Abstract
The diet in the elderly does not provide a sufficient level of nutrients needed to maintain an adequate healthy status leading to micronutrient deficiencies and impaired immune response with subsequent development of degenerative diseases. Nutrient “zinc” is a relevant micronutrient involved in maintaining a good integrity of many body homeostatic mechanisms, including immune efficiency, owing to its requirement for the biological activity of many enzymes, proteins and for cellular proliferation and genomic stability. Old people aged 60–65 years and older have zinc intakes below 50% of the recommended daily allowance on a given day. Many causes can be involved: among them, altered intestinal absorption, inadequate mastication, psychosocial factors, drugs interactions, altered subcellular processes (zinc transporters (Zip and ZnT family), metallothioneins, divalent metal transporter-1). Zinc supplementation may remodel the immune alterations in elderly leading to healthy ageing. Several zinc trials have been carried out with contradictory data, perhaps due to incorrect choice of an effective zinc supplementation in old subjects showing subsequent zinc toxic effects on immunity. Old subjects with specific IL-6 polymorphism (GG allele carriers; named C−) are more prone for zinc supplementation than the entire old population, in whom correct dietary habits with foods containing zinc (Mediterranean diet) may be sufficient in restoring zinc deficiency and impaired immune response. We summarise the main causes of low zinc dietary intake in elderly reporting an update on the impact of zinc supplementation upon the immune response also on the basis of individual IL-6 polymorphism.
Keywords: Dietary zinc intake, Zinc intestinal absorption, Zinc supplementation, IL-6 polymorphism, Ageing, Immunosenescence
Introduction
The elderly population above the age of 60–65 years shows a higher risk of developing nutritional disorders caused by the ageing process itself coupled with a series of physiological, biochemical, biological and psychological changes, which in turn alter the individual physical activity as well as general behaviour, dietary habits and social interactions (Meunier et al. 2005). Several clinical symptoms are linked to this situation, including dermatitis, diarrhoea and especially alterations in immunocompetence (Hambidge 2000; Cunningham-Rundles et al. 2005).
Generally, adequate nutrition plays a pivotal role in maintaining healthy status (Hambidge 2010) and immunocompetence in humans (High 1999) with possible extension of the life span (Chernoff 2005). Among the micronutrients, zinc is essential in the elderly in terms of its impact on biological, biochemical and immune functions (Shankar and Prasad 1998; Mocchegiani et al. 1998; Haase et al. 2006b).
Zinc deficiency in humans is quite prevalent, affecting over two billion people (Prasad 2008). Nutritional zinc deficiency is widespread throughout developing countries. A lot of evidences support the belief that the main factor associated with zinc deficiency seems to be an inadequate zinc dietary intake influenced in turn by other several intrinsic and extrinsic factors (Gibson et al. 2008). Indeed, zinc is well recognised as an essential trace element for all organisms and plays an important role in the development and integrity of the immune system affecting both innate (T, NK, and NKT cells) and adaptive (anti/pro-inflammatory cytokine production) immune responses (Prasad 2000; Ibs et al. 2003; Bogden 2004; Haase et al. 2006b; Mocchegiani et al. 2009). Zinc is required for DNA synthesis, RNA transcription, cell division and activation (Prasad 2007) as well as in preventing apoptosis (Fraker 2005). Zinc has also a significant role as “zinc signal” because affecting the signal transduction for immune cell functions (Haase and Rink 2009a). All these effects have been identified in experimental animals and humans where an altered zinc status can affect the immunocompetence (Prasad 1998; Haase and Rink 2009b; Mocchegiani et al. 2008d). Zinc deficiency coupled with altered immune response, as occurring in ageing, leads to an increased susceptibility for some age-related diseases (Vasto et al. 2006; Prasad 2009). As a consequence, several studies suggest the usefulness of a zinc supplementation in the prevention and/or treatment of diseases associated with zinc deficiency (Prasad 2009; Haase and Rink 2009b; Mocchegiani et al. 2008d). The aim of this review is to evidence some possible causes of low zinc dietary intake in ageing and the main effects of zinc on immunosenescence. Moreover, we discuss the potential role of the zinc supplementation in elderly in order to restore the immune response in relation to individual genetic background that is represented by IL-6 polymorphism. Such an assumption is based on the fact that the production of IL-6 increases in ageing leading to chronic inflammation, named “inflammaging” (Franceschi 2007), with subsequent altered intracellular zinc homeostasis (Mocchegiani et al. 2006).
Dietary zinc deficiencies
Zinc deficiency is an important factor in the origin of certain common diseases that affect and cause morbidity among the elderly. Zinc is a critical trace element in human health for tissue growth, taste acuity, connective tissue growth and maintenance, immune response, prostaglandin production, bone mineralisation, proper thyroid function, blood clotting, cognitive functions, foetal growth and sperm production (Sandstead 1994). Zinc is also required for the biological activity of enzymes, for cell proliferation and for “zinc finger” DNA motifs (Mocchegiani et al. 1998). Clinical evidences support the pathological consequences that can occur during zinc deficiency that is a serious public health problem from young up to old age (Table 1). Such a deficiency in ageing is typically the result of an inadequate zinc dietary intake that may occur as a response to reduced energy requirements or age-related sensory impairment (Stewart-Knox et al. 2005). It has been reported that mild zinc deficiency is a significant clinical problem in free-living elderly people: only 42.9% have a sufficient intake of zinc (defined as >67% of the recommended daily allowance (RDA)) (Prasad et al. 1993). These data have been confirmed by other studies (ZENITH project, Andriollo-Sanchez et al. 2005; ZINCAGE project, Mocchegiani et al. 2008a; Japan study, Kogirima et al. 2007 and German study from the Max Ruben Institute, MRI 2008). In the latter, 44% of men and 27% of women (age range, 65–80 years) do not reach the recommendation. Moreover, the Third National Health and Nutrition Examination Survey (NHANES) documented a decrease in zinc intake with advancing age, and only 42.5% of old participants (age, ≥71 years) showed an adequate zinc intake (defined as ≥77% of the RDA) (Briefel et al. 2000).
Table 1.
Clinical consequences and risk factors in zinc deficiency from young up to old age
| Reference | Clinical risk factors and pathologies associated with Zn deficiency |
|---|---|
| Vasto et al. (2006) | Increased total cholesterol |
| Grahn et al. (2001) | Macular degeneration |
| Bunk et al. (1989) | Decreased plasma concentrations of vitamin E |
| Cipriano et al. (2009) | Accumulation of senescent cells with short telomeres |
| Mocchegiani et al. (1998), Dardenne (2002), Bogden (2004), Haase et al. (2006b), and Prasad (2008) | Impairment of the immune response (cell-mediated immunity and humoral immunity) and impairment of thymic and extrathymic T cell pathways (NKT cell number and function) |
| Eberle et al. (1999) | Abnormalities in bone growth and bone formation and mineralisation |
| Gur et al. (2002) | Osteoporosis |
| Hambidge (1992) | Diarrhoea |
| Hambidge (2000) | Pneumonia |
| Kury et al. (2002) | Acrodermatitis enteropathica |
| García et al. (2009) | Obesity especially in children |
| Gibson et al. (2002) | Lack of sexual development in females |
| Leek et al. (1988) | Delayed skeletal maturation and defective mineralisation of bone (monkeys) |
| Little et al. (1989) | Possible contributor to loss of appetite |
| Evans (1986) | hair loss, impotence, skin lesions, weight loss and delayed wound healing |
| Lyon et al. (2003) and Giacconi et al. (2004) | Hypertension and increased risk factor for atherosclerosis and cardiovascular diseases |
| Mackenzie et al. (2007) | Altered neonatal and infant behaviour and cognitive and motor performance |
| Fraker (2005) | Decreased cell proliferation and increased cell death (apoptosis) |
| Markovits et al. (1990) | Decreased taste acuity |
| Cousins (1985) and Mocchegiani et al. (2008b) | Reduced concentration of blood metal transport proteins (ceruloplasmin, albumin and α2-macroglubulin) |
| Meunier et al. (2005) | High susceptibility to oxidative damage of membrane fractions |
| Marcellini et al. (2006) | Negative consequences on human behaviour |
| Meunier et al. (2005) | Decreased absorption of dietary folate |
| Malavolta et al. (2010) and Mocchegiani et al. 2011) | Increased Cu/Zn ratio as predictor of frailty and mortality in old age |
| Mocchegiani et al. (2006) | Increased inflammation and limited zinc release by Metallothioneins |
| Taysi et al. (2008) | Decreased erythrocyte and cardiac antioxidant capacity |
| Marcellini et al. (2006) | Mood disorders |
| Tinker and Rucker (1985) | Defective connective tissue |
| Prasad (1985) | Weight loss |
| Prasad (1985) | Hypogonadism in males |
| Prasad (1985) | Growth retardation |
| Prasad (1985) | Delayed puberty in adolescents |
| Prasad (1985) | Mental lethargy |
| Prasad (1985), Dardenne (2002), and Mocchegiani et al. (2007) | Altered hormone productions (insulin, glucocorticoids, thyroid hormones, IGF-1, growth hormone, melatonin, testosterone, progesterone and thymulin) |
| Cakman et al. (1996) and Prasad (2000) | Decreased resistance to infection, imbalance of Th1/Th2 paradigm |
| Mariani et al. (2006) and Haase and Rink (2007) | Abnormalities in cytokine and chemokine secretions and functions |
| Sandstead (2000) and Marcellini et al. (2006) | Neuropsychological impairment |
| Sato et al. (2002) | Exacerbated hypertension and cardiovascular diseases |
| Sayeg Porto et al. (2000) | Short stature |
| Moroni et al. (2005), Mariani et al. (2008), and Haase and Rink (2009b) | Decreased functionality in monocytes, neutrophils, natural killer cells, granulocytes and phagocytosis |
| Mocchegiani et al. (2008c) | Risk factor for the development of diabetes |
| Varela et al. (1992), Su and Birmingham (2002), and Nova et al. (2004) | Possible contributor to anorexia nervosa |
| Karaca et al. (2007) | Dwarfism |
Such a reduced zinc dietary intake in ageing leads to low intracellular zinc ion availability, which has been well documented using specific fluorescent zinc probe (Haase et al. 2006a). The low intracellular zinc ion availability occurs despite the plasma zinc levels may be in the normal range, suggesting that the determination of plasma zinc can be misleading to detect a real zinc deficiency in elderly (Mocchegiani et al. 2006) that in turn does not reflect the reduced dietary zinc intake (ZINCAGE project) (Mocchegiani et al. 2008a). Such a reduction may be due to many factors related to the ageing process. Among them, altered intestinal absorption, alteration in zinc transporter proteins, inadequate mastication, psychosocial factors, drug interactions and competition between zinc and other bivalent minerals (copper, iron, calcium and selenium) or vitamins may be involved. Old subjects display also a reduction in zinc cellular uptake in comparison to young-adults perhaps due to the cellular senescence, which is less responsive to zinc because altered gene expressions of some zinc transporters (Zip1, Zip2 and Zip3) on cellular membrane occur (Giacconi et al. 2011). Alternatively, epigenetic mechanisms might occur in the promoter region of the zinc transporters leading to an hypermetylation of the gene with subsequent decreased zinc absorption in the intestinal lumen, as supposed for the zinc transporter ZnT5 (Coneyworth et al. 2009). Anyway, regardless of the mechanism involved, the zinc dietary intake and intestinal zinc absorption are deficient in aging leading to an increased risk for the appearance of degenerative age-related diseases.
