Abstract
Survivin, an important anti-apoptotic protein, is highly expressed in most cancers, which generally arise in cells of older individuals. We have shown here accumulation of survivin and phospho-survivin in aged normal human skin fibroblasts and mice organs. This age-related accumulation of survivin was due to protein stabilization through association with the molecular chaperone Hsp90 protein, which was also up-regulated during aging. Interestingly, Hsp90 binds preferentially to phospho-survivin, which explains its higher stability. In addition, we provide clear evidence that aged cells exhibit apoptosis resistance when challenged with UV light, cisplatin, γ-rays or H2O2 as compared to their younger counterparts. In response to γ-rays and H2O2, the levels of Bcl-2 and both forms of survivin were up-regulated in old cells, but not in their corresponding young ones. This repression of survivin and phospho-survivin in young cells is p53 dependent. Importantly, survivin inhibition/down-regulation with flavopiridol or specific shRNAs increased the apoptotic response of old fibroblasts to various genotoxic agents, and restored the pro-apoptotic Bax/Bcl2 ratio and the increase in the levels of cleaved caspase-3 and PARP in old cells. These results show the role of survivin in the age-dependent resistance of human fibroblasts, and provide new insights into the molecular mechanisms that underlie the complex relationship between aging, apoptosis, and cancer.
Keywords: Aging, Apoptosis, Cancer, DNA damage, Survivin
Introduction
Aging is a complex process driven by genetic and extrinsic factors that ultimately lead to the accumulation of deleterious modifications in various vital biomolecules, change in gene expression, and resistance to various stresses (Partridge and Gems 2002). Several reports have shown that damaged cells, which are normally removed from the body, accumulate during aging, which can lead to various age-related diseases such as cancer (Kourtis and Tavernarakis 2011). Apoptosis normally plays a critical role in maintaining tissue homeostasis over age and consequently suppresses the carcinogenesis process (Tan et al. 2009). However, the nature of the link between aging and apoptosis, and the molecular mechanisms underlying this link are still not well understood.
Among the regulators of apoptosis, interest has been recently focused on survivin, a structurally unique member of the inhibitors of apoptosis proteins (IAP) family. Abundantly expressed during embryonic development but undetectable in most normal adult tissues, survivin is over-expressed in most human cancers and functionally implicated in apoptosis inhibition, control of cell division, and cellular adaptation to stress (Altieri 2006, 2010). High levels of survivin correlate with abbreviated patient survival, unfavorable prognosis, resistance to therapy, and accelerated rates of tumor recurrence (Altieri 2003b, 2006; Li et al. 1998). It is noteworthy that survivin phosphorylation on Thr 34 by the mitotic kinase Cdk1 is important for the anti-apoptotic function of the protein (O'Connor et al. 2000).
In the present report, we have shown that the levels of survivin and its active phosphorylated form increase in an age-dependent manner in normal human and mice skin fibroblast cells as well as in aged mice heart, liver, and lung tissues. Interestingly, old human and mice fibroblast cells showed survivin-dependent resistance to various genotoxic agents. This is the first report implicating survivin in apoptosis resistance of aged fibroblasts.
Materials and methods
Cell culture and chemicals
Normal human skin fibroblasts (HFSN1) and mouse embryonic fibroblasts (MEF) were routinely cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum. Cisplatin (cis diamminedicloroplatinum II), hydrogen peroxide (H2O2), flavopiridol, and cycloheximide were purchased from Sigma (MO, USA). 17-AAG (17-Allylaminogeldanamycin) was purchased from Selleck Chemicals (USA).
Animals and homogenization of tissues
Three young (1 month) and three old (6 months) female mice (C57BL/6 J) were sacrificed and heart, liver, and lung were extracted and frozen in liquid nitrogen and kept at −80°C. The breeding, care, and sacrifice of the animals were in accordance with the protocols approved by the Animal Care and Use Committee of the King Faisal Specialist Hospital and Research Centre. Frozen tissues were mechanically homogenized using a mortar and pestle. Samples were then solubilized in lysis buffer containing 9 M urea, 2 M thiourea, 1 mM EDTA, 65 mM DTT, 0.2 mM PMSF, 0.8 mM benzamidine, 25 mM CHAPS, 5% Resolyte (pH 4–8), and 5% Nonidet P40. Ten percent SDS including 33.3% mercaptoethanol, DNase I (3.72 U/ml), and RNase A (0.036 U/ml) was also added. The mixture was incubated on a shaker at room temperature for 3 h and then centrifuged at 12,000 rpm for 15 min to remove any insoluble material. Protein concentration was determined using the Bradford method.
UV light and γ-ray treatments
For UV irradiation, the medium was removed and cell culture monolayers were covered with phosphate-buffered saline (PBS; 138 mM NaCl, 2.7 mM KCl pH 7.4) and exposed to a germicidal UV lamp (254 nm) at fixed distance. The UV dosimetry was performed using an ultraviolet meter (Spectronics Corporation, NY, USA). Gamma radiation was done using cobalt (Co) source at a dose rate of 0.30 Gy/min.
Calculation of cumulative population doublings (PD)
The population doublings of cells grown in vitro were determined by direct counting of cell numbers at passages as previously described (Cristofalo et al. 1998).
Cell cycle analysis by flow cytometry
Propidium iodide (PI) stained cells were analyzed for DNA content and the percentage of cells in various cell cycle phases was determined by the Cell Quest software (Becton Dickinson, Mississauga, ON, Canada).
Analysis of protein half-life
Sub-confluent cells were treated with 20 μg/ml cycloheximide (Sigma) for various periods of time (0–150 min) and then lysed. Cellular extracts from each time point were subjected to immunoblotting using specific antibody.
Cellular lysate preparation
Cells were washed and scraped in lysis buffer supplemented with 40 μg/ml aprotinin, 20 μg/ml leupeptin, and 5 μg/ml pepstatin. Lysates were homogenized using a Polytron homogenizer and then centrifuged at 14,000 rpm in an Eppendorf microcentrifuge tube for 20 min. The supernatant was removed, aliquoted, and stored at −80°C.
