lipid hydroperoxide formation, as well as reduced GSH levels. Similarly, RONS (Donato et al. 2007) and inflammatory cytokines (Pierce et al. 2009) have both been shown to stimulate the redox-sensitive transcription factor NF-κB, and although NF-κB is a relatively non-specific transcription factor, this does link oxidative stress and inflammation with a common integrative source. NF-κB is a transcription factor expressed in mammalian cells that mediates the expression of genes controlling cellular inflammation, redox status and tissue specific enzymes (Ungvari et al. 2007). Different oxidative and inflammatory signals can have markedly different effects on NF-κB transcription in different cell and tissue types. The propagation of signals from upstream kinases such as the mitogen-activated protein kinase (MAPK) can transmit extracellular to intracellular signals with a great degree of variety or specificity (Seger and Krebs 1995), differentially activating NF-κB.
Evidence in support of the mechanistic pathways between oxidative stress and inflammation are lacking. It has been established in vitro that neutrophils present during an inflammatory response may directly produce ROS, such as O·−2 (Babior et al. 1973). Of the limited studies that assess oxidative stress-induced inflammation, the majority have done so in vitro, linking enzymes and intermediate signalling molecules with an enhanced inflammatory response, during periods of heightened oxidative stress. However, the role of prostaglandins and thioredoxin (TRX) in the interactions between oxidative stress and inflammation are worth noting. Prostaglandins are compounds derived from fatty acids that regulate cellular growth and differentiation near the site of their release. It has been proposed that prostaglandins may directly modify components of the electron transport chain, thus disrupting ATP generation and elevating radical leakage (Kondo et al. 2001). Equally, prostaglandins have been shown to be elevated 1,000 times above their baseline concentrations at the site of both acute (Offenbacher et al. 1986) and chronic inflammation (Gilroy et al. 1999). Of interest are the interactions of 15-deoxy-delta-12,14-prostaglandin J2 (15 d-PGJ2) with NF-κB. 15 d-PGJ2 is a dehydrated product of prostaglandin D2 and a ligand for the transcription factor PPARγ. It appears that radicals produced from 15 d-PGJ2-induced disruption of the electron transport chain can signal via MAPK to directly upregulate expression of IL-8 cytokines and initiate inflammation (Fu et al. 2002). It is well documented that RONS can signal through the MAPK family (Cuadrado and Nebreda 2010), and IL-8 is a cytokine known to recruit neutrophils at the beginning of the inflammatory response (Utgaard et al. 1998).
TRX is a ubiquitous oxidoreductase protein present within the cytoplasm, nuclei and mitochondria of cells (Go et al. 2007). TRX can exist in a reduced or oxidised state, dependent on the level of systemic oxidative stress and activity of TRX reductase and peroxidase. When in a reduced state, TRX can act as an antioxidant, utilising its thiol group to directly scavenge ROS, as well as activating the expression of various antioxidant enzymes (Burke-Gaffney et al. 2005) and regenerating GSH (Tan et al. 2010) and vitamin C (May et al. 1997). However, recent evidence has implicated a TRX binding protein, thioredoxin-interacting protein (TXNIP), as a link between elevated oxidative stress and inflammation (Zhou et al. 2010; World et al. 2011). In resting cells, TXNIP is bound to TRX via a disulphide bound, promoting TRX in its oxidised form (Patwari et al. 2006). An increase in ROS has been shown to promote dissociation of TXNIP from TRX, enabling TRX to scavenge ROS, whilst TXNIP activates the inflammatory cytokine, IL-1β via the NLP3 inflammasome (Zhou et al. 2010). Previous evidence supports TRX involvement with inflammatory cytokine production (IL-1α, IL-6, IL-8, TNF-α and IL-2) (Yamada et al. 2003; Schenk et al. 1996). It therefore appears that despite the antioxidant properties of TRX, it may also act indirectly via TXNIP to enhance the inflammatory signal during periods of heightened oxidative stress. Additionally, overexpression of TXNIP has been associated with impaired NO-dependent endothelial cell function (Schulze et al. 2006) and is thus of interest in the progression of cardiovascular pathologies (Spindel et al. 2012).