Altered intestinal absorption
The amount of zinc in a meal affects zinc absorption (Lonnerdal 2000). The higher the amount of zinc in a meal, the less the fractional absorption of zinc as a percentage. With the use of radiolabelled zinc solutions in water and measuring the zinc absorption by the whole body in human adults, 40 μmol of zinc produces 73% of absorption vs. 46% when 200 μmol was administered (Sandström and Cederblad 1980). Zinc absorption also consists of a specific saturable carrier-mediated component and a nonspecific unsaturable diffusion-mediated component (Menard and Cousins 1983; Steel and Cousins 1985). Taking into account that zinc is predominantly transported via the specific saturable transport mechanism (Sandström 1992), the fractional zinc absorption decreases when dietary zinc increases, whereas the amount of zinc absorbed increases linearly at higher dietary levels. However, in presence of dietary ligands the concentration of absorbed zinc is lower (Sandström 1992). In old humans, zinc absorption, especially in the small intestine, is lower than young-adult individuals but it is independent by zinc dietary intake (August et al. 1989). Therefore, other factors can influence zinc absorption. Among them, the amount of zinc present in the intestinal lumen, the presence of dietary promoters (e.g. human milk and animal proteins) and altered physiological states have been reported (Lonnerdal 2000). Another factor influencing the zinc absorption is the presence of phytates and other minerals (iron and calcium) in the diet that may act as inhibitors binding zinc or blocking its action (Lonnerdal 2000; Hambidge 2010). Recently, a cross-over study in young and elderly healthy women using two different diets containing two phytate concentrations (phytate/Zn molar ratio = 23 (high phytate content) and phytate/Zn molar ratio = 10 (low phytate content)) for 9 days showed that phytates did not alter plasma zinc concentrations and urinary zinc excretion in both groups (Kim et al. 2007).
Although this last finding suggests that phytate may not be as important in zinc absorption, the age-related changes in intestinal architecture may play a key role. Studies in old animals have shown that the ageing process is accompanied by alterations entailing some of the following intestinal changes: alterations in villus shape (cilia), increased collagen alteration, mitochondrial changes, crypt elongation and prolonged replication time of cryptal cells (Thomson 2009). These changes might at least explain the altered zinc absorption in the elderly, as suggested by August et al. (1989) and Turnlund et al. (1982), with a reduction of 30% in old individuals with respect to the young-adult ones.
Decreased zinc absorption in elderly may also occur in large intestine owing to degenerative alterations in enterocytes and intestinal microvilli (Elmes and Jones 1980). The causes of these physio-pathological alterations in large intestine are still unknown. Anyway, regardless of these factors, when the capacity of the intestinal absorption diminishes, the zinc deficiency develops with subsequent loss of appetite, impaired immune functions followed by the appearance of many clinical evidences (Table 1), including hair loss, diarrhoea, impotence, eye and skin lesions, weight loss, delayed wound healing, taste abnormalities and mental lethargy (Evans 1986).
Mastication and changes in oral structures
Poor dentition and loss of dental pieces are common events among the elderly, while the satisfactory prosthetic replacement option decreases. The inability to masticate properly leads to several modifications in dietary models since there is a tendency to avoid and substitute certain foods often zinc rich, such as red meat or hard cheese, with other more soft zinc-poor foods, such as bread. These losses may be also generally caused by periodontal diseases, which might in turn be originated by deficits of some minerals in the diet (calcium, phosphorous and zinc) (van der Putten et al. 2009). Although few oral diseases are characteristic in the elderly, some pathological states are frequent in old individuals representing a critical factor for a correct zinc dietary intake. The most common oral diseases in old people are especially those ones related to mucosa membrane inflammation and/or atrophy, xerostomia (dry mouth), leukoplakia and malignant neoplasia (Swoboda et al. 2008). On the other hand, old subjects with mucosal erythema, stomatitis, angular chielitis and atrophic glossitis, display low plasma zinc concentrations (Sweeney et al. 1994). Therefore, an improved mastication through individual prosthetic replacement is strongly recommended in elderly in order to increase dietary zinc consumption and, at the same time, to reduce oral mucosa inflammation through an improved mastication.
Psychosocial factors
Although the energy requirement is diminished in ageing owing to low physical activity (Starling and Poehlman 2000) with subsequent higher incidence of diet-related illnesses (Drewnowski and Shultz 2001), some psychosocial factors can have a strong impact on dietary habits and ultimately low zinc dietary intake. Marital status, depression, mental status, education, socioeconomic status, dietary habits and convenience play an important role as well. Under this profile, psychological–social factors are crucial in determining the “frailty syndrome” in old people (Lang et al. 2009). An incorrect or non-intake of certain foods containing zinc together with psychological-social factors leads to determine specific phenotype biomarkers for “frailty”. In this context, high Cu/Zn ratio is a strong predictor of “frailty” and mortality in old people associated with marital status, depression, dietary habits and physical activity (Malavolta et al. 2010; Mocchegiani et al. 2011). These findings give further support to the relevance of the interrelationship among correct diet, psychological factors and physical activity for healthy ageing (Drewnowski and Evans 2001; Topinková 2008). On the other hand, increased score of depression and impaired cognitive performances were associated with low serum zinc levels in a cohort of elderly people (Marcellini et al. 2006).
Drug interactions
There are numerous cases of adverse drug/nutrition interactions in the elderly. While several drugs (i.e. cefuroxime, erythromycin ethylsuccinate and HMGCoA-reductase inhibitor lovastatin) should be taken with foods to maximise their absorption and efficacy, other drugs (ampicillin, ciprofloxacin, doxycycline and captopril) should not be taken with food in order to have an optimal absorption and efficacy of the drugs (Genser 2008). However, drug therapy may frequently interfere with digestion, absorption, utilisation or excretion of essential nutrients altering enzyme biosynthesis, coenzyme or protein transport and hormones metabolism. Moreover, medication can produce appetite, olfactory and taste abnormalities, which in turn affect the nutritional status (Genser 2008). Elderly patients usually have more than one permanent daily medication treatments leading to a high risk of interaction between drugs and zinc absorption (Basu and Donaldson 2003). One of the mechanisms by which some drugs may interfere with zinc absorption is due to the presence of oxidised Metallothioneins that, acting as antioxidant agents to protect the cells against drug toxicity, provoke a limited zinc capture by enterocytes and no storage of zinc in specific cellular organelles named “zincosomes” (Maret and Sandstead 2006). As a consequence, the absorption of intestinal zinc is strongly limited and the majority of zinc ions is excreted by urine and the zinc signals, indispensable for cell functions, are quenched (Krezel et al. 2007). Therefore, old people under prolonged drug treatments may need zinc supplement in order to reduce the risk of this interaction. However, Costarelli et al. (2008), using microarray analysis, have been recently reported the existence of positive interactions between HMGCoA-reductase inhibitors and zinc against stress and inflammation in old atherosclerotic patients, in whom some genes related to zinc and inflammatory/immune response are up-regulated or down-regulated depending on their function. In particular, HMGCoA-reductase inhibitors increase intracellular zinc ion bioavailability associated with an up-regulation of Metallothionein 2A and PPARα and a down-regulation of IL-8. Moreover, the zinc transporters ZnT6 and Zip4 are up and down-regulated, respectively, by HMGCoA-reductase inhibitors. These findings suggest, on one side, the existence of positive effects by this interaction against inflammation and oxidative stress; on the other side, they pin-point a better cellular zinc efflux/influx by zinc transporters. Therefore, the zinc–drug interaction may be also positive. The measure of intracellular zinc ion bioavailability and the effect of the drugs on individual genetic background, using microarray analysis, may be valid tools to discern between negative and positive effects of the drugs. As a result, the best individual therapy or prevention may be performed in ageing and age-related diseases.
Dietary components (zinc and other mineral and vitamin interactions)
Interaction of zinc–calcium
The interactions between zinc and other minerals are relevant for studying zinc absorption. The interaction between zinc and calcium or iron is of interest. Although the long-term use of calcium supplements has limited effect on the zinc status (Sandström 2001), old literature reports that the content of calcium in the diet might affect zinc absorption from phytate-containing meals (Oberleas et al. 1966). Ellis et al. (1987) have shown that this interference is dependent on the balance between calcium and phytate in the diet: the phytate × calcium/zinc millimolar ratios ≤200 is recommended in order to obtain an adequate zinc bioavailability from human diets. However, an excess of calcium does not seem to interfere on zinc absorption by phytate because the zinc absorption was inhibited of about only 25% by high dietary phytate (Hunt and Beiseigel 2009), suggesting that the absorption of zinc may not be as influenced by increased ingestion of calcium, even when dietary phytate is high. Other authors instead report in postmenopausal women that high calcium may interfere in zinc absorption with a negative zinc balance (measured as the difference between zinc intake and faecal zinc recovery) owing to the presence of high phytates in the diet (Wood and Zheng 1997). Although different mechanisms of zinc and calcium absorption in small intestine have been proposed through the binding with prostaglandin E and vitamin D, respectively (Song and Adham 1978), experiments in rats have however shown that calcium may interfere in intestinal zinc absorption because a competition between zinc and calcium for the same transcellular transporting carriers on the membrane surface occurs (Dursun and Aydoğan 1994).
From these findings, the effects of zinc–calcium interaction are still unclear and require further studies. Anyway, the possible presence of a negative zinc balance by high calcium intake is strongly suggestive because a negative zinc balance is already present in ageing (Mocchegiani et al. 1998), with thus a strong caution when giving calcium supplements to elderly individuals without performing a first clinical control on calcium and zinc status.
Interaction of zinc–iron
Situations that seem to also encounter problematic interactions are those related to zinc–iron interaction especially when iron is administered in solution or as a separate supplement rather than being incorporated into a meal. Studies showed that high concentrations of iron can have a negative effect on zinc absorption in human adults when zinc and iron are given in solution and on an empty stomach (Sandstrom et al. 1985). It has been suggested that suppression of zinc absorption by iron occurs when given in an aqueous medium because of a competition for common nonspecific pathways. This suppression cannot occur when iron and zinc are given during a meal because zinc can be absorbed via an alternate pathway with the aid of ligands formed during protein digestion (Whittaker 1998). Anyway, an antagonism between iron and zinc exists because both of them use for their transport the divalent metal transporter-1 (DMT1) found in enterocytes of the small intestine (Gunshin et al. 1997). Experiments conducted in Caco-2 cells in presence or absence of foetal bovine serum in the incubating medium have shown that the apical uptake of 65Zn+2 was significantly reduced in presence of iron and serum, suggesting that Fe interferes with the absorption of Zn. The absorption of 55Fe+2 was also decreased by excess iron, both in presence and absence of serum. Only in absence of serum, however, the reduction in Fe absorption correlated with a decrease in DMT1 expression (Tallkvist et al. 2000). This study implies that Zn uptake may be independent by the DMT1 mechanism. Other mechanisms may be involved. A family of human intestinal Zn transporters (Zip family) was recently identified. Zip14 seems the more involved in zinc and iron uptake in the gastrointestinal tract (Liuzzi et al. 2006; Cousins 2010).