Immunoblotting
SDS–PAGE was performed using 12% separating minigels. Equal amounts of protein extract (30 μg) from different samples were placed in boiling water for 5 min in the presence of SDS gel sample buffer (0.5 M Tris pH 6.8, 10% glycerol, 10% SDS, 5% 2-mercaptoethanol, 1% bromophenol) and electrophoresed for 2 h at 125 V. After transfer onto polyvinylidene difluoride membrane (PVDF), the membrane was incubated overnight with the appropriate antibodies. Visualization of the secondary antibody was performed using the enhanced chemiluminescence detection system (Amersham Biosciences, NJ, USA). The antibodies directed against β-actin (C-11), GAPDH (FL-335), α-tubulin (B-5-1-2), Bax (B-9), Bcl-2 (C-2), and survivin (C-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); phospho-survivin (Thr34) was purchased from Abcam (Cambridge, MA, USA); cleaved PARP (Asp214) and cleaved caspase-3 (Asp175) from Cell Signalling (UK) and Hsp90 (4F10) was purchased from Transduction Laboratories (Lexington, KY, USA).
Quantification of protein expression level
The expression levels of the immunoblotted proteins were measured using a densitometer (BIO-RAD GS-800 Calibrated Densitometer). X-ray films were scanned and protein signal intensity of each band was determined. Next, dividing the obtained value of each band by the values of the corresponding internal control allowed a correction of the loading differences. The fold of induction in the protein levels was determined by dividing the corrected values that corresponded to the treated samples by that of the non-treated one (time 0).
Immunocytochemical analysis
Cells were cytospin as described (Ghebeh et al. 2007) and fixed in 1:1 acetone/methanol, and a standard indirect immunoperoxidase procedure was applied, using mouse monoclonal antibody for Ki-67 (Dako, CA, USA) followed by peroxidase-conjugated rabbit anti-mouse Ig (Dako) followed by the mouse-specific avidin–biotin–peroxidase (ABC) system (Vector Laboratories, Burlingame, CA, USA). Sites of antibody binding were visualized by the deposition of brown polymer following incubation in diaminobezidine–hydrogen peroxide, and nuclei were visualized with a light hematoxylin counterstain.
Immunoprecipitation
Cell lysates from young and old cells were prepared using RIPA buffer containing protease inhibitors, and then centrifuged at 14,000 rpm at 4°C. Three hundred micrograms of protein extracts was pre-cleaned with 20 μl protein A/G agarose for 2 h at 4°C and then incubated for 2 h with 2 μg of specific antibody at 4°C. Subsequently, 50 μl of A/G agarose was added for 2 h at 4°C. After centrifugation, the pellet was washed with the RIPA buffer and the proteins were recovered by boiling in Laemmli buffer.
shRNA/siRNA transfection
Sure Silencing shRNA plasmids against survivin and the control plasmid (SA Biosciences, Frederick, MD, USA), and HSP90-siRNA and plasmid bearing the HSP90 ORF as well as their respective controls (Ambion, Carlsbad, USA) were used to transfect HFSN1 cells. Transfection was carried out by mixing 8 μg of the plasmid DNA in 1.5 ml of Opti-MEM I medium without serum. A mixture of 1.5 ml of Opti-MEM I medium with 36 μl Lipofectamine (Invitrogen) was then added to the DNA, followed by incubation for 20 min before mixing with cells. Cells were incubated for 12 h, and the media was changed to remove the remaining transfection reagent. Forty-eight hours later, transfected cells were selected with 100 μg/ml G418.
RNA purification and RT–PCR
Total RNA was purified using the TRI reagent (Sigma) according to the manufacturer’s instructions. The concentration of RNA was determined using NanoDrop® ND-1000 Spectrophotometer (NanoDrop Inc., Wilmington, DE, USA). Single stranded complementary DNA (cDNA) was obtained from reverse transcription of 1 μg of RNA using RT–PCR kit (Clontech, CA, USA) following the manufacturer’s protocol. cDNA was then amplified with 1 U Taq polymerase, dNTPs (50 mM), and primers (25 pmol each). The mixture was first heated at 95°C for 5 min and then 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, then 72°C for 10 min. PCR products were seen on 2% agarose gel. The respective primers were as follows: survivin—5′-CAGAGGAGGCGCCAAGACAG-3’ (forward) and 5′-CCTGACGGCGGAAAACGC-3′ (reverse); GAPDH—5′-ATGGATGACGATATCGCTGCGC-3′ (forward) and 5′-ACAGGGCAAGGGAGGTAGAT-3′ (reverse). The intensity of the bands was determined with the Quantity One program (Bio-RAD) and was normalized against GAPDH.
Apoptosis analysis by annexin V/flow cytometry
Cells were either not treated or challenged with cisplatin (60 μg/μl), γ-rays (30 Gy), UV light (10 J m−2), and H2O2 (0.2 μM). Detached and adherent cells were then harvested after 72 h, unless otherwise stated, centrifuged, and re-suspended in 1 ml phosphate buffered saline (PBS). Cells were then stained by propidium iodide (PI) and Alexa Flour 488 annexin V. Annexin V staining was performed using Vybrant Apoptosis Assay kit #2 (Molecular Probes, Eugene, OR, USA) following the manufacturer’s recommendations. Annexin V-stained cells were analyzed by flow cytometry, measuring the fluorescence emission at 530 nm and >575 nm. The percentage of cells was determined by the FACSCalibur apparatus and the Cell Quest Pro software from Becton Dickinson. For each cell culture, three independent experiments were performed using 104 cells in each experiment.
Statistical analysis
Student’s t test was performed and results were considered to be statistically significant when P <0.05.
Results
Increased expression of survivin in aged cells and organs
After serial passaging of human fibroblast HFSN1 cells, we first confirmed that the late passage cells (PD 40) were actively proliferating and not senescent (Fig. 1a). Therefore, young (PD 20) and old (PD 40) fibroblast cells were stained with the proliferation marker Ki-67, and the number of stained nuclei was calculated. Figure 1b shows that both young and old cells stained positive with Ki-67 and that the Ki-67 labeling index was similar (89%), indicating that late passage cells are not replicatively senescent. Next, we investigated age-related expression of both the phosphorylated (Thr34) and non-phosphorylated forms of survivin. Figure 1c shows that the expression of both forms progressively increased in the serially passaged HFSN1 cells, reaching levels 5.1- and 3.8-fold higher in old cells (PD 40) as compared to their younger counterparts (PD 11), respectively. Similar results were also obtained in mouse embryonic fibroblasts (MEF) and human breast fibroblasts (data not shown). It is noteworthy that both young and old human fibroblasts exhibited similar proportions of cells in G0/G1 (p = 0.151) and G2/M (p = 0.641) phases (Fig. 1d), indicating that the aging-dependent accumulation of survivin is not due to the accumulation of cells in the G2/M phase of the cell cycle. Similarly, the levels of survivin and phospho-survivin were also higher in aged heart, liver, and lung mice tissues as compared to their levels in the same tissues extracted from young mice (Fig. 1c), which shows age-dependent up-regulation of survivin in various cell types and organs.