It may be possible that TRX, prostaglandins and other unidentified mediators are responsible for amplifying the inflammatory signal under conditions where oxidative stress is enhanced, rather than RONS inducing inflammation directly. However due to a lack of studies in this area, this is mainly a speculation. Additionally, oxidative stress-induced damage to cellular biomolecules such as proteins, lipids and DNA may well initiate inflammation in response to damage (Ungvari et al. 2010).
NF-κB and vascular dysfunction
In addition to NF-κB being a transcription factor common to both inflammation and oxidative stress, it has been established that NF-κB activity correlates with the reduced EDD seen in ageing (Pierce et al. 2009), thus making NF-κB an integral factor in the proposed vascular health triad (Donato et al. 2009b). The role of NF-κB in age-related vascular dysfunction is reviewed in greater detail by Donato et al. (2009b). Donato et al. (2007) showed that older men had an increased activity of NF-κB, higher CRP and 3-NT levels and reduced endothelial function than younger men. A subsequent study by Pierce et al. directly underlined the role of NF-κB in vascular dysfunction by inactivating NF-κB with salsalate in overweight adults (52–68 years) (Pierce et al. 2009). Endothelial function was markedly increased (74 %) following salsalate administration, concurrent with reduced 3-NT and NADPH oxidase activity. Taken together, there is overwhelming evidence to suggest that redox-sensitive NF-κB is poignant in the transcriptional activation of further pro-inflammatory and pro-oxidative genes, which culminates in reduced vascular function.
The vascular health triad
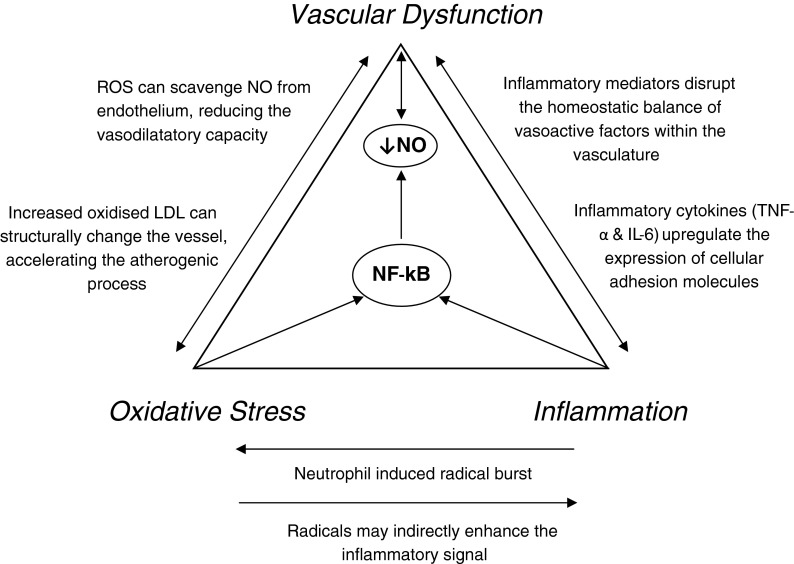
Evidence strongly suggests that a pro-inflammatory phenotype throughout the vascular tree is paralleled by higher levels of cellular and tissue oxidative stress. From the evidence discussed in this review, it is clear that this predisposes the vasculature to impaired vascular function through multiple mechanisms, notably reduced NO, damaged LDL and enhanced adhesiveness for circulating leukocytes. We therefore propose a tightly regulated model (Fig. 2), integrating inflammatory and oxidative processes with vascular dysfunction, primarily mediated by the transcriptional factor NF-κB and targeting downstream NO bioavailability. This model is cyclic in nature, with vascular dysfunction creating an environment that favours further inflammation and oxidative stress.
Fig. 2.
The Vascular Health Triad. The relationships between oxidative stress, inflammation and vascular health are outlined briefly in this newly proposed model of vascular health
Evidence in support of this model has been demonstrated previously (Clapp et al. 2004). Clapp et al. (2004) induced an inflammatory response in healthy subjects and observed a decrease in total antioxidant status as well as reduced endothelial function. Despite no direct measure of radical production, this study lends support to our model (Fig. 2), indicating that inflammation-induced oxidative stress may impair endothelial function, through reductions in NO availability.