Interaction of zinc–copper/selenium
Zinc also interferes with other micronutrients, such as copper and selenium, in which Metallothioneins (MT) are engaged. Since zinc induces the synthesis of MT, copper entering the cell displaces zinc from the protein because copper is more tightly bound to MT than zinc. This bound copper becomes unavailable for transfer out of the cell thereby decreasing zinc absorption (Cousins 1985). With regard to selenium, the interaction with zinc is of benefit because selenium through glutathione peroxidise reduces oxidised MT with subsequent zinc release by MT and antioxidant effect of free zinc ions, via activation of antioxidant zinc-dependent enzymes (superoxide dismutase) (Maret 2003). Of interest is the s-glutathionylation of MT that it may occur under nitrosative and oxidative stress in ageing leading to oxidised MT in the thiol groups. Glutathione, via selenium, reverses this phenomenon leading to functional MT with possible re-release of zinc by MT (Casadei et al. 2008).
Interaction of zinc–vitamins
Finally, of interest is the interaction of zinc with some vitamins (A, D and E) because zinc affects the transporters of the vitamins, as for example retinol-binding protein for vitamin A (Smith 1980) or tocopherol for vitamin E (Bunk et al. 1989). The interaction between zinc and vitamin D is suggestive because zinc participates in the constitution of vitamin D (especially D3 isoform) receptor DNA binding domain through two zinc finger-like motifs (Freedman and Towers 1991), favouring the re-absorption of calcium at kidney level and phosphorus at intestinal level. Therefore, the interaction between zinc and vitamin D is also crucial for a good functioning of many organs and tissues, including brain and bone.
Absorption of zinc (subcellular processes)
Absorption can be considered as the processes of influx into the enterocyte and through the basolateral membrane and of transport into the portal circulation. The subcellular mechanisms involve zinc transporters, MT and the transmembrane transporter DMT1.
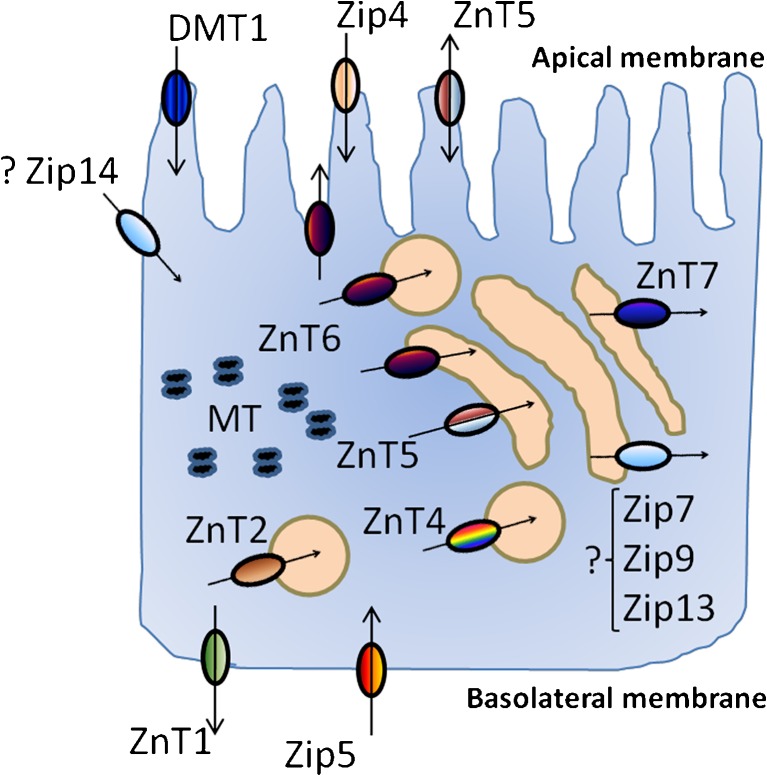
Two recently identified families of zinc transporter proteins, ZnT (SLC30) and Zip (SLC39), are responsible in maintaining intracellular zinc homeostasis. The prevailing view of zinc transporter functionality is that transporters in the ZnT family function to reduce cytosolic zinc concentration, either by efflux across the plasma membrane or by intracellular sequestration in subcellular compartments (Palmiter and Huang 2004). Zip family transporters function acts in the opposite direction, to increase cytosolic zinc concentration (Eide 2004). Effects of ageing on zinc homeostasis and dietary requirements mediated through effects on zinc transporters are most likely to be through transporters with a particular direct role in intestinal absorption and/or endogenous secretion. Current evidence indicates that, within the ZnT family, ZnT1, ZnT5 and ZnT6 may be of particular importance in intestinal zinc transport processes. The localisation of ZnT1 to the basolateral membrane of the intestinal enterocyte in rat and mouse (McMahon and Cousins 1998; Yu et al. 2007), coupled with its function in reducing cytosolic zinc concentration (Palmiter and Findley 1995), indicates a role for ZnT1 in the efflux of zinc absorbed from the intestinal lumen across the basolateral enterocyte membrane.
The same task also occurs for ZnT5 and ZnT6 that act also in the zinc uptake across the enterocyte apical membrane, other than efflux direction (Cragg et al. 2005). The localisation and functional and regulatory properties of Zip4 and Zip5 indicate that they play a particular role in the absorption of dietary zinc. Zip4 is expressed at the apical enterocyte membrane in mouse at increased levels in animals fed a zinc-deficient when compared with a zinc-replete diet (Dufner-Beattie et al. 2003), consistent with a role in dietary zinc absorption. Mutations in Zip4 are associated with the human zinc deficiency disease, such as acrodermatitis enteropathica, which displays a severe reduction in zinc uptake (Kury et al. 2002). Zip5 is believed to play a role in zinc homeostasis through regulated endogenous secretion, by mediating the transport of zinc from the serosa into the enterocyte. This view is supported by the expression of Zip5 at the basolateral membrane in the zinc-replete, but not zinc-deficient, mouse small intestine (Dufner-Beattie et al. 2004; Wang and Zhou 2010). Although the specific localization in enterocyte (basolateral or apical membrane) is still unclear, another zinc transporter Zip14, expressed in duodenum and jejunum and mediated by IL-6, plays a relevant role because involved in zinc and iron uptake with a task in regulating compensatory mechanisms in order to avoid iron overload that can be toxic in ageing. Suppression of endogenous Zip14 expression by using Zip14 siRNA reduces the uptake of both iron and zinc, suggesting the peculiar role played by Zip14 in the uptake of both minerals (Liuzzi et al. 2006). However, it is currently unclear how ageing affects the function and the gene expression of zinc transporters in enterocytes. It may be supposed that it might depend by a less efficient membrane localization of these proteins or related to an impaired activity of the zinc transporters with advancing aging due to the presence of chronic inflammation. Such an assumption may be supported by the findings in experimental models of chronic inflammation (airway inflammation) showing a positive correlation between altered mRNA zinc transporters (Zip1, Zip14, Zip4 and ZnT4) and high gene expression of macrophage, monocyte, eosinophil inflammatory-related proteins (cc16, cc18, cc19 and cc111) (Lang et al. 2007). Alternatively, DNA methylation, known as an epigenetic event and modified by age, might alter the gene expressions. Age-related modifications in the promoter methylation status of specific Zn transporters, as supposed for ZnT5, may contribute to diminished Zn absorption with age. Since there is a CpG island in the ZnT5 gene promoter region, the expression of ZnT5 may potentially be regulated by its promoter methylation status. Preliminary studies in three adult cohorts from northern England show correlations between methylation of specific CpG sites in the ZnT5 promoter and age, supporting the hypothesis that the age-related reduction in ZnT5 expression in the intestine may contribute to the decline in Zn status observed with ageing (Coneyworth et al. 2009). The expression of other Zn transporters involved in dietary Zn absorption may also be potentially regulated by DNA methylation. Indeed, a CpG island is present within the promoter region of ZnT1 (Balesaria and Hogstrand 2006). Thus, it is reasonable to speculate that ZnT1 expression may be modified as a consequence of age-related changes in DNA methylation status. Despite of these studies, no definitive deduction may be still made either on the effect of ageing on zinc transporter functions in enterocytes or on gene regulatory responses to zinc. In this last context, however, a double-blind, randomised, crossover trial in adult-old humans (examining zinc transporter responses in the small intestine to zinc supplement (25 mg day−1 14 days−1 as zinc sulphate)), reveals a possible effect of age with a normalization of zinc transporters in old individuals. A down-regulation of ZnT1, ZnT5 and Zip4 in intestinal mucosa was observed (Cragg et al. 2005). These findings are consistent with the homeostatic response to the zinc supplement in order to prevent the absorption of surplus of zinc, as also shown in Caco-2 cells adding high zinc concentrations (200 μM) (Cragg et al. 2005).
The role of MT in the regulation of zinc absorption, particularly in conjunction with the zinc transporters, has been also studied. Hepatic and intestinal MT synthesis is stimulated by dietary zinc supplementation, by intraperitoneal zinc injection and by the acute phase response. Dietary restriction also results in diminished MT synthesis. Experiments in MT knockout mice show that the rise in serum zinc after a single dose of zinc was much greater than in the control animals. In contrast, the serum zinc response of the MT transgenic animals was blunted when compared with control animals. The expression of ZnT-1 was also found to be directly related to serum zinc levels but unaffected by MT levels (Davis et al. 1998). Thus, MT may work in cellular responses to limit free zinc concentrations within quite narrow ranges (Cousins 1996) and to act as a zinc pool (Mocchegiani et al. 2008d). On the other hand, recent data have shown that in vitro zinc supply in lymphocytes from old donors restores the gene expression of MT (Mazzatti et al. 2007), suggesting a role of MT in normalizing intracellular zinc homeostasis and, at the same time, in maintaining its redox properties for antioxidant function. Another transporter potentially involved in zinc and other metal uptakes is DMT1: a transmembrane polypeptide found in the duodenum in the crypts and lower villi and responsible for the uptake of several metal ions (McMahon and Cousins 1998). As these transport proteins are identified and characterised, further investigations in the whole animal as well as in humans under conditions of a range of dietary intake, are however necessary in order to elucidate the amount of absorbed zinc to the amount of excreted zinc together with these subcellular processes. Figure 1 reports the main proteins involved in zinc absorption (influx), intracellular zinc homeostasis and zinc efflux in enterocytes.
Fig. 1.