Fig. 1.
Survivin and phospho-survivin protein levels are higher in old cells. a Cumulative population doublings for HFSN1 cells in culture. b In vitro passaged HFSN1 cells [Population Doubling 20 (PD 20), Population Doubling 40 (PD 40)] were cytospin attached to slides and stained with anti-Ki-67 antibody. Scale bars represent 500 μm. c Proteins were extracted from serially passaged HFSN1 cells as well as from young and old mice tissues, and then used for western blot analysis utilizing the indicated antibodies. The histogram shows the expression level of survivin (PD population doubling, Y young, O old). Error bars represent standard deviation of at least three different experiments. d Cells were harvested and analyzed for DNA content by flow cytometry. The percentage of G0/G1 and G2/M cells are indicated. e Total RNA was extracted from young and old cells, and then RT–PCR was used to assess the level of the survivin mRNA. GAPDH was used as internal control. f Cells were treated with cycloheximide (20 μg/ml), and then were harvested at the indicated periods of time for protein extraction and western blotting. Thirty micrograms of proteins was loaded and the indicated antibodies were used. Signals were quantitated by densitometry and were normalized against GAPDH. The graph shows the proportion of the remaining survivin per time. Error bars indicate standard errors from three different experiments
Next, total RNA was prepared and the survivin mRNA level was assessed by RT–PCR. Figure 1e shows that the mRNA level of survivin was similar in young and old cells, suggesting that the age-dependent up-regulation of the survivin protein did not result from increase in the corresponding mRNA, but seems to occur at the protein level.
Therefore, the turn-over of the survivin and phospho-survivin (Thr34) proteins were tested in the young and old cells by treating them with cyclohexamide (20 μg/ml) for different periods of time (0–150 min). While survivin half-life was only 30 min in the young cells, it reached 95 min in their old counterparts (Fig. 1f). The turn-over of phospho-survivin (Thr34) was also assessed in young and old cells. Interestingly, phospho-survivin was much more stable than survivin in young cells. However, the stability of phospho-survivin was similar in young and old cells up to 150 min (Fig. 1f). This suggests that the age-related increase in survivin level occurs at the post-translational level through increasing the stability of the protein.
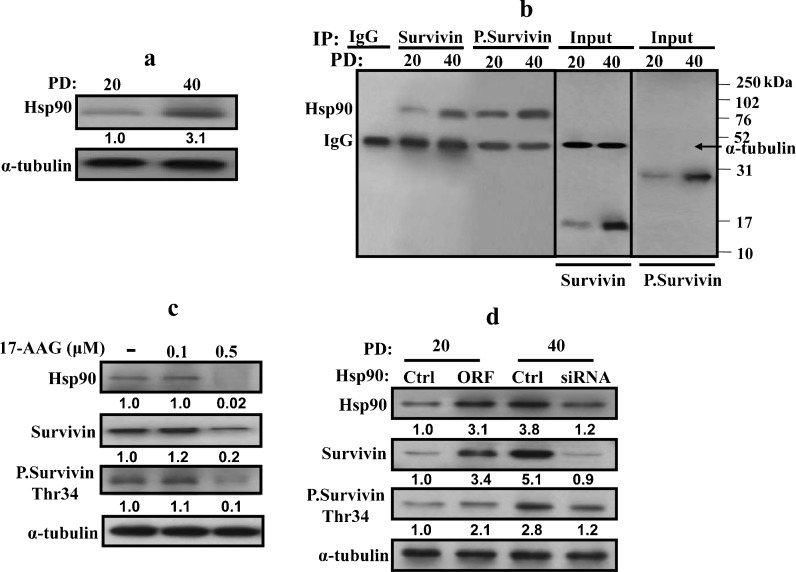
Age-dependent up-regulation of Hsp90 and association with survivin
It has been previously shown that survivin is degraded through the ubiquitin–proteasome pathway (Zhao et al. 2000) and that the binding of the Hsp90 protein to survivin inhibits this degradation (Fortugno et al. 2003). Therefore, it is possible that increased survivin stability during aging is due to its binding to Hsp90. To test this hypothesis, we first investigated age-dependent expression of Hsp90. Figure 2a shows that the Hsp90 protein level increased 3.1-fold in old cells as compared to their younger counterparts. Next, the same protein extracts were used for immunoprecipitation using specific anti-survivin antibody, and specific anti-Hsp90 antibody was utilized for immunoblotting. Figure 2b, left panel, shows that concomitant to the age-related increase in the survivin and Hsp90 protein levels, the level of the Hsp90–survivin complex was also higher in old cells as compared to their younger counterparts. Subsequently, we investigated whether Hsp90 binds preferentially to phospho-survivin. Figure 2b, right panel, shows that the levels of both forms of survivin were similar in the input of each age group. However, the level of Hsp90 bound to phospho-survivin was more pronounced than the level of Hsp90 associated with survivin in both young and old cells. This indicates an age-dependent preferential binding of Hsp90 to phospho-survivin.
Fig. 2.
Hsp90 is up-regulated during aging. Whole-cell extracts were prepared from young (PD 20) and old (PD 40) HFSN1 cells. a Western blot analysis using the indicated antibodies. The numbers below the bands indicate the expression level of Hsp90. b Immunoprecipitation with either anti-survivin or anti-phospho-survivin (Thr34) antibodies and mouse IgG was used as control. IP materials were then immunoblotted with anti-Hsp90 antibody. c HFSN1 cells (PD 40) were treated with different concentration of the Hsp90 inhibitor (17-AAG) and then re-incubated for 24 h. Whole-cell extracts were prepared and analyzed by western blot using the indicated antibodies. d Young HFSN1 cells (PD 20) were transfected with a plasmid bearing the HSP90 ORF and a control plasmid, and old HFSN1 cells (PD 40) were transfected with Hsp90-siRNA and control siRNA. Seventy-two hours post-transfection, cells were collected and whole-cell extracts were prepared and analyzed by immunoblotting using the indicated antibodies. The numbers below the bands represent the corresponding fold of variation in the expression levels relative to their respective controls and α-tubulin
To confirm this age-related relationship between Hsp90 and survivin, the level of Hsp90 was down-regulated with different concentrations of 17-AAG and the levels of Hsp90, survivin, and phospho-survivin (Thr34) were analyzed by western blot. Figure 2c shows that the decrease in the level of Hsp90 after treatment with 0.5 μM of 17-AAG was accompanied by a decrease in the level of both the survivin and phospho-survivin proteins. This suggests that the age-related accumulation of survivin and phospho-survivin is Hsp90 dependent.