The effect of exercise on oxidative stress and inflammation in ageing
Exercise is beneficial for health and is known to improve cardiovascular function, physical fitness and psychosocial health (Metsios et al. 2008). Regular exercise is associated with a reduction in multiple cardiovascular risk factors such as correction of lipoprotein profiles, lowered fat mass and blood pressure (Shephard and Balady 1999) as well as improved vascular function (Clarkson et al. 1999) and enhanced NO release (Lewis et al. 1999). Implicated within the Vascular Health Triad, low-grade systemic inflammation (Colbert et al. 2004) and oxidative stress (Donato et al. 2010) are two processes also seen to independently decline as a result of regular exercise.
The interactions between oxidative stress and inflammation during and after exercise are frequently researched, primarily concerning the links between muscle metabolism and muscle damage (Peake et al. 2007). At a cellular level, exercise in humans is known to stimulate an outflow of inflammatory mediators and free radicals across the active muscle bed (Bailey et al. 2003), with markers of both oxidative stress and inflammation seen for hours, even days following cessation of exercise (Michailidis et al. 2007; Petersen and Pedersen 2005). From a number of recent studies using antioxidant supplementation, the essential role of free radicals and inflammatory cytokines has been outlined in the body’s adaptations to exercise. These studies aimed to attenuate exercise-induced muscle damage via decreases in both oxidative stress and inflammation. However results showed that antioxidant supplementation blocked essential adaptations, such as mitochondrial biogenesis and endurance capacity (Gomez-Cabrera et al. 2008). Interestingly also, antioxidant supplementation has been shown to block the anti-inflammatory response to exercise, by reducing muscle-derived IL-6 production (Fischer et al. 2004; Vassilakopoulos et al. 2003). This underpins further the links between oxidative stress and inflammation. Evidence suggests that it is only when the exercise intensity is high or exhaustive, then it may be associated with an overwhelming burst of oxidants and inflammatory mediators, that may subsequently damage cellular biomolecules such as proteins and lipids (Packer 1997; Radak et al. 1999).
Age-related changes in cellular structure and function, combined with the enhanced exercise-induced muscle damage and reduced muscle repair and regeneration seen with age (McArdle et al. 2002), may well exaggerate cellular and vascular oxidative damage and inflammation in response to exercise in older age (Ji 2001). Indeed, there is evidence to suggest that the age-related decline in vascular function is present not only under resting conditions but also in response to acute exercise (Donato et al. 2010). However, an adaptive vascular response to exercise still exists in older age (DeSouza et al. 2000; Donato et al. 2010; Wray et al. 2009). Indeed, regular exercise has been shown to attenuate multiple factors associated with age-related vascular dysfunction, independent of traditional cardiovascular profile changes (Green et al. 2003). Colbert et al. (2004) determined lower levels of TNF-α, IL-6 and CRP in physically active older subjects than sedentary counterparts (70–79 years). Similarly, Donato et al. (2010) assessed brachial artery vasodilation in young and old subjects before and after 6 weeks of training, concurrent with either pre-exercise placebo or antioxidant supplementation. Prior to training, brachial artery vasodilation was impaired in older subjects; however, antioxidant supplementation restored normal vascular function comparable to that in younger subjects. Training independently improved brachial artery vasodilation, but repeated antioxidant supplementation had no effect on vascular responsiveness. Training in older subjects appears to have induced an increase in antioxidant capacity, therefore diminishing the effect of exogenous antioxidants. Elderly muscle therefore appears to have a hemodynamic reserve (Wray et al. 2009) that is masked by a high background of oxidative stress but reversed with regular exercise.
Exercise in the ageing population would appear to be a beneficial behavioural intervention to improve cardiovascular health. However, future studies are still required to ascertain the optimal exercise dose (i.e. intensity, frequency, duration and modality) to achieve optimal vascular adaptations in individuals with higher baseline levels of oxidative stress and inflammation. The relationship between the type of exercise performed and the magnitude of free radical production has yet to be established. In ageing in particular, a fine line may well exist between the production of free radicals that can achieve optimal adaptations within skeletal muscle and an amount that may cause damage.