Zinc transporter pathways, zinc carrier (DMT1) and MT in a polarised enterocyte. Zip4 and Zip5 are involved in zinc influx and efflux, respectively. ZnT5 in both influx and efflux. ZnT1 in zinc efflux. ZnT6 is involved in various tasks: zinc efflux and zinc storage in specific vesicles named “zincosomes” and in Golgi apparatus. ZnT2 and ZnT4 in zinc storage in zincosomes. ZnT7 in zinc storage in Golgi apparatus. DMT1 is involved in zinc uptake. MT are involved in intracellular zinc homeostasis for zinc signalling. Zip4, ZnT5, and DMT1 are present in apical membrane, whereas ZnT1 and Zip5 in basolateral membrane. The specific localization of Zip14, involved in zinc and iron uptake in enterocyte, is still unclear and remains to be established as well as the specific function of Zip7, Zip9 and Zip13 expressed in the Golgi apparatus of enterocyte (question marks)
Zinc status of the elderly
Although the upper limit of the dietary zinc intake has not to exceed 25–40 mg/day (Food and Nutritional Board 2001; SCF 2002), the RDA for zinc in young-adult individuals and older is 11 mg/day for men and 8 mg/day for women (Maret and Sandstead 2006). An uptake below the RDA can only be seen as an indicator of potential zinc deficiency, because many other factors also play a role in decreased zinc intake. Hence, it is necessary to analyse the zinc status of each individual. The parameter of choice is often serum or plasma zinc. However, this is not the ideal parameter to determine the zinc status taking into account that many old individuals, despite increased pro-inflammatory cytokines known as factors for zinc depletion (Shankar and Prasad 1998), display circulating plasma zinc levels within the normal range (about 85–90 μg/dl) (Mocchegiani et al. 2003). Other parameters may be useful to test the zinc status, such as for example the intracellular zinc ion bioavailability with specific zinc probes (Haase et al. 2006a) and the testing the capacity of zinc release by MT using NO-donors (Mocchegiani et al. 2006). Recently, the determination of the zinc score has been validated in ZINCAGE project (based on the determination of the zinc content in the foods and the individual quantity of the food intake). This score may represent a valid test for determining the zinc status being well correlated with the age-dependent plasma zinc levels (Kanoni et al. 2010). However, a plethora of studies have reported that, in general, plasma zinc levels decrease with advancing age (Haase et al. 2006b) as well as in some cell types, such as erythrocytes and lymphocytes (Prasad et al. 1993; Andriollo-Sanchez et al. 2005). These findings have been confirmed by data from the second NHANES, in which serum zinc levels increased into the third decade of life and declined from that age (Hotz et al. 2003). Such a decrement may depend on dietary habits and life style conditions (Mocchegiani et al. 2008a), and it varies from country to country where zinc deficiency can be more or less severe or marginal. The European Nutrition and Health Report summarise data regarding the nutritional zinc uptake in elderly from Austria, Denmark, Germany, Hungary and the UK, where the zinc uptake is particularly low in UK elderly (Fabian and Elmadfa 2008). Recently, it has been reported that in Italy and France the zinc dietary intake by different foods may be sufficient in maintaining a satisfactory zinc status with however marginal zinc deficiency in old people. By contrast, it is insufficient in Greece elderly coupled with severe zinc deficiency and enhanced inflammatory status in comparison with elderly people living in France, Italy and Germany (Mocchegiani et al. 2008a). Other studies in a large number of middle-aged (age range, 55–70 years) individuals from Italy, France (Andriollo-Sanchez et al. 2005) and old ones (age range, 70–85 years) from Germany (Volkert et al. 2004) have reported a marginal zinc deficiency and the zinc dietary intake quite similar to that one recommended by RDA (10–12 mg/day) both in male and female. Also in the USA, one study in a large number of old individuals (age range, 60–90 years) showed that the marginal zinc deficiency appeared in more than 90% of old subjects despite the zinc dietary intake is quite similar to RDA (11.5 mg/day) both for men and women (Ma and Betts 2000). Instead, a study performed in old institutionalised individuals (age range, 65–98 years) in León (Spain), showed severe zinc deficiency coupled with lower zinc dietary intake (9.1 mg/day) than that one recommended by RDA (Villarino Rodríguez et al. 2003).
Although the majority of these studies reports that the zinc dietary intake may be satisfactory in many Countries because meeting the dose recommended by RDA, old people of both sexes display marginal zinc deficiency. Since the “Mediterranean diet” is variegated and contains foods (especially fish) rich of zinc (Sofi et al. 2010) usually consumed in Italy and France and some foods rich of zinc (read meat and legumes) are consumed in Northern European countries and in the USA, might in part justify the quite sufficient zinc dietary intake and the relative marginal zinc deficiency in elderly. But, it does not explain the reason of a remarkable subgroup in all countries of elderly subjects who achieve “successful ageing” (centenarian subjects) without suffering from age-related diseases despite of the presence low zinc dietary intake and zinc deficiency (Mocchegiani et al. 2003). Taking into account that severe zinc deficiency is strictly related to the chronic inflammation (Mocchegiani et al. 2006; Prasad 2009), the reason may be related to a lower inflammatory status in centenarians with subsequent still capacity in zinc release by MT, suggesting that the available quota of free zinc ions, despite reduced, is still sufficient to maintain good performances in immune response and antioxidant activity (Mocchegiani et al. 2003), furtherly confirming the relevance of zinc for immunosenescence and in keeping under control the inflammation.
Zinc and immunosenescence
Ageing is a continuous multidimensional process of physical, psychological and social changes that compromises the normal functioning of various organs and systems, including several immunological alterations named immunosenescence, which is characterised by increased susceptibility to infections, autoimmune diseases and cancer (Pawelec et al. 2010). The immune efficiency decreases with advancing aging, starting around 60–65 years. Alterations of the immune system during ageing and zinc deprivation show many similarities, indicating the existence of a strict relationship between immunosenescence and zinc deficiency (Mocchegiani et al. 1998; Bogden 2004). The similarity is in adaptive and innate immunity as well as in neutrophil functions (chemotaxis, phagocytosis, oxidative burst). Although the total number of neutrophils is not different between old and young-adult subjects, phagocytosis, oxidative burst, and intracellular killing are impaired in ageing and neutrophils from the elderly show reduced chemotaxis and a lower resistance to apoptosis, as shown by impaired anti-apoptotic effects after specific stimuli (lipopolysaccharide (LPS), G-CSF and GM-CSF) (Schroder and Rink 2003). In this context, zinc may play a key role because a satisfactory intracellular zinc ion bioavailability preserves the oxidative burst by neutrophils, via reduction of IL-6 signalling, as observed in centenarians, who in turn display satisfactory intracellular zinc content and low grade of inflammation (Moroni et al. 2005).
Adaptive immunity
With regard to adaptive immunity, the plasma concentrations of IL-6, IL-8, MCP-1, MIP-1α and TNF-α were positively correlated with age with a progressive elevation in very old age (Mariani et al. 2006). Th1 (IFN-γ and IL-2) cytokines decrease whereas Th2 (IL-4 and IL-10) cytokines increase (Cakman et al. 1996). The same trend was also observed after LPS stimulation (Gabriel et al. 2002; Cakman et al. 1997). These alterations in Thl and Th2 cytokine productions lead to an imbalance of Th1/Th2 paradigm with a shift towards Th2 production and subsequent chronic low grade of inflammation, named “inflammaging” (Franceschi 2007). Alterations in the balance of Th1/Th2 cytokines also occurs in zinc deficiency (Uciechowski et al. 2008) that is characterised by decreased IFN-γ, IL-2 and TNF-α production (Th1 cells) and increased IL-6 production by Th2 cells and macrophages (Mocchegiani et al. 1998). However, in very old age, the high levels of IL-6 are not so detrimental because of the low gene expression of IL-6 subunit receptor (gp130) that allows the presence of an inactive quota of IL-6 with subsequent reduced inflammation and good intracellular zinc content (Moroni et al. 2005).
T cell functions
A similarity between ageing and zinc deficiency exists also in T cell pathway. The more characteristic T cell pathway abnormalities in ageing are: (a) reduced T cell proliferation in response to T cell receptor or CD3 or mitogen stimulations (Pawelec et al. 1998); (b) altered CD4/CD8 ratio (Pawelec et al. 1998); (c) higher expression of CD95 (Fas) and lower expression of BCL-2 and p53 leading to increased apoptosis (McLeod 2000); (d) lower number of naïve (CD45RA+) and a higher number of activated memory (CD45RO+) T cells (Gregg et al. 2005); and (e) thymic involution producing reduced number of naïve T cells and also immature because of the lack of thymic hormone activity required for T cell maturation and differentiation (Arnold et al. 2011). The same age-related T cell pathway alterations also occur in zinc deficiency (Mocchegiani et al. 1998; Dardenne 2002) as well as thymic involution, regardless of age, due to increased thymocyte apoptosis provoked by elevating glucocorticoid production and by the negative regulatory function of zinc in immune cell apoptosis (Taub and Longo 2005). Zinc supplementation in old mice increases the thickness of the thymic gland (especially cortical part) (Sbarbati et al. 1998) and restores the number of viable thymocytes and serum thymic hormone (thymulin) activity (Dardenne et al. 1993; Mocchegiani et al. 1995). Thus, the zinc deficiency in the elderly may also contribute to the thymic involution by augmenting apoptosis during T cell maturation and differentiation, as observed in old (Provinciali et al. 1998a) and in young zinc-diet deprived mice (King et al. 2002). On the other hand, the thymic output (measured using T cell receptor rearrangement excision circles) is strongly reduced during ageing and in zinc deficiency leading to a reduced number of naïve mature T cells in the circulation with subsequent inability to substitute activated memory T cells that undergo to apoptosis after exposure to “foreign” antigens (Mitchell et al. 2006). By contrast, in centenarian subjects with a satisfactory zinc pool (Mocchegiani et al. 2003), the thymic output is still sufficiently maintained by IL-7 (Nasi et al. 2006), and IL-7 and its receptor act via zinc finger protein Miz-1 and SOCS1 (Saba et al. 2011), which the latter is in turn regulated by another zinc finger protein TRIM8/GERP (Toniato et al. 2002). Therefore, zinc is relevant in ageing for thymic output signalling with possible new T cell maturation and differentiation.
Innate immunity
Of particular interest is the involvement of zinc in innate immunity, such as NK cells, NKT cells and their cytotoxicity in ageing. The total number of NK cells and their percentage among circulating cells is increased in old people, but this effect is compensated by a reduced cytotoxic activity and reduced proliferation in response to IL-2 (Solana and Mariani 2000; Mocchegiani et al. 2009). Because the main functions of NK cells are the elimination of cancer- or virus-infected cells, the higher incidence of viral infections and cancer in the elderly may well be related to impairment of NK cell function. In this context, the role played by zinc may be pivotal. First of all, zinc may affect the new production of NK cells by stem cells. Zinc in vitro (10 μM) improves the development of CD34+ cell progenitors towards NK cells both in young (expressing CD56+ CD16− phenotype) and old age (expressing CD56− CD16+ or CD56+ CD16+ phenotypes), via increased expression of GATA-3 transcription factor (Muzzioli et al. 2009). Moreover, several studies in old animals and humans describe decreased NK cell cytotoxicity related to zinc deficiency (Mocchegiani et al. 2009) through different mechanisms involving NF-kB or Ap-1 transcriptional factors or A20 protein (Prasad 2007; Bao et al. 2010). In vitro (1 μM) and in vivo zinc treatments (12 mg Zn++/day) for a short period (1 month) induce complete recovery of NK cell cytotoxicity both in old mice and humans (Mocchegiani et al. 1995; Mariani et al. 2008). In addition, a physiological zinc treatment (15 Zn++/day)for 1 month in old infected patients, other than an increased NK cell cytotoxicity, recovers the IFN-γ production leading to 50% reduction of infection relapses (Mocchegiani et al. 2003). Findings in centenarians and in very old mice confirm the relevance of zinc in restoring NK cell function in elderly. Indeed, they have a well preserved NK cell cytotoxicity, a good zinc ion bioavailability and satisfactory IFN-γ production (Mocchegiani et al. 2003; Miyaji et al. 2000). A very intriguing aspect is the NKT cells bearing TCRαβ or TCRγδ that are the first defence of the organisms against virus and bacteria from early in life. NKT cells produce Th1 (IFN-γ) and Th2 (IL-4) cytokines and are functionally linked to NK cells, via IFN-γ (Biron and Brossay 2001). A dysregulation in IL-4 production by NKT cells leads to pathology, as it occurs during a chronic inflammation and autoimmune diseases (Araujo et al. 2004). However, the main task of NKT cells is to produce IFN-γ with thus a pivotal role in anti-tumour cytotoxic response (Cui et al. 1997). NKT cells have been found in the thymus, liver, spleen and bone morrow. Despite the existence of a thymus-independent differentiation pathway located in the liver for NKT cell lineage, as demonstrated in athymic nude mice, the thymus is also a site for NKT development, and the liver for NKT homing (Emoto and Kaufmann 2003). During ageing, the thymus is atrophic. Therefore, the liver extrathymic function becomes prominent in order to compensate the thymic failure during ageing (Abo et al. 2000). Therefore, liver NKT cell function becomes relevant in ageing for host defence. Zinc also improves the liver NKT cell (mainly bearing TCRγδ) cytotoxicity in old and in very old mice, suggesting that good zinc ion bioavailability and the function of these types of NKT cells are fundamental to achieve successful ageing (Mocchegiani et al. 2004).