To further confirm this relationship between Hsp90 and survivin, HSP90 was specifically down-regulated by siRNA in old cells. After 72 h, the levels of the Hsp90, survivin, and phospho-survivin (Thr34) proteins were analyzed by immunoblotting. Figure 2d shows that HSP90-siRNA decreased the level of the protein 3.1-fold as compared to the control cells. Concomitantly, the level of survivin and phospho-survivin (Thr34) also decreased 5.6-fold and 2.3-fold as compared to their levels in the control counterparts (Fig. 2d). On the other hand, over-expression of Hsp90 in young cells increased the level of survivin and phospho-survivin (Thr34) 3.4-fold and 2.1-fold as compared to the control cells, respectively. This shows that the age-related up-regulation of survivin and phospho-survivin is Hsp90 dependent.
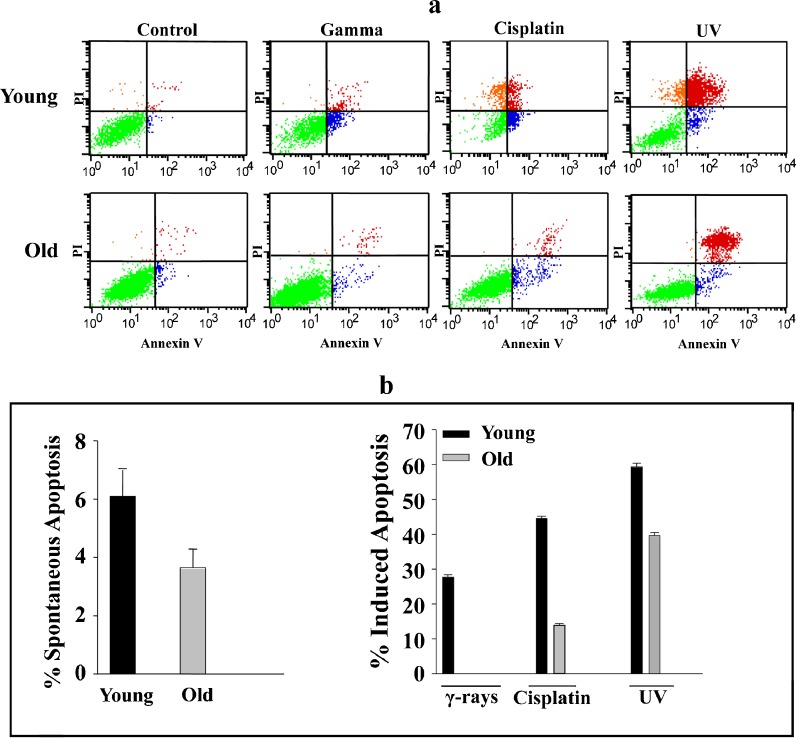
Aged human fibroblast cells are resistant to cisplatin, γ-rays, and UV light
To investigate the effect of aging on the response of fibroblast cells to various genotoxic stresses, young and old HFSN1 cells were either sham-treated or challenged with γ-rays (30 Gy), cisplatin (60 μg/ml), or UV light (10 J m−2) and re-incubated for 72 h. Subsequently, cells were collected and apoptosis was assessed using the flow cytometry/annexin V + PI technique. Only annexin V positive cells were considered as apoptotic. Figure 3a shows the spontaneous and induced apoptosis of HFSN1 cells in response to the various agents. The proportion of apoptosis depicted in Fig. 3b was considered as the sum of both early and late apoptosis after deduction of the proportion of spontaneous apoptosis (Shinwari et al. 2008).
Fig. 3.
Aged human skin fibroblasts are resistant to DNA damaging agents. a HFSN1 cells were either mock-treated or challenged with γ-rays (30 Gy), cisplatin (60 μg/ml), or UV light (10 J m−2) and then re-incubated for 72 h. Apoptosis was analyzed by annexin V/PI flow cytometry. b Histograms showing the proportions of spontaneous and induced apoptosis (early + late). The error bars represent standard deviations of at least three different experiments
Spontaneous apoptosis was very low in both young and old cells (Fig. 3a, b). Interestingly, in response to DNA damage, the apoptotic potential of old cells was significantly reduced as compared to young cells. Indeed, in response to γ-rays, 29.65% of young cells and only 4.26% of old cells underwent apoptosis (p value, 0.00047). Similarly, in response to cisplatin, the proportion of apoptosis was higher in young cells (49.4%) as compared to their older counterparts (17.93%) with a p value of 0.0004 (Fig. 3a, b). Likewise, UV light induced apoptosis in 58.58% of young cells, but only 39.11% of old cells underwent apoptosis, with a p value of 0.01 (Fig. 3a, b). This indicates that aging significantly reduces the apoptotic potential of human skin fibroblasts in response to various DNA damaging agents.