NO availability and exercise in ageing
Despite the outlined reduction in NO bioactivity with age, improvements in vascular function have been seen in exercise studies in older age (DeSouza et al. 2000). This suggests that improvements in NO release (i.e. increased substrate and cofactor) and reduced scavenging of NO may be achieved using exercise interventions. Recent evidence from studies investigating NOx has shown an impaired ability of aged vasculature to produce NO (Lauer et al. 2008; Brinkley et al. 2009a). Lauer et al. (2008) showed that exercise stress to exhaustion increased plasma levels of NO·−2in younger subjects (+38 %), but there was a reduced capacity in older subjects (+13 %), concurrent with reduced endothelial function. Extending these findings to ascertain whether training-induced improvements in NO bioactivity could occur in older subjects, Brinkley et al. (2009a) assessed NOx before and after a 24-week exercise intervention (75 % VO2MAX) in older subjects (50–75 years), with simultaneous measures of forearm blood flow (FBF). Surprisingly, regular exercise was associated with no changes in plasma NOx or FBF, despite associated improvements in maximum oxygen consumption, HDL cholesterol, triglycerides and body fat. In contrast to these observations, training-induced improvements in vascular function have been recently demonstrated in older subjects (Donato et al. 2010).
Future studies are required to clarify how levels of NOx are perturbed in response to exercise, both acutely and chronically, and in relation to the vascular health triad model. Determining markers of oxidative stress and inflammation in response to exercise, alongside markers of vascular function will help ascertain whether: (1) the exercise intensity utilised was too high, thus inducing higher levels of oxidative stress that may mask the increases in NO production in older adults or (2) that the exercise stimulus utilised was insufficient to stimulate enhanced NO production. Our proposed model (Fig. 2) suggests that all factors regarding vascular health are integrative, and hence it would be advantageous to measure oxidative stress (adduct formation and antioxidant capacity), inflammation and vascular function (both functionally and metabolically) in the same study.
Summary and future perspectives
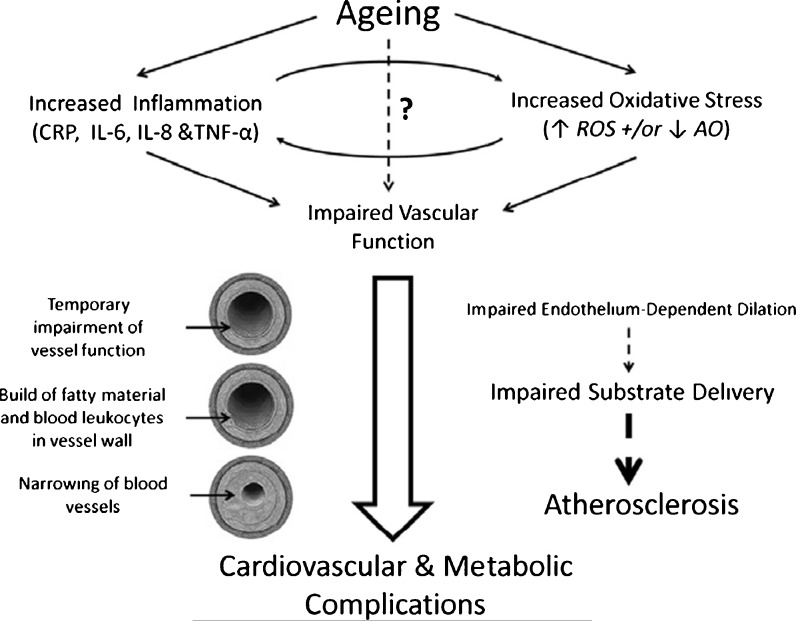
The vascular health triad implicates high levels of inflammation and oxidative stress with impaired vascular function. This model is intrinsically accelerated by the ageing process, promoting multiple cardiovascular and metabolic complications (Fig. 3). It seems clear that insufficient generation and availability of NO is the primary factor linking these three processes. Despite this, there is ample evidence to suggest that exercise could act to restore the age-related decline in vascular function, through reductions in both oxidative stress and inflammation. Future studies need to examine the associations between all these factors in ageing and ascertain the optimal intensity and duration of exercise in the ageing population, with the aim to improve vascular function and reduce the risk of age-related vascular pathologies.
Fig. 3.
The Progression of Ageing. Overview of the mechanisms that contribute to cardiovascular pathologies with age. Question mark represents potential additional mechanisms not discussed in current review
Contributor Information
Alex J. Wadley, Email: ajw614@bham.ac.uk
Jet J. C. S. Veldhuijzen van Zanten, Email: VeldhuJJ@bham.ac.uk
Sarah Aldred, Phone: +44-121-4147284, FAX: +44-121-4144121, Email: s.aldred.1@bham.ac.uk.