Humoral immunity
With regard to humoral immunity, changes during aging are also found with a reduction in B cell number. Such a reduction seems to be not affected by zinc deficiency, via apoptosis mechanisms (King et al. 2005). However, increased immunoglobulin productions (IgA and IgG subclasses) have been observed (Paganelli et al. 1992) and the response to vaccination with several antigens is instead diminished due to an impaired interaction with T helper cells (Weksler and Szabo 2000). In this context, zinc might be relevant in affecting humoral immunity through its influence in cytokine production by T cell repertoire, (IL-6, IFN-γ), suppressing the release of IL-6 (von Bulow et al. 2005) and promoting IFN-γ release by PBMCs (Driessen et al. 1994). Although the role of zinc in humoral immunity is still unclear, it does not exclude the relevance of zinc for a correct inflammatory/immune response against external noxae. In particular, the zinc–gene (IL-6) interaction is pivotal in keeping the inflammatory/immune response under control with subsequent longevity, indicating this gene as “robust” for “healthy ageing” (Mocchegiani et al. 2006).
Zinc–interleukin-6 (IL-6) gene interaction
IL-6 −174G/C locus variability has been suggested: (1) to be capable of modulating on one hand the individual susceptibility to common causes of morbidity and mortality among elderly and (2) to play a crucial role in longevity (Franceschi et al. 2005). Therefore, the genetic variations of this locus of IL-6 gene are fundamental in elderly population in order to better understand the intrinsic causes of the longevity. The association of these genetic variations to the possible different immune responses is an attractive focus in elucidating the molecular mechanisms involved in immunosenescence.
The genetic variations of the IL-6 −174G/C locus have been extensively studied by different groups with, however, contradictory data. Bonafe et al. (2001) studied IL-6 promoter genetic variability at the −174C/G locus and its effect on IL-6 levels in Italian 700 people aged 60 to 110 years, including n. 323 centenarians. Individuals who are genetically predisposed to produce high levels of IL-6 during ageing, i.e. C− men (GG genotype) at IL-6 −174C/G locus, are disadvantaged for longevity. On the other hand, the capability of C+ individuals (CC and CG genotypes) to produce low levels of IL-6 throughout life span appears to be beneficial for longevity, at least in men. The women have, conversely, high IL-6 serum levels later in life with respect to men independently from −174C/G locus polymorphism (Bonafe et al. 2001). The inhibitory tone of estrogens on IL-6 gene expression could explain the gender difference (Bruunsgaard et al. 1999), assuming that its long-term effects last until the extreme limits of human life-span.
The major production of IL-6 in C− subjects for the whole life, including centenarians, has been also confirmed by other in vivo longitudinal studies (Rea et al. 2003). A more recent study in old and nonagenarian subjects has confirmed that IL-6 production is higher in C− carriers and that these subjects are prone to contract one of the more usual age-related inflammatory pathologies, such as atherosclerosis (Giacconi et al. 2004). Interestingly, in this last study C− old and nonagenarian subjects display also impaired innate immune response (NK cell cytotoxicity), increased MT, zinc deficiency and low zinc ion availability in comparison to C+ carriers (Giacconi et al. 2004). These findings clearly suggest that the genetic variations of the IL-6 −174G/C locus play a key role for the longevity at immune functional level. Moreover, they suggest that the determination of the genetic variations of the IL-6 −174G/C locus associated to a comprehensive evaluation of the zinc status are an useful strategy to identify old subjects who can benefit of zinc supplementation without health risks (see below “Effect of zinc supplementation in immunosenescence on the basis of IL-6 genetic background”).
Potential usefulness of Zn supplementation in elderly subjects
Since zinc deficiency may alter age-associated health changes, zinc supplementation is of critical relevance because helping to prevent certain age-related diseases due to the effect of zinc upon the immune functions, metabolic harmony and antioxidant effect with an extension of also the maximum life span, at least in mice (Mocchegiani et al. 2007). In this respect, the National Eye Institute has reported that zinc significantly reduces the odds of developing advanced age-related diseases, reducing oxidative stress in elderly subjects (Clemons et al. 2004). Various mechanisms of action of zinc upon the immune system have been well elucidated. Both direct and indirect mechanisms of zinc are involved in affecting the immune response in ageing from inducing DNA proliferation, to maintaining membrane stability, to restoring Th1/Th2 paradigm up to preventing apoptosis, via activation of nuclear factors NF-kB and AP-1, caspase-3 inhibition or induction of A20 protein with subsequent reduction of pro-inflammatory cytokines (Table 2) (Shankar and Prasad 1998; Mocchegiani et al. 2000; Prasad 2007; Bao et al. 2010; Prasad et al. 2011). The most intriguing mechanism of zinc upon the immune system is however the existing interrelationship among pro-inflammatory cytokines (IL-6)-Metallothioneins (MT)-Nitric Oxide (NO)-PARP-1 taking into account that IL-6 promotes MTmRNA induction, NO is involved in zinc release by MT and PARP-1 (a nuclear enzyme codified by two zinc finger motifs) prevents apoptosis with subsequent cellular genomic stability (Mocchegiani et al. 2000). Alterations or changes in the various steps of these mechanisms owing to zinc deficiency, as it occurs in ageing, leads to the appearance of some degenerative age-related diseases, including infection and cancer (Mocchegiani et al. 2006). Therefore, zinc supplementation may be of benefit in prevention or in reducing the risk or in delaying the appearance of diseases and the subsequent disability.
Table 2.
Main biochemical pathways of zinc relevant to immunosenescence
| Direct effects | Indirect effects |
|---|---|
| DNA-RNA polymerases activation | |
| Thymidine–kinase activation | |
| Terminal deoxyribonucleotidyl transferase activation | |
| Ornithine decarboxylase activation | |
| Ecto-5 nucleotidase activation | |
| Protein kinase-C activation | Endocrine cell activation (pineal gland, thyroidgland, adenohypophysis and Beta-cells of pancreas) |
| Membrane stability (competing with thiols) | Hormone receptor superfamily activation (melatonin, growth hormone, nerve growth factor, insulin, IGF-I and thyroid hormones) |
| Transcriptional factor activation (NF-kB and AP-1) | Metallothioneins-nitric oxide-PARP homeostasis |
| Activation of A20 protein | |
| IL-2 receptor activation | |
| Apoptosis prevention | |
| Thymulin activation (ZnFTS) | |
| Balance of Th1:Th2:Th3 paradigm (with subsequent cytokine production) | |
| MHC class II restricted activation | |
| p56lck autophosphorylation |
Evidences of the usefulness of zinc supplementation for immunity in the elderly began to emerge three decades ago with however inconsistent data on its beneficial effect upon the immune efficiency due to different doses and duration of zinc treatment as well as the form of zinc to be supplemented (Mocchegiani et al. 2008d). Although zinc was used at the doses recommended by RDA (from 10 up to 40 mg/day) (U.S. Department of Agriculture 1996) improvements of both innate and adaptive immune responses are not so exciting and also contradictory. However, the best results were obtained when zinc is supplemented at the doses recommended by RDA as zinc gluconate or aspartate or acetate, less when zinc was used as zinc sulphate (Haase and Rink 2009b). No effects on cell-mediated and humoral immunity were observed when zinc was used at high doses and as zinc sulphate (90 mg/day for 3–6 months) (Provinciali et al. 1998b; Stewart-Knox et al. 2005). Anyway, improvements upon the immune system in general occur with however many limitations. An exhaustive picture of the zinc supplementation in elderly by different studies has been recently reported by Haase and Rink (2009b).
From all the studies, physiological doses of zinc applied for a long period or high doses of zinc for short periods might induce limited effects on the immune response perhaps due to a zinc accumulation in various organs and tissues with subsequent toxic effect of zinc upon the immune functions (Sandstead 1995). In this context, it is also useful to remind that high doses of zinc trigger apoptosis of the immune cells in presence of high oxidative stress and inflammation (Fraker and Telford 1997). Therefore, caution is advised for the management of zinc supplementation with the suggestion to perform the trial for short periods and on alternate cycles only. Following this concept, zinc treatment (even if as zinc sulphate form) at the dose of 15 mg Zn++/day for 1 month at alternating cycles in Down’s syndrome subjects, in elderly and in old infected patients restores thymic endocrine activity, lymphocyte mitogen proliferative response, CD4(+) cell number, NK cell cytotoxicity, pro-inflammatory cytokine production (Mocchegiani et al. 2003; Franceschi et al. 1988; Kahmann et al. 2006; Kahmann et al. 2008) and DNA repair (Chiricolo et al. 1993). Clinically, significant reductions of relapsing infections occur in Down’s syndrome subjects (Licastro et al. 1994), in elderly and in old infected patients (Mocchegiani et al. 2003; Prasad et al. 2007).
An intriguing point of the zinc supplementation is the augmented gene expression of some zinc transporters. Elderly women treated with zinc gluconate (22 mg−1 day/27 days−1) display significant increases of ZnT1 gene expression in peripheral leukocytes (Andree et al. 2004), even if the gene expression of the zinc transporters is sensitive in relation to the immune cells considered (Whitney et al. 2003). Such increases of ZnT1 have been also observed in human lymphoblastoid cells adding in vitro 50 or 100 μmol/L of zinc (Andree et al. 2004), furtherly suggesting the relevance of zinc supplementation in affecting the gene expression of zinc transporters and, consequently, the correct maintenance of intracellular zinc homeostasis. For example, zinc supplementation might be important to restore ZnT8 gene expression in pancreatic vesicles being involved in the aetiology of type 2 diabetes (Sladek et al. 2007): a pathology related to ageing, zinc deficiency and altered immune response (Mocchegiani et al. 2008c).
Since zinc affects also the cytotoxicity of liver NKT cells bearing TCRγδ (extrathymic T cell pathway) with higher production of IFN-γ in old mice (Mocchegiani et al. 2004), the presence of satisfactory zinc ion bioavailability, coupled with increased NKT cell cytotoxicity and enhanced IFN-γ production in centenarians with respect to elderly (Mocchegiani et al. 2003; Miyaji et al. 2000), strengthens the pivotal role of zinc supplementation in maintaining or improving the global immune response (thymic and extrathymic T cell pathways) and in fighting oxidative stress and inflammation. Some authors have also shown that zinc supplementation in combination with other micronutrients may enhance immunity without interfering on vitamin metabolism. Zinc supplementation (15 or 30 mg/day as zinc gluconate for 6 months) has no deleterious effects on folate or vitamin B12 (Ducros et al. 2009) or vitamin E status increasing instead vitamin A (Intorre et al. 2008) in healthy free-living old subjects (age range, 55–85 years). In this context, however, it is useful to remind that vitamin and mineral supplements (including zinc) in older women are associated with increased total mortality risk compared with non-users (Mursu et al. 2011; Bjelakovic and Gluud 2011). This phenomenon may be related to more enhanced copper than zinc (Mursu et al. 2011) leading to a possible imbalance of Cu/Zn ratio, which is in turn a high mortality risk factor especially in older women (Mocchegiani et al. 2011). Therefore, the doses of minerals to use for supplements has to be at physiological doses (for zinc = 10–12 mg/day) in order to maintain a correct balance among minerals because of their close interactions (see “Dietary components (zinc and other mineral and vitamin interactions)”). Otherwise, an imbalance may occur with an excessive accumulation of some minerals, such as zinc, that is toxic because it may lead to an abnormal activation of some zinc-dependent enzymes, such as PARP-1, or favouring the entering of excessive calcium into the cells, with subsequent cell death in both cases (Mocchegiani et al. 2000; Frazzini et al. 2006).