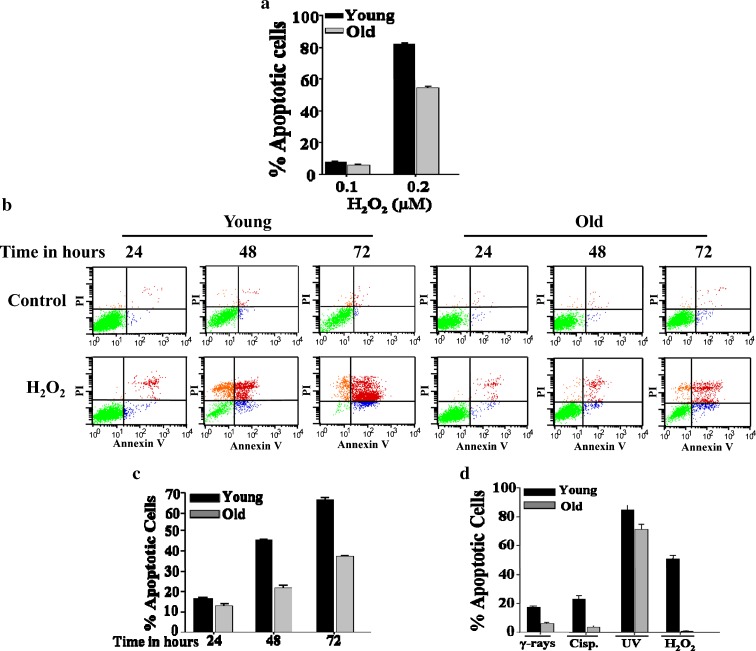
Aged fibroblasts develop resistance to oxidative stress
Next, we tested the susceptibility of old fibroblasts to the oxidative stress induced by H2O2. Thereby, young and old cells were exposed to different doses (0–0.2 μM) of hydrogen peroxide for 72 h, and then cells were collected and were subjected to annexin V/flow cytometry analysis. Figure 4a shows that, in response to H2O2 (0.1 μM), the proportion of apoptosis induced in young and old cells was similar about 10%. However, in response to 0.2 μM the proportion of apoptotic cells increased in young cells reaching 82%, but was only 58% in old cells, with a p value of 0.0035 (Fig. 4a). Next, the time-dependent response of young and old cells to 0.2 μM H2O2 was tested. Figure 4b and c shows time-dependent increase in apoptosis with the maximum proportion occurred after 72 h of treatment. There was a significant difference in the response of young cells as compared to the old ones. At 48 h, 0.2 μM H2O2 induced apoptosis in about 45% of young cells, while only 21% of old cells were apoptotic, with a p value of 0.01 (Fig. 4c). After 72 h of treatment, 66% of young cells were apoptotic, whereas only 37% of old cells underwent apoptosis (Fig. 4c). These results clearly show that H2O2 induces apoptosis in human fibroblasts, and that during aging cells develop resistance to this oxidative stress.
Fig. 4.
Aged fibroblasts are resistant to H2O2. a HFSN1 cells were either mock-treated or challenged with serial concentrations of H2O2 and then re-incubated for 72 h. Apoptosis was analyzed by annexin V/PI flow cytometry. Histogram showing the proportions of apoptosis in response to the indicated doses of H2O2. b Young and old fibroblasts were either mock-treated or challenged with 0.2 μM H2O2 for the indicated periods of time and then apoptosis was assessed by annexin V/PI flow cytometry. c Histogram showing the proportions of apoptosis after treatment with H2O2 (0.2 μM). The error bars represent standard deviation of at least three different experiments. d Young (PD 6) and old (PD 20) MEF cells were either mock-treated or challenged with the indicated genotoxic agents then re-incubated for 72 h. Apoptosis was analyzed by annexin V/PI flow cytometry. Histogram showing the proportions of induced apoptosis. The error bars represent standard deviation of at least three different experiments
Similar to HFSN1 cells, old MEF cells (PD 20) were also more resistant than their younger counterparts (PD 6) to γ-rays, cisplatin, UV light, and H2O2 (Fig. 4d). This indicates that the age-related resistance of fibroblast cells to genotoxic stresses is not restricted to human fibroblasts.
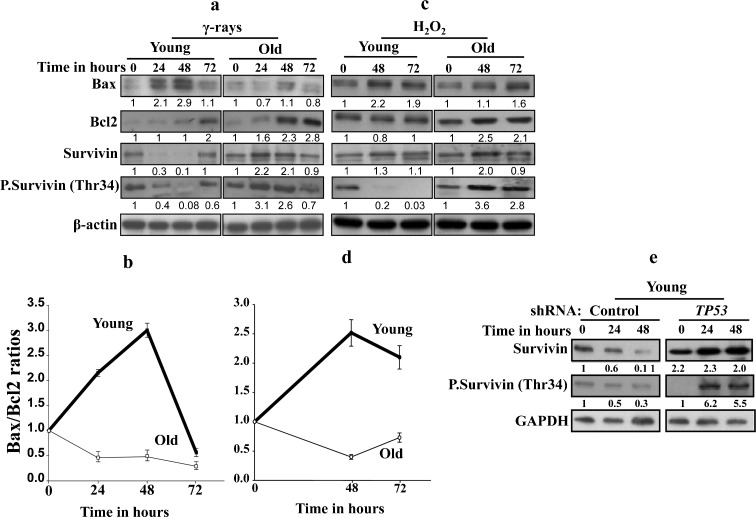
γ-Rays and H2O2 up-regulate survivin and Bcl-2 in old cells
Next, we investigated the possible implication of survivin in the resistance of old cells to the killing effect of γ-rays and H2O2. Therefore, the expression of the anti-apoptotic survivin protein was investigated in response to both genotoxic agents in old and young cells. Figure 5a shows that in young cells, the expression level of survivin decreased 3.3-fold and 10-fold 24 h and 48 h post γ-irradiation, respectively, and then returned to the basal level at 72 h after γ-irradiation. However, in old cells, the level of survivin showed 2.2- and 2.1-fold increase 24 h and 48 h post-γ-irradiation, respectively, and returned to the basal level at 72 h. In response to H2O2, survivin was also up-regulated in old cells (2-fold after 48 h of treatment), while the effect was only marginal in the young ones (Fig. 5c). Interestingly, the level of phospho-survivin decreased sharply in young cells but increased in old ones in response to both γ-rays and H2O2, with different kinetics (Fig. 5a, c), indicating that γ-rays and H2O2 modulate survivin expression in an age-dependent manner.
Fig. 5.