References
- Aldred S, Griffiths HR. Oxidation of protein in human low-density lipoprotein exposed to peroxyl radicals facilitates uptake by monocytes; protection by antioxidants in vitro. Environ Toxicol Pharmacol. 2004;15(2–3):111–117. doi: 10.1016/j.etap.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol. 2003;94(5):1714–1718. doi: 10.1152/japplphysiol.01024.2002. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich FC. Oral l-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Fenty-Stewart NM, Park JY, Brown MD, Hagberg JM. Plasma nitrate/nitrite levels are unchanged after long-term aerobic exercise training in older adults. Nitric Oxide. 2009;21(3–4):234–238. doi: 10.1016/j.niox.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley TE, Nicklas BJ, Kanaya AM, Satterfield S, Lakatta EG, Simonsick EM, Sutton-Tyrrell K, Kritchevsky SB. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension. 2009;53(5):846–852. doi: 10.1161/HYPERTENSIONAHA.108.127043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78(4):819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26(8):398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction. Ann N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33(5):1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens Suppl. 1999;17(5):S41–44. [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition study. J Am Geriatr Soc. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Singh U, Rao LV, Jialal I. C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo. Atherosclerosis. 2009;203(1):67–74. doi: 10.1016/j.atherosclerosis.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Pierce GL, Lesniewski LA, Seals DR. Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging (Albany NY) 2009;1(8):678–680. doi: 10.18632/aging.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. 2010;298(2):H671–678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568(Pt 3):1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, Sjoberg LB, Pedersen BK. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558(Pt 2):633–645. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-delta12, 14 prostaglandin J2 (15 d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002;160(1):11–20. doi: 10.1016/s0021-9150(01)00541-x. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Go YM, Ziegler TR, Johnson JM, Gu L, Hansen JM, Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Radic Biol Med. 2007;42(3):363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR, O'Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285(6):H2679–2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–487. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- Hensley K, Maidt ML, Pye QN, Stewart CA, Wack M, Tabatabaie T, Floyd RA. Quantitation of protein-bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. Anal Biochem. 1997;251(2):187–195. doi: 10.1006/abio.1997.2281. [DOI] [PubMed] [Google Scholar]
- Jacob C, Ba LA. Open season for hunting and trapping post-translational cysteine modifications in proteins and enzymes. ChemBioChem. 2011;12(6):841–844. doi: 10.1002/cbic.201100068. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293(3):H1344–1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J Biol Chem. 2001;276(15):12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82(8):2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lauer T, Heiss C, Balzer J, Kehmeier E, Mangold S, Leyendecker T, Rottler J, Meyer C, Merx MW, Kelm M, Rassaf T. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Res Cardiol. 2008;103(3):291–297. doi: 10.1007/s00395-008-0714-3. [DOI] [PubMed] [Google Scholar]
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hardy MM, Hazen SL, Wagner P, Oh-ishi S, Steinbrecher UP, Heinecke JW. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272(3):1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JP, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19(11):2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- May JM, Mendiratta S, Hill KE, Burk RF. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J Biol Chem. 1997;272(36):22607–22610. doi: 10.1074/jbc.272.36.22607. [DOI] [PubMed] [Google Scholar]
- Mayrovitz HN, Wiedman MP, Tuma RF. Factors influencing leukocyte adherence in microvessels. Thromb Haemost. 1977;38(4):823–830. [PubMed] [Google Scholar]
- McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1(1):79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, Treharne GJ, Panoulas VF, Douglas KM, Koutedakis Y, Kitas GD. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology (Oxford) 2008;47(3):239–248. doi: 10.1093/rheumatology/kem260. [DOI] [PubMed] [Google Scholar]
- Michailidis Y, Jamurtas AZ, Nikolaidis MG, Fatouros IG, Koutedakis Y, Papassotiriou I, Kouretas D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med Sci Sports Exerc. 2007;39(7):1107–1113. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41(6 Pt 1):892–896. [PubMed] [Google Scholar]
- Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21(2):101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Packer L. Oxidants, antioxidant nutrients and the athlete. J Sports Sci. 1997;15(3):353–363. doi: 10.1080/026404197367362. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281(31):21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake JM, Suzuki K, Coombes JS. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J Nutr Biochem. 2007;18(6):357–371. doi: 10.1016/j.jnutbio.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119(9):1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani P, Petruzzi E, Orlando C, Stefanescu A, Antonini MF, Serio M, Pazzagli M. Reduced serum antioxidant capacity in healthy centenarians. Clin Chem. 1997;43(5):855–856. [PubMed] [Google Scholar]
- Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268(5 Pt 1):L699–722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvari M, Nyakas C, Goto S. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27(1–2):69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409(1):79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski LA, Connell M, LaRocca T, Donato A, Seals DR. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9(3):304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22(Suppl 4):S1–14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H, Vogt M, Droge W, Schulze-Osthoff K. Thioredoxin as a potent costimulus of cytokine expression. J Immunol. 1996;156(2):765–771. [PubMed] [Google Scholar]
- Schulze PC, Liu H, Choe E, Yoshioka J, Shalev A, Bloch KD, Lee RT. Nitric oxide-dependent suppression of thioredoxin-interacting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol. 2006;26(12):2666–2672. doi: 10.1161/01.ATV.0000248914.21018.f1. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- Seven A, Guzel S, Aslan M, Hamuryudan V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin Biochem. 2008;41(7–8):538–543. doi: 10.1016/j.clinbiochem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99(7):963–972. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB (2011) Inflammatory markers in population studies of aging. Ageing Res Rev 10(3):319–329. doi:10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed]
- Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43(6):780–791. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluiter W, Pietersma A, Lamers JM, Koster JF. Leukocyte adhesion molecules on the vascular endothelium: their role in the pathogenesis of cardiovascular disease and the mechanisms underlying their expression. J Cardiovasc Pharmacol. 1993;22(Suppl 4):S37–44. [PubMed] [Google Scholar]
- Spindel ON, World C, Berk B (2012) Thioredoxin interacting protein (TXNIP): redox dependent and independent regulatory mechanisms. Antioxid Redox Signal 16(6):587–596. doi:10.1089/ars.2011.4137 [DOI] [PMC free article] [PubMed]
- Stevens RJ, Douglas KM, Saratzis AN, Kitas GD. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med. 2005;7(7):1–24. doi: 10.1017/S1462399405009154. [DOI] [PubMed] [Google Scholar]
- Tan SX, Greetham D, Raeth S, Grant CM, Dawes IW, Perrone GG. The thioredoxin–thioredoxin reductase system can function in vivo as an alternative system to reduce oxidized glutathione in Saccharomyces cerevisiae. J Biol Chem. 2010;285(9):6118–6126. doi: 10.1074/jbc.M109.062844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tracy RP. Inflammation in cardiovascular disease: cart, horse, or both? Circulation. 1998;97(20):2000–2002. doi: 10.1161/01.cir.97.20.2000. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293(1):H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel–Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188(9):1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoughby DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91(6):2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Karatza MH, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C. Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol. 2003;94(3):1025–1032. doi: 10.1152/japplphysiol.00735.2002. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, Dhillon B, Mickle DA. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105(16):1890–1896. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Wang W, Hein TW, Zhang C, Zawieja DC, Liao JC, Kuo L. Oxidized low-density lipoprotein inhibits nitric oxide-mediated coronary arteriolar dilation by up-regulating endothelial arginase I. Microcirculation. 2011;18(1):36–45. doi: 10.1111/j.1549-8719.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- World C, Spindel ON, Berk BC. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-alpha: a key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arterioscler Thromb Vasc Biol. 2011;31(8):1890–1897. doi: 10.1161/ATVBAHA.111.226340. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol. 2009;297(5):H1870–1875. doi: 10.1152/ajpheart.00709.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. Superoxide generation from nitric oxide synthases. Antioxid Redox Signal. 2007;9(10):1773–1778. doi: 10.1089/ars.2007.1733. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nakamura H, Adachi T, Sannohe S, Oyamada H, Kayaba H, Yodoi J, Chihara J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol Lett. 2003;86(2):199–205. doi: 10.1016/s0165-2478(03)00006-3. [DOI] [PubMed] [Google Scholar]
- Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297(5):H1829–1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116(3):219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]