Moreover, it is also important to highlight that zinc also affects MT gene expression (Maret 2003). Therefore, the question arises whether zinc supplementation in old age may further increase MT, with more limited zinc release by MT, as it usually occurs in old age (Mocchegiani et al. 2006). This fact may be avoided because zinc lowers the inflammation (Haase and Rink 2007) and, as such, MT can be still able to release zinc with subsequent good immune performances (Cipriano et al. 2006). Therefore, the potential limited zinc release by MT may be excluded during physiological zinc supplementation in ageing.
Effect of zinc supplementation in immunosenescence on the basis of IL-6 genetic background
One possible cause of the discrepancy existing in literature on the effect of zinc supplementation upon the immune response in elderly may be the choice of old subjects who effectively need zinc supplementation in strict relationship with dietary habits and inflammatory status (Mocchegiani et al. 2007). This fact is supported by the discovery that old subjects carrying GG genotypes (named C− carriers) in IL-6 −174G/C locus display increased IL-6 production, low intracellular zinc ion availability and impaired innate immune response (Mocchegiani et al. 2008b). By contrast, old subjects carrying GC and CC genotypes (named C+ carriers) in the same IL-6 −174 locus display satisfactory intracellular zinc as well as innate immune response. However, the most intriguing finding is that male carriers of C+ allele are more prone to reach centenarian age than C− carriers. Therefore, old C− subjects are more prone for zinc supplementation than old C+ carriers. Zinc supplementation in old C− subjects restores NK cell cytotoxicity to values present in old C+ carriers and considerably improves zinc status (Mocchegiani et al. 2008b; Mariani et al. 2008). The benefit of the zinc supplementation in old people selected on the basis of IL-6 polymorphism is also observed for some other immune parameters (Table 3). The inflammation is best kept under control in C− carries after zinc supplementation because especially reducing the gene expression of genes related to the inflammation, such as IL-1 and its receptor (Mazzatti et al. 2008) and improving, in general, the balance between Th1 (IFN-γ) and Th2 (IL-10) cytokine production (Kahmann et al. 2008). As such, the organism is more prompt to respond to external noxae with a satisfactory inflammatory/immune response, via a better JAK/STAT signalling (Varin et al. 2008). Although further studies according to IL-6 polymorphism are needed, these results open the hypothesis that the daily requirement of zinc might be different in elderly harbouring a different genetic background with thus a specific and personalised dietary zinc intake.
Table 3.
Effect of zinc supplementation on immunosenescence in elderly according to IL-6 polymorphism
| Parameter | Effect | Reference | |
|---|---|---|---|
| Plasma cytokines/chemokines | IL-6, IL-8, TNF-α and MIP-1α | –↑ | Mariani et al. (2008) and Mocchegiani et al. (2008b) |
| MCP-1 and RANTES | – | Mariani et al. (2008) | |
| Immune functions | NK cell cytotoxicity | ↑↑ | Mocchegiani et al. (2008b) and Mariani et al. (2008) |
| Cytokines (IL-1ra) gene expression | ↓ | Mazzatti et al. (2008) |
The dose of zinc used was 10 mg/day of zinc aspartate (Unizink 50, KOHLER PHARMA Corp., Alsbach-Hahnlein, Germany) for 45 days
“↑↑” strongly increased, “↑” increased, “–” not modified, “–↑” slightly increased at least in some sub-groups, “↓” decreased
Conclusions and future perspectives
Zinc deficiency in elderly, resulting mainly from the reduced zinc dietary intake together with some age-related factors (intestinal absorption, mastication, psychosocial factors, drugs interactions and subcellular processes), could compromise immune functions leading to the appearance of some degenerative diseases. Since zinc deficiency is a common event in the elderly, several researchers have documented the impact of zinc supplementation in old people in order to restore the zinc status and, as such, to prevent the disability caused by the diseases. Clinical evidences have also suggested that zinc-rich foods, as occurring in the Mediterranean diet, may be useful in the prevention of zinc deficiencies in old people, as shown in some European countries (Italy and France).
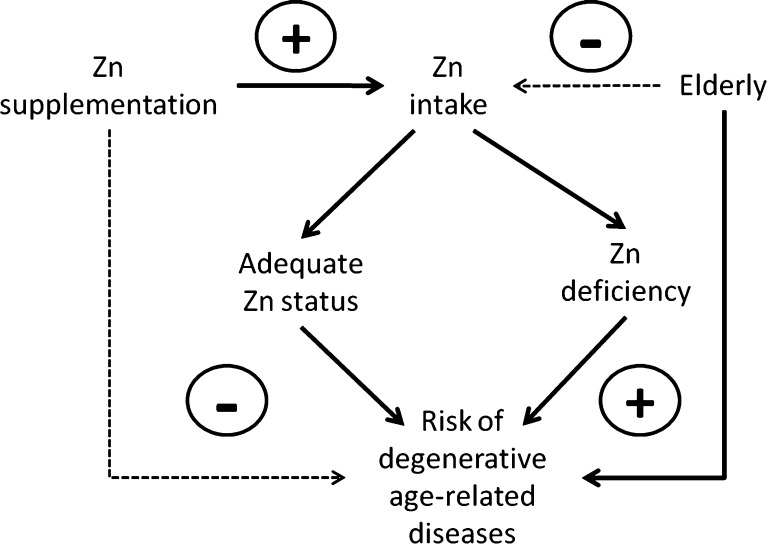
However, controversial findings exist on the “real” necessity of zinc supplementation because the major problem for zinc supplementation in old people is related to the choice of old subjects who effectively need zinc supplementation. The sole determination of plasma zinc is not sufficient because zinc is bound to many proteins. The intracellular zinc ion availability measured with specific zinc probes as well as the ratio Cu/Zn can be used as complementary methods to test zinc and inflammatory states. These methods may be used in daily clinical practice. The polymorphisms of IL-6 may be the added value to screen effective old subjects for zinc supplementation in restoring the immune response and to avoid zinc toxic effects. As a consequence, the healthy ageing and longevity may be achieved. Thus, zinc supplementation can have a great impact on healthy ageing especially in subjects carrying specific IL-6 polymorphisms and, as such, at high risk of zinc deficiency. Taking into account the low cost of zinc supplementation, a higher consideration by the international health organisations is therefore required with specific zinc fortification programmes. On the other hand, the physiological dose of zinc (10–12 mg/day), the length of the treatment (short period = 1 month in alternate cycles) and the form of zinc (zinc aspartate) are strongly recommended, as well as the genetic screening of IL-6 polymorphism by RT-PCR method, for the successful of the zinc supplementation. Otherwise zinc is toxic to high doses and for long continue treatment because inducing cell death. However, some points require further investigations. First of all, the precise biochemical mechanisms involved, in particular addressing NO-related intracellular pathways. Moreover, some aspects of the zinc absorption have to be better studied because it is dependent by the interactions with other nutrients. Anyway, zinc supplementation or more correct diet with foods containing zinc may have significant clinical therapeutic benefits in elderly by preventing the risk of degenerative age-related diseases, such as cancer and infection (Fig. 2).
Fig. 2.
Potential effect of Zn supplementation in preventing risk (minus sign) of degenerative age-related diseases in the elderly in comparison to zinc deficiency and risk of degenerative diseases (plus sign)
Acknowledgements
This study was supported by unrestricted educational grant from Danone, by INRCA, Cariverona Foundation (Italy) and ZINCAGE European Project (n. FOOD-2004-506850; Coordinator: Eugenio Mocchegiani).
Footnotes
Javier Romeo passed away 8 August 2011. This manuscript is dedicated to his memory.