The effect of γ-rays and H2O2 on survivin expression is age dependent. a, c Young and old cells were either sham-treated or exposed to γ-rays (30 Gy) or H2O2 (0.2 μM) and then were incubated for the indicated periods of time, before being harvested and proteins extracted. Thirty micrograms of proteins was used for immunoblotting analysis using the indicated antibodies. The numbers below the gels indicate the induction/reduction folds in the expression levels after normalization against β-actin. b, d Graphs showing the age-dependent level of Bax/Bcl-2 ratio after treatment with γ-rays or H2O2, respectively. Error bars represent standard deviation of at least three different experiments. e Western blot analysis using the indicated antibodies in young TP53 shRNA-expressing cells and their control counterparts treated with γ-rays (30 Gy). The numbers below the gels indicate the fold of induction/reduction in the expression levels as compared to the basal level (time 0)
We next sought to assess the expression level of the pro-apoptotic (Bax) as well as the anti-apoptotic (Bcl-2) proteins by immunoblotting following treatment with γ-rays (30 Gy) and H2O2 (0.2 μM) for different periods of time. Treatment of young cells with γ-rays led to 2.9-fold increase in the level of Bax, while the level of Bcl-2 remained stable, which resulted in 3-fold increase in the Bax/Bcl-2 ratio at 48 h post-treatment (Fig. 5a, b). On the other hand, treatment of old cells with the same dose of γ-rays led to a slight decrease in Bax expression level and 2.8-fold increase in Bcl-2 level, which resulted in 2-fold decrease in the Bax/Bcl-2 ratio 48 h post-γ-ray treatment (Fig. 5a, b). Similar results were obtained when cells were treated with H2O2 (Fig. 5c, d), indicating that γ-rays and H2O2 trigger apoptosis in HFSN1 fibroblasts through the internal mitochondrial pathway.
γ-Ray-dependent modulation of survivin during aging is p53-related
Since the transcription of survivin is negatively regulated by the tumor suppressor p53 protein at the basal level (Hoffman et al. 2002; Mirza et al. 2002) and in response to DNA damage (Ikeda et al. 2007), we sought to investigate the potential role of p53 in survivin up-regulation in old cells in response to γ-rays. With this objective, TP53 was down-regulated using specific shRNA in young cells and a scrambled sequence shRNA was used as control (Fig. 5e). Cells were irradiated with γ-rays (30 Gy) and then were re-incubated for different periods of time, and used for protein preparation. Figure 5e shows that while the level of survivin decreased 10-fold in the control cells, it increased 2.3-fold in p53-deficient cells. Similarly, phospho-survivin at Thr34, which was down-regulated in the control cells, was also up-regulated in response to γ-rays in young p53-deficent cells (Fig. 5e). This indicates that, in young cells, the down-regulation of survivin following DNA damage is p53 dependent.
Age-related resistance to genotoxic stress is survivin dependent
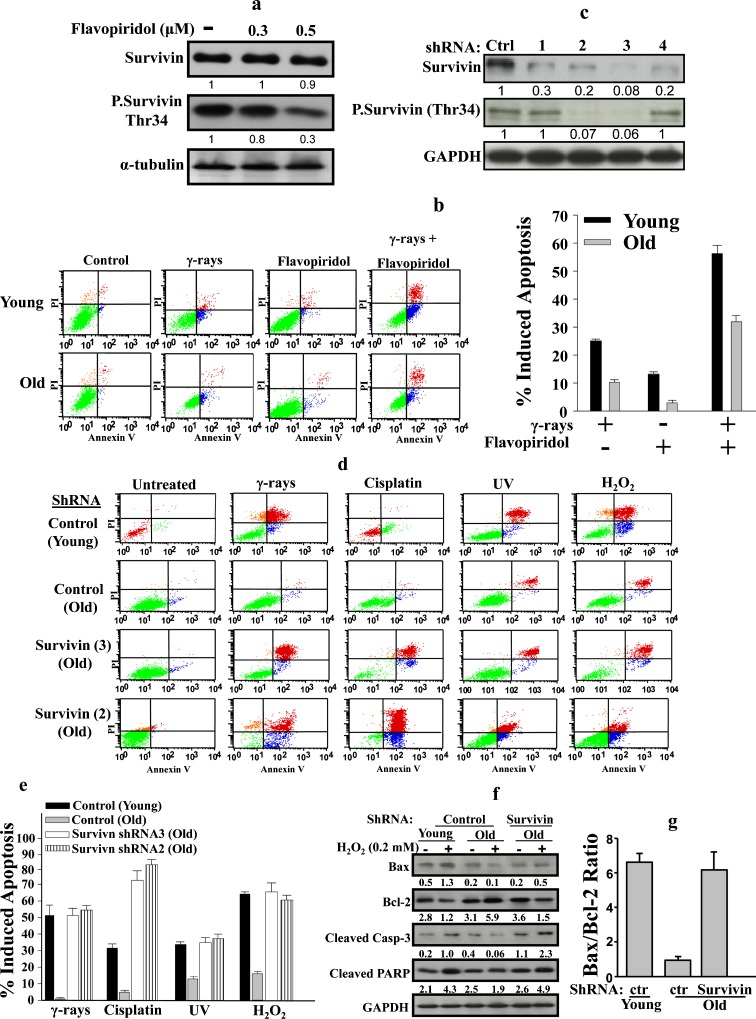
To address the role of survivin in age-related resistance to DNA damaging agents, we first inhibited the activation of survivin by phosphorylation using flavopiridol, which has been shown to suppress the phosphorylation of survivin at Thr34 by targeting Cdk1 (Wall et al. 2003). Thereby, old HFSN1 cells were treated with different concentrations of flavopiridol for 24 h. Figure 6a shows that while the level of survivin did not change after treatment with flavopiridol, there was 3-fold decrease in the level of phospho-survivin (Thr34) in response to 0.5 μM falvopiridol (Fig. 6a). Next, young and old cells were either mock-treated or challenged with γ-rays (30 Gy), flavopiridol (0.5 μM), or a combination of both. Cells were re-incubated for 72 h, and then cell death was assessed by the flow cytometry/annexin V + PI technique. Figure 6b shows that γ-rays or flavopiridol alone triggered only low proportion of apoptosis in both young and old cells, with the young ones more sensitive than their old counterparts. When combined, flavopiridol significantly enhanced the apoptotic response of old cells to γ-rays, reaching a level close to that exhibited by the young ones treated with γ-rays alone (35% and 25%, respectively). This suggests that phospho-survivin could be involved in the resistance of old cells to the killing effect of γ-rays. To further investigate this possibility, survivin was specifically down-regulated using four different shRNAs that were introduced independently into HFSN1 fibroblast cells, with a scrambled sequence served as control. The expression levels of both survivin and phospho-survivin (Thr34) were assessed by immunoblotting. Figure 6c shows that compared to the control, the third sequence (shRNA3) shows a significant down-regulation of survivin. Similarly, a sharp decrease in the level of phospho-survivin (Thr34) was also obtained (Fig. 6c). Subsequently, young and old HFSN1 fibroblasts expressing the control plasmid and old fibroblasts expressing survivin-shRNA3 were then treated as described above. Figure 6d and e shows that down-regulation of survivin in old cells increased the proportion of apoptotic cells to 49%, similar to that exhibited by young cells (Fig. 6d, e). Interestingly, similar results were obtained in response to UV light, cisplatin, and H2O2 (Fig. 6d, e). Similar results were also obtained using old HFSN1 expressing survivin-shRNA sequence 2 (Fig. 6d, e).
Fig. 6.
Inhibition of survivin enhances apoptotic response of old fibroblasts to DNA damage. a HFSN1 cells (PD 40) were treated with different concentration of flavopiridol and then re-incubated for 24 h. Whole-cell extracts were prepared and analyzed by western blot using the indicated antibodies. b Young and old HFSN1 cells were either mock-treated or challenged with γ-rays (30 Gy), flavopiridol (0.5 μM), or a combination of both and re-incubated for 72 h. Apoptosis was then assessed by annexin V/PI flow cytometry. The histogram shows the proportions of apoptosis induced by the indicated agents. The error bars represent standard deviations of three different experiments. c Western blot showing the expression of the indicated proteins in HFSN1 cells expressing either four shRNA against survivin, independently, or control shRNA (Ctrl). The numbers below the gels indicate the expression level of the indicated proteins as compared to the control. d Young and old HFSN1 fibroblasts expressing either survivin-shRNA or the control shRNA were either mock-treated or challenged with the indicated agents and then cell death was assessed by annexin V/PI flow cytometry. e Histogram showing the proportions of apoptosis induced by the indicated agents. The error bars represent standard deviations of three different experiments. f Young and old HFSN1 fibroblasts expressing the control plasmid and old fibroblasts expressing survivin-shRNA3 were either mock-treated or challenged with H2O2 (0.2 μM) for 72 h. Cells were then collected and cell lysates were prepared to assess the level of the indicated apoptotic proteins. The numbers below the gels indicate the expression levels of the indicated proteins after normalizing against GAPDH. g Graph showing the Bax/Bcl-2 ratio in the indicated cells after treatment with H2O2 (0.2 μM). Error bars represent standard deviation of at least three different experiments
To further show the effect of survivin down-regulation on apoptosis, we assessed the effect of H2O2 (0.2 μM) on the expression of pro- and anti-apoptotic proteins in young and old cells. Figure 6f shows that, similar to survivin, the levels of cleaved caspase-3 and PARP increased in young cells, while they decreased in the old ones. Importantly, specific down-regulation of survivin in old cells enhanced their expression to a level similar to that observed in young ones (Fig. 6f). Similarly, upon H2O2 treatment, the Bax/Bcl-2 ratio increased in survivin-defective old cells as compared to their control counterparts, reaching a level similar to that observed in young cells (Fig. 6g). Together, these results strongly suggest that survivin is the main inhibitor of the age-related resistance to genotoxic stress-induced apoptosis.
Discussion
In the present report, we present the first indication that the expression of survivin and phospho-survivin (Thr34) increases with age in human and mouse skin fibroblast cells as well as in aged mice tissues. This up-regulation is mainly due to a decrease in the protein turn-over. Indeed, survivin half-life increased from 30 min in young cells to 95 min in their old counterparts. This rapid turn-over of survivin in young cells parallels what has been previously reported (Zhao et al. 2000). However, we have shown here that survivin stability is regulated in an age-dependent manner. Furthermore, we have found an age-dependent increase in the level of the phosphorylated survivin at Thr34, which is indeed much more stable than the non-phosphorylated form of the protein (Fig. 1e). This result confirms what has been previously shown that non-phosphorylatable survivin, which harbor alanine instead of threonine-34, exhibited accelerated clearance as compared to the wild-type protein (Wall et al. 2003). Importantly, we have also shown that the level of Hsp90, known to stabilize survivin (Fortugno et al. 2003), significantly increases with age and that Hsp90 binds survivin in an age-dependent manner with preference to the phosphorylated form of the protein. This binding stabilized survivin during the aging process (Fig. 2). Together, these results indicate that survivin and Hsp90 accumulate during aging, which leads to the stabilization of survivin and the consequent inhibition of apoptosis, a major hallmark of cancer cells (Hanahan and Weinberg 2011). Like survivin, Hsp90 is also known to be up-regulated in cancer cells and linked to apoptotic resistance (Altieri 2003a, 2010).
Besides, we have found a significant decrease in the apoptotic response of old human and mouse cells as compared to their young counterparts when challenged with γ-rays, UV light, cisplatin, and H2O2. This is in agreement with other studies that have reported a decrease in induced apoptosis in old cells in response to different stimuli, including oxidative stress (Higami and Shimokawa 2000; Lee and Wei 2007) and γ-rays (Polyak et al. 1997). Likewise, previous studies have shown that senescent fibroblasts are resistant to apoptosis induced by UV light (Ryu et al. 2006) oxygen radicals (Gansauge et al. 1997), serum withdrawal, H2O2, staurosporine, and thapsigargin (Wang 1995; Ryu et al. 2006, 2007). On the other hand, other studies have shown that aging enhances apoptosis triggered by various challenges (Higami and Shimokawa 2000; Lee and Wei 2007). Indeed, fibroblasts cultured from old individuals were more sensitive to H2O2-induced apoptosis than those cultured from younger ones (Miyoshi et al. 2006). Apoptosis also increased with age in cells of AL-fed mice incubated with H2O2 (Avula and Fernandes 2002). These discrepancies are probably due to the use of different species, different strains, or different cell types (Higami and Shimokawa 2000). It is also possible that these differences result from the differences between mitotic and post-mitotic tissues, as aging of post-mitotic tissues is known to be associated with a general enhancement of apoptosis, and this trend seems to be essential for the removal of damaged and dysfunctional non-replaceable cells (Higami and Shimokawa 2000; Campisi 2003). These considerations suggest that the decline of the apoptotic response in mitotic tissues, human and mouse skin fibroblasts in this study, may decrease longevity as a consequence of higher incidence of cancer. Hence, our findings support the hypothesis set out by Polyak et al. (1997) and Suh et al (2002), based on rodent data, that a decrease in apoptosis efficiency may play an important role in age-associated disease processes.