References
- Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–149. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- Andree KB, Kim J, Kirschke CP, Gregg JP, Paik H, Joung H, Woodhouse L, King JC, Huang L. Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr. 2004;134:1716–1723. doi: 10.1093/jn/134.7.1716. [DOI] [PubMed] [Google Scholar]
- Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandolini-Bunlon M, Polito A, O’Connor JM, Ferry M, Coudray C, Roussel AM. Zinc intake and status in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59:S37–S41. doi: 10.1038/sj.ejcn.1602296. [DOI] [PubMed] [Google Scholar]
- Araujo LM, Lefort J, Nahori MA, Diem S, Zhu R, Dy M, Leite-de-Moraes MC, Bach JF, Vargaftig BB, Herbelin A. Exacerbated Th2-mediated airway inflammation and hyperresponsiveness in autoimmune diabetes-prone NOD mice: a critical role for CD1d-dependent NKT cells. Eur J Immunol. 2004;34:327–335. doi: 10.1002/eji.200324151. [DOI] [PubMed] [Google Scholar]
- Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age—age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- August D, Janghorbani M, Young VR. Determination of zinc and copper absorption at three dietary Zn–Cu ratios by using stable isotope methods in young adult and elderly subjects. Am J Clin Nutr. 1989;50:1457–1463. doi: 10.1093/ajcn/50.6.1457. [DOI] [PubMed] [Google Scholar]
- Balesaria S, Hogstrand C. Identification, cloning and characterization of a plasma membrane zinc efflux transporter TrZnT-1, from fugu pufferfish (Takifugu rubripes) Biochem J. 2006;394:485–493. doi: 10.1042/BJ20050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC, Knoell DL. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu TK, Donaldson D. Intestinal absorption in health and disease: micronutrients. Best Pract Res Clin Gastroenterol. 2003;17:957–979. doi: 10.1016/s1521-6918(03)00084-2. [DOI] [PubMed] [Google Scholar]
- Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Gluud C. Vitamin and mineral supplement use in relation to all-cause mortality in the Iowa Women’s Health Study. Arch Intern Med. 2011;171:1633–1634. doi: 10.1001/archinternmed.2011.459. [DOI] [PubMed] [Google Scholar]
- Bogden JD. Influence of zinc on immunity in the elderly. J Nutr Health Aging. 2004;8:48–54. [PubMed] [Google Scholar]
- Bonafe M, Olivieri F, Cavallone L, Giovagnetti S, Marchegiani F, Cardelli M, Pieri C, Marra M, Antonicelli R, Lisa R, Rizzo MR, Paolisso G, Monti D, Franceschi C. A gender-dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol. 2001;31:2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::aid-immu2357>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Briefel RR, Bialostosky K, Kennedy-Stephenson JJ, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population. Findings from the third National Health and Nutrition Examination Survey 1988–1994. J Nutr. 2000;130:1367S–1373S. doi: 10.1093/jn/130.5.1367S. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin Exp Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunk MJ, Dnistrian AM, Schwartz MK, Rivlin RS. Dietary Zn deficiency decreases plasma concentrations of vitamin E. Proc Soc Exp Biol Med. 1989;190:379–384. doi: 10.3181/00379727-190-42876. [DOI] [PubMed] [Google Scholar]
- Cakman I, Rohwer J, Schutz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- Cakman I, Kirchner H, Rink L. Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J Interferon Cytokine Res. 1997;17:469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- Casadei M, Persichini T, Polticelli F, Musci G, Colasanti M. S-glutathionylation of metallothioneins by nitrosative/oxidative stress. Exp Gerontol. 2008;43:415–422. doi: 10.1016/j.exger.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Chernoff R. Micronutrient requirements in older women. Am J Clin Nutr. 2005;81:1240S–1245S. doi: 10.1093/ajcn/81.5.1240. [DOI] [PubMed] [Google Scholar]
- Chiricolo M, Musa AR, Monti D, Zannotti M, Franceschi C. Enhanced DNA repair in lymphocytes of Down syndrome patients: the influence of zinc nutritional supplementation. Mutat Res. 1993;295:105–111. doi: 10.1016/0921-8734(93)90012-r. [DOI] [PubMed] [Google Scholar]
- Cipriano C, Malavolta M, Costarelli L, Giacconi R, Muti E, Gasparini N, Cardelli M, Monti D, Mariani E, Mocchegiani E. Polymorphisms in MT1a gene with longevity in Italian Central female population. Biogerontology. 2006;7:357–365. doi: 10.1007/s10522-006-9050-x. [DOI] [PubMed] [Google Scholar]
- Cipriano C, Tesei S, Malavolta M, Giacconi R, Muti E, Costarelli L, Piacenza F, Pierpaoli S, Galeazzi R, Blasco M, Vera E, Canela A, Lattanzio F, Mocchegiani E. Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. J Gerontol A Biol Sci Med Sci. 2009;64:745–751. doi: 10.1093/gerona/glp048. [DOI] [PubMed] [Google Scholar]
- Clemons TE, Kurinij N, Sperduto RD, AREDS Research Group (2004) Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13 Arch Ophthalmol 122:716–726 [DOI] [PMC free article] [PubMed]
- Coneyworth LJ, Mathers JC, Ford D. Does promoter methylation of the SLC30A5 (ZnT5) zinc transporter gene contribute to the ageing-related decline in zinc status? Proc Nutr Soc. 2009;68:142–147. doi: 10.1017/S0029665109001104. [DOI] [PubMed] [Google Scholar]
- Costarelli L, Muti E, Malavolta M, Giacconi R, Cipriano C, Sartini D, Emanuelli M, Silvestrini M, Provinciali L, Gobbi B, Mocchegiani E. Modulation of genes involved in zinc homeostasis in old low-grade atherosclerotic patients under effects of HMG-CoA reductase inhibitors. Rejuvenation Res. 2008;11:287–291. doi: 10.1089/rej.2008.0665. [DOI] [PubMed] [Google Scholar]
- Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Cousins RJ. Zinc. In: Filer LJ, Ziegler EE, editors. Present knowledge in nutrition. 7. Washington, DC: International Life Science Institute-Nutrition Foundation; 1996. pp. 93–306. [Google Scholar]
- Cousins RJ. Gastrointestinal factors influencing zinc absorption and homeostasis. Int J Vitam Nutr Res. 2010;80:243–248. doi: 10.1024/0300-9831/a000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg RA, Phillips SR, Piper JM, Varma JS, Campbell FC, Mathers JC, Ford D. Regulation of zinc transporters in the human small intestine by dietary zinc supplementation. Gut. 2005;54:469–478. doi: 10.1136/gut.2004.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56(Suppl 3):S20–S23. doi: 10.1038/sj.ejcn.1601479. [DOI] [PubMed] [Google Scholar]
- Dardenne M, Boukaiba N, Gagnerault MC, Homo-Delarche F, Chappuis P, Lemonnier D, Savino W. Restoration of the thymus in aging mice by in vivo zinc supplementation. Clin Immunol Immunopathol. 1993;66:127–135. doi: 10.1006/clin.1993.1016. [DOI] [PubMed] [Google Scholar]
- Davis SR, McMahon RJ, Cousins RJ. Metallothionein knockout and transgenic mice exhibit altered intestinal processing of zinc with uniformzinc-dependent zinc transporter-1 expression. J Nutr. 1998;128:825–831. doi: 10.1093/jn/128.5.825. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Evans WJ. Nutrition, physical activity, and quality of life in older adults: summary. J Gerontol A Biol Sci Med Sci. 2001;56:89–94. doi: 10.1093/gerona/56.suppl_2.89. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Shultz JM. Impact of aging on eating behaviors, food choices, nutrition, and health status. J Nutr Health Aging. 2001;5:75–79. [PubMed] [Google Scholar]
- Driessen C, Hirv K, Rink L, Kirchner H. Induction of cytokines by zinc ions in human peripheral blood mononuclear cells and separated monocytes. Lymphokine Cytokine Res. 1994;13:15–20. [PubMed] [Google Scholar]
- Ducros V, Andriollo-Sanchez M, Arnaud J, Meunier N, Laporte F, Hininger-Favier I, Coudray C, Ferry M, Roussel AM. Zinc supplementation does not alter plasma homocysteine, vitamin B12 and red blood cell folate concentrations in French elderly subjects. J Trace Elem Med Biol. 2009;23:15–20. doi: 10.1016/j.jtemb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem. 2003;278:33474–33481. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–49090. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- Dursun N, Aydoğan S. Comparative effects of calcium deficiency and supplements on the intestinal absorption of zinc in rats. Jpn J Physiol. 1994;44:157–166. doi: 10.2170/jjphysiol.44.157. [DOI] [PubMed] [Google Scholar]
- Eberle J, Schindmayer S, Erben RG, Stangassinger M, Roth HP. Skeletal effects of zinc deficiency in growing rats. J Trace Elem Med Biol. 1999;13:21–26. doi: 10.1016/S0946-672X(99)80019-4. [DOI] [PubMed] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- Ellis R, Kelsay JL, Reynolds RD, Morris ER, Moser PB, Frazier CW. Phytate:zinc and phytate X calcium:zinc millimolar ratios in self-selected diets of Americans, Asian Indians, and Nepalese. J Am Diet Assoc. 1987;87:1043–1047. [PubMed] [Google Scholar]
- Elmes ME, Jones JG. Ultrastructural changes in the small intestine of zinc deficient rats. J Pathol. 1980;130:37–43. doi: 10.1002/path.1711300106. [DOI] [PubMed] [Google Scholar]
- Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- Evans GW. Zinc and its deficiency diseases. Clin Physiol Biochem. 1986;4:94–98. [PubMed] [Google Scholar]
- Fabian E, Elmadfa I. Nutritional situation of the elderly in the European Union: data of the European Nutrition and Health Report (2004) Ann Nutr Metab. 2008;52(Suppl 1):57–61. doi: 10.1159/000115352. [DOI] [PubMed] [Google Scholar]
- Zinc. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001. pp. 442–501. [PubMed] [Google Scholar]
- Fraker PJ. Roles for cell death in zinc deficiency. J Nutr. 2005;135:359–362. doi: 10.1093/jn/135.3.359. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Telford WG. A reappraisal of the role of zinc in life and death decisions of cells. Proc Soc Exp Biol Med. 1997;215:229–236. doi: 10.3181/00379727-215-44132. [DOI] [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Chiricolo M, Licastro F, Zannotti M, Masi M, Mocchegiani E, Fabris N. Oral zinc supplementation in Down’s syndrome: restoration of thymic endocrine activity and of some immune defects. J Ment Defic Res. 1988;32:169–181. doi: 10.1111/j.1365-2788.1988.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, Salvioli S, Valensin S, De Benedictis G, Di Iorio A, Caruso C, Paolisso G, Monti D. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev. 2005;126:351–361. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Frazzini V, Rockabrand E, Mocchegiani E, Sensi SL. Oxidative stress and brain aging: is zinc the link? Biogerontology. 2006;7:307–314. doi: 10.1007/s10522-006-9045-7. [DOI] [PubMed] [Google Scholar]
- Freedman LP, Towers TL. DNA binding properties of the vitamin D3 receptor zinc finger region. Mol Endocrinol. 1991;5:1815–1826. doi: 10.1210/mend-5-12-1815. [DOI] [PubMed] [Google Scholar]
- Gabriel P, Cakman I, Rink L. Overproduction of monokines by leukocytes after stimulation with lipopolysaccharide in the elderly. Exp Gerontol. 2002;37:235–247. doi: 10.1016/s0531-5565(01)00189-9. [DOI] [PubMed] [Google Scholar]
- García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutr Rev. 2009;67:559–572. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- Genser D. Food and drug interaction: consequences for the nutrition/health status. Ann Nutr Metab. 2008;52(Suppl 1):29–32. doi: 10.1159/000115345. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Cipriano C, Albanese F, Boccoli G, Saba V, Olivieri F, Franceschi C, Mocchegiani E. The −174G/C polymorphism of IL-6 is useful to screen old subjects at risk for atherosclerosis or to reach successful ageing. Exp Gerontol. 2004;39:621–628. doi: 10.1016/j.exger.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Malavolta M, Costarelli L, Busco F, Galeazzi R, Bernardini G, Gasparini G, Mocchegiani E (2011) Comparison of intracellular zinc signals in non-adherent lymphocytes from young-adult and elderly donors: role of zinc transporters (Zip-family) and pro-inflammatory cytokines. J Nutr Biochem (in press) [DOI] [PubMed]
- Gibson RS, Heath AL, Ferguson EL. Risk of suboptimal iron and zinc nutriture among adolescent girls in Australia and New Zealand: causes, consequences, and solutions. Asia Pac J Clin Nutr. 2002;11(Suppl 3):S543–S552. doi: 10.1046/j.1440-6047.11.supp3.10.x. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008;99:S14–S23. doi: 10.1017/S0007114508006818. [DOI] [PubMed] [Google Scholar]
- Grahn BH, Paterson PG, Gottschall-Pass KT, Zhang Z. Zinc and the eye. J Am Coll Nutr. 2001;20(Suppl 2):106–118. doi: 10.1080/07315724.2001.10719022. [DOI] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25 high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Gur A, Colpan L, Nas K, Cevik R, Saraç J, Erdoğan F, Düz MZ. The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab. 2002;20:39–43. doi: 10.1007/s774-002-8445-y. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. Functional significance of zinc-related signalling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9–15. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Ann Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–428. doi: 10.1007/s10522-006-9057-3. [DOI] [PubMed] [Google Scholar]
- Hambidge KM. Zinc and diarrhea. Acta Paediatr Suppl. 1992;381:82–86. doi: 10.1111/j.1651-2227.1992.tb12377.x. [DOI] [PubMed] [Google Scholar]
- Hambidge KM. Human zinc deficiency. J Nutr. 2000;130(Suppl 5S):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- Hambidge KM. Micronutrient bioavailability: dietary reference intakes and a future perspective. Am J Clin Nutr. 2010;91(Suppl 1):1430S–1432S. doi: 10.3945/ajcn.2010.28674B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP. Micronutrient supplementation and immune function in the elderly. Clin Infect Dis. 1999;28:717–722. doi: 10.1086/515208. [DOI] [PubMed] [Google Scholar]
- Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78:756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr. 2009;89:839–843. doi: 10.3945/ajcn.2008.27175. [DOI] [PubMed] [Google Scholar]
- Ibs KH, Gabriel P, Rink L. Zinc and the immune system of elderly. Adv Cell Aging Gerontol. 2003;13:243–259. [Google Scholar]
- Intorre F, Polito A, Andriollo-Sanchez M, Azzini E, Raguzzini A, Toti E, Zaccaria M, Catasta G, Meunier N, Ducros V, O’Connor JM, Coudray C, Roussel AM, Maiani G. Effect of zinc supplementation on vitamin status of middle-aged and older European adults: the ZENITH study. Eur J Clin Nutr. 2008;62:1215–1223. doi: 10.1038/sj.ejcn.1602844. [DOI] [PubMed] [Google Scholar]
- Kahmann L, Uciechowski P, Warmuth S, Malavolta M, Mocchegiani E, Rink L. Effect of improved zinc status on T helper cell activation and TH1/TH2 ratio in healthy elderly individuals. Biogerontology. 2006;7:429–435. doi: 10.1007/s10522-006-9058-2. [DOI] [PubMed] [Google Scholar]
- Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM, Malavolta M, Mocchegiani E, Rink L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008;11:227–237. doi: 10.1089/rej.2007.0613. [DOI] [PubMed] [Google Scholar]
- Kanoni S, Dedoussis GV, Herbein G, Fulop T, Varin A, Jajte J, Rink L, Monti D, Mariani E, Malavolta M, Giacconi R, Marcellini F, Mocchegiani E. Assessment of gene-nutrient interactions on inflammatory status of the elderly with the use of a zinc diet score—ZINCAGE study. J Nutr Biochem. 2010;21:526–531. doi: 10.1016/j.jnutbio.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Karaca Z, Tanriverdi F, Kurtoglu S, Tokalioglu S, Unluhizarci K, Kelestimur F. Pubertal arrest due to Zn deficiency: the effect of zinc supplementation. Hormones (Athens) 2007;6:71–74. [PubMed] [Google Scholar]
- Kim J, Paik HY, Joung H, Woodhouse LR, King JC. Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J Am Coll Nutr. 2007;26:1–9. doi: 10.1080/07315724.2007.10719579. [DOI] [PubMed] [Google Scholar]
- King LE, Osati-Ashtiani F, Fraker PJ. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J Nutr. 2002;132:974–979. doi: 10.1093/jn/132.5.974. [DOI] [PubMed] [Google Scholar]
- King LE, Frentzel JW, Mann JJ, Fraker PJ. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J Am Coll Nutr. 2005;24:494–502. doi: 10.1080/07315724.2005.10719495. [DOI] [PubMed] [Google Scholar]
- Kogirima M, Kurasawa R, Kubori S, Sarukura N, Nakamori M, Okada S, Kamioka H, Yamamoto S. Ratio of low serum zinc levels in elderly Japanese people living in the central part of Japan. Eur J Clin Nutr. 2007;61:375–381. doi: 10.1038/sj.ejcn.1602520. [DOI] [PubMed] [Google Scholar]
- Krezel A, Hao Q, Maret W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys. 2007;463:188–200. doi: 10.1016/j.abb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- Lang C, Murgia C, Leong M, Tan LW, Perozzi G, Knight D, Ruffin R, Zalewski P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L577–L584. doi: 10.1152/ajplung.00280.2006. [DOI] [PubMed] [Google Scholar]
- Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55:539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- Leek JC, Keen CL, Vogler JB, Golub MS, Hurley LS, Hendrickx AG, Gershwin ME. Long-term marginal zinc deprivation in rhesus monkeys. IV. Effects on skeletal growth and mineralization. Am J Clin Nutr. 1988;47:889–895. doi: 10.1093/ajcn/47.5.889. [DOI] [PubMed] [Google Scholar]
- Licastro F, Chiricolo M, Mocchegiani E, Fabris N, Zannoti M, Beltrandi E, Mancini R, Parente R, Arena G, Masi M. Oral zinc supplementation in Down’s syndrome subjects decreased infections and normalized some humoral and cellular immune parameters. J Intellect Disabil Res. 1994;38:149–162. doi: 10.1111/j.1365-2788.1994.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Little KY, Castellanos X, Humphries LL, Austin J. Altered Zn metabolism in mood disordered patients. Biol Psychol. 1989;26:646–648. doi: 10.1016/0006-3223(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults the 1994–96 continuing survey of food intakes by individuals (CSFII) J Nutr. 2000;130:2838–2843. doi: 10.1093/jn/130.11.2838. [DOI] [PubMed] [Google Scholar]
- MacKenzie GG, Zago MP, Aimo L, Oteiza PI. Zinc deficiency in neuronal biology. IUBMB Life. 2007;59:299–307. doi: 10.1080/15216540701225966. [DOI] [PubMed] [Google Scholar]
- Malavolta M, Giacconi R, Piacenza F, Santarelli L, Cipriano C, Costarelli L, Tesei S, Pierpaoli S, Basso A, Galeazzi R, Lattanzio F, Mocchegiani E. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology. 2010;11:309–319. doi: 10.1007/s10522-009-9251-1. [DOI] [PubMed] [Google Scholar]
- Marcellini F, Giuli C, Papa R, Gagliardi C, Dedoussis G, Herbein G, Fulop T, Monti D, Rink L, Jajte J, Mocchegiani E. Zinc status, psychological and nutritional assessment in old people recruited in five European countries: ZINCAGE study. Biogerontology. 2006;7:339–345. doi: 10.1007/s10522-006-9048-4. [DOI] [PubMed] [Google Scholar]
- Maret W. Cellular zinc and redox states converge in the metallothionein/thionein pair. J Nutr. 2003;133(Suppl 1):1460S–1462S. doi: 10.1093/jn/133.5.1460S. [DOI] [PubMed] [Google Scholar]
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Mariani E, Cattini L, Neri S, Malavolta M, Mocchegiani E, Ravaglia G, Facchini A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: relationship with zinc status. Biogerontology. 2006;7:449–459. doi: 10.1007/s10522-006-9060-8. [DOI] [PubMed] [Google Scholar]
- Mariani E, Neri S, Cattini L, Mocchegiani E, Malavolta M, Dedoussis GV, Kanoni S, Rink L, Jajte J, Facchini A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: interactive influence of +647 MT1a and −174 IL-6 polymorphic alleles. Exp Gerontol. 2008;43:462–471. doi: 10.1016/j.exger.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Markovits PM, Sankey AW, James DK, McCabe R, Mahomed K, Golding J. Zn taste test and postnatal depression. Br J Psychol. 1990;156:451–452. doi: 10.1192/bjp.156.3.451. [DOI] [PubMed] [Google Scholar]
- Max Rubner-Institut (MRI) (editor) (2008) Nationale Verzehrstudie; Federal Research Institute of Nutrition and Food. Karlsruhe, Germany
- Mazzatti DJ, Malavolta M, White AJ, Costarelli L, Giacconi R, Muti E, Cipriano C, Powell JR, Mocchegiani E. Differential effects of in vitro zinc treatment on gene expression in peripheral blood mononuclear cells derived from young and elderly individuals. Rejuvenation Res. 2007;10:603–620. doi: 10.1089/rej.2007.0553. [DOI] [PubMed] [Google Scholar]
- Mazzatti DJ, Malavolta M, White AJ, Costarelli L, Giacconi R, Muti E, Cipriano C, Powell JR, Mocchegiani E. Effects of interleukin-6 −174C/G and metallothionein 1A +647A/C single-nucleotide polymorphisms on zinc-regulated gene expression in ageing. Exp Gerontol. 2008;43:423–432. doi: 10.1016/j.exger.2008.01.007. [DOI] [PubMed] [Google Scholar]
- McLeod JD. Apoptotic capability in ageing T cells. Mech Ageing Dev. 2000;121:151–159. doi: 10.1016/s0047-6374(00)00206-2. [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ. Mammalian zinc transporters. J Nutr. 1998;128:667–670. doi: 10.1093/jn/128.4.667. [DOI] [PubMed] [Google Scholar]
- Menard MP, Cousins RJ. Zinc transport by brush border membrane vesicles from rat intestine. J Nutr. 1983;113:1434–1442. doi: 10.1093/jn/113.7.1434. [DOI] [PubMed] [Google Scholar]
- Meunier N, Feillet-Coudray C, Rambeau M, Andriollo-Sanchez M, Brandolini-Bunlon M, Coulter SJ, Cashman KD, Mazur A, Coudray C. Impact of micronutrient dietary intake and status on intestinal zinc absorption in late middle-aged men: the ZENITH study. Eur J Clin Nutr. 2005;59:S48–S52. doi: 10.1038/sj.ejcn.1602298. [DOI] [PubMed] [Google Scholar]
- Mitchell WA, Meng I, Nicholson SA, Aspinall R. Thymic output, ageing and zinc. Biogerontology. 2006;7:461–470. doi: 10.1007/s10522-006-9061-7. [DOI] [PubMed] [Google Scholar]
- Miyaji C, Watanabe H, Toma H, Akisaka M, Tomiyama K, Sato Y, Abo T. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–916. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Santarelli L, Muzzioli M, Fabris N. Reversibility of the thymic involution and of age-related peripheral immune dysfunctions by zinc supplementation in old mice. Int J Immunopharmacol. 1995;17:703–718. doi: 10.1016/0192-0561(95)00059-b. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Cipriano C, Giacconi R. Zinc, T-cell pathways, aging: role of metallothioneins. Mech Ageing Dev. 1998;106:183–204. doi: 10.1016/s0047-6374(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Giacconi R. Zinc and immunoresistance to infection in aging: new biological tools. Trends Pharmacol Sci. 2000;21:205–208. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Muzzioli M, Giacconi R, Cipriano C, Gasparini N, Franceschi C, Gaetti R, Cavalieri E, Suzuki H. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003;124:459–468. doi: 10.1016/s0047-6374(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, Gasparini N, Bernardini G, Malavolta M, Menegazzi M, Cavalieri E, Muzzioli M, Ciampa AR, Suzuki H. The variations during the circadian cycle of liver CD1d-unrestricted NK1.1 + TCR gamma/delta + cells lead to successful ageing. Role of metallothionein/IL-6/gp130/PARP-1 interplay in very old mice. Exp Gerontol. 2004;39:775–788. doi: 10.1016/j.exger.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Tesei S, Malavolta M. Nutrient-gene interaction in ageing and successful ageing. A single nutrient (zinc) and some target genes related to inflammatory/immune response. Mech Ageing Dev. 2006;127:517–525. doi: 10.1016/j.mad.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, Costarelli L, Muti E, Tesei S, Giuli C, Papa R, Marcellini F, Mariani E, Rink L, Herbein G, Varin A, Fulop T, Monti D, Jajte J, Dedoussis G, Gonos ES, Trougakos IP, Malavolta M. Zinc, metallothioneins, and longevity—effect of zinc supplementation: ZINCAGE study. Ann NY Acad Sci. 2007;1119:129–146. doi: 10.1196/annals.1404.030. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Bürkle A, Fulop T. Zinc and ageing (ZINCAGE project) Exp Gerontol. 2008;43:361–362. doi: 10.1016/j.exger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Costarelli L, Muti E, Cipriano C, Tesei S, Pierpaoli S, Giuli C, Papa R, Marcellini F, Gasparini N, Pierandrei R, Piacenza F, Mariani E, Monti D, Dedoussis G, Kanoni S, Herbein G, Fulop T, Rink L, Jajte