What is the molecular mechanism that underlies age-dependent resistance to genotoxic stress? Previous studies have shown that failure to down-regulate Bcl-2 is responsible for the resistance of senescent human diploid fibroblasts to apoptosis (Wang 1995; Ryu et al. 2007). We have shown here that indeed Bcl-2 level increases in response to γ-rays and H2O2 in old cells while its modulation was only marginal in young ones. By contrast, we have found that γ-rays and H2O2 increases the level of the pro-apoptotic Bax protein in young cells but not in their old counterparts. This led to a significant difference in the Bax/Bcl-2 ratio favoring apoptosis in young cells (Fig. 5). Furthermore, we have shown that the levels of both survivin and phospho-survivin decreased in young cells, but rather increased in their old counterparts following treatment with γ-rays and H2O2. Interestingly, p53-defective young cells also up-regulate survivin and phospho-survivin following treatment with γ-rays, which shows the role of p53 in γ-ray-dependent modulation of survivin expression during aging.
Together, these results prompted us to speculate that sustained down-regulation of survivin might have the ability to re-sensitize these cells to genotoxic stress. Indeed, we have shown that sustained down-regulation of survivin by means of shRNA sensitized old fibroblasts to UV light, γ-rays, cisplatin, and H2O2. In addition, suppressing phosphorylation of survivin by flavopiridol enhanced the apoptotic response of old fibroblasts to γ-rays. This points to a synergism between flavopiridol and γ-rays, and shows that the age-dependent resistance to induced apoptosis is indeed survivin dependent. Interestingly, survivin affects also the Bax/Bcl-2 levels and ratio in aged cells following treatment with H2O2. While this ratio is 7-fold higher in young cells than in the old ones, it increased to a level similar to that observed in young cells when survivin was knocked down in old cells. This suggests that Bax/Bcl-2 expression is under the control of survivin. This effect could be mediated through negative regulation of p53 known to control the expression of Bax and Bcl-2. Indeed, Wang et al. (2004) have shown that survivin inhibits the accumulation of p53 in response to adriamycin. Therefore, the effect of survivin on Bax/Bcl-2 could be mediated through p53. In addition, down-regulation of survivin in old cells increased the levels of cleaved caspase-3 and PARP (Fig. 6g). The molecular mechanism underlying the increase in apoptosis is still not clear, but it is plausible that survivin down-regulation does not normalize apoptosis in old cells, but rather enable the killing process by affecting the apoptotic gene network, wherein p53 plays a major role. These results shed light on the role of survivin in age-related resistance to genotoxic agents; however, further experiments are required to elucidate the complex molecular link between survivin, apoptosis, and predisposition to cancer.
Acknowledgments
We thank Dr. K. Al-Hussein and P.S. Manogaran for their help with flow cytometry. We are also grateful to F. Mahyoub and K. Alhadyan for their help with γ-ray treatment, and to Dr. I. Al-Jammaz and R. El-Sayed for their help with animals. We are also very thankful to the Research Centre administration and the Office of Research Affairs at the KFSH&RC for their continuous support.
References
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula CP, Fernandes G. Inhibition of H2O2-induced apoptosis of lymphocytes by calorie restriction during aging. Microsc Res Tech. 2002;59:282–292. doi: 10.1002/jemt.10206. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansauge S, Gansauge F, Gause H, Poch B, Schoenberg MH, Beger HG. The induction of apoptosis in proliferating human fibroblasts by oxygen radicals is associated with a p53- and p21WAF1CIP1 induction. FEBS Lett. 1997;404:6–10. doi: 10.1016/S0014-5793(97)00059-8. [DOI] [PubMed] [Google Scholar]
- Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, Dermime S. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–132. doi: 10.1007/s004419900156. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Okamoto I, Tamura K, Satoh T, Yonesaka K, Fukuoka M, Nakagawa K. Down-regulation of survivin by ultraviolet C radiation is dependent on p53 and results in G(2)-M arrest in A549 cells. Cancer Lett. 2007;248:292–298. doi: 10.1016/j.canlet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med. 2007;232:592–606. [PubMed] [Google Scholar]
- Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen LL, Pickett CB, Liu S. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Oubrahim H, Chock PB, Stadtman ER. Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad Sci U S A. 2006;103:1727–1731. doi: 10.1073/pnas.0510346103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci U S A. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D. The evolution of longevity. Curr Biol. 2002;12:R544–R546. doi: 10.1016/S0960-9822(02)01048-5. [DOI] [PubMed] [Google Scholar]
- Polyak K, Wu TT, Hamilton SR, Kinzler KW, Vogelstein B. Less death in the dying. Cell Death Differ. 1997;4:242–246. doi: 10.1038/sj.cdd.4400226. [DOI] [PubMed] [Google Scholar]
- Ryu SJ, Cho KA, Oh YS, Park SC. Role of Src-specific phosphorylation site on focal adhesion kinase for senescence-associated apoptosis resistance. Apoptosis. 2006;11:303–313. doi: 10.1007/s10495-006-3978-9. [DOI] [PubMed] [Google Scholar]
- Ryu SJ, Oh YS, Park SC. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007;14:1020–1028. doi: 10.1038/sj.cdd.4402091. [DOI] [PubMed] [Google Scholar]
- Shinwari Z, Manogaran PS, Alrokayan SA, Al-Hussein KA, Aboussekhra A. Vincristine and lomustine induce apoptosis and p21(WAF1) up-regulation in medulloblastoma and normal human epithelial and fibroblast cells. J Neurooncol. 2008;87:123–132. doi: 10.1007/s11060-007-9502-4. [DOI] [PubMed] [Google Scholar]
- Suh Y, Lee KA, Kim WH, Han BG, Vijg J, Park SC. Aging alters the apoptotic response to genotoxic stress. Nat Med. 2002;8:3–4. doi: 10.1038/nm0102-3. [DOI] [PubMed] [Google Scholar]
- Tan ML, Ooi JP, Ismail N, Moad AI, Muhammad TS. Programmed cell death pathways and current antitumor targets. Pharm Res. 2009;26:1547–1560. doi: 10.1007/s11095-009-9895-1. [DOI] [PubMed] [Google Scholar]
- Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230–235. [PubMed] [Google Scholar]
- Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55:2284–2292. [PubMed] [Google Scholar]
- Wang Z, Fukuda S, Pelus LM. Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146–8153. doi: 10.1038/sj.onc.1207992. [DOI] [PubMed] [Google Scholar]
- Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113:4363–4371. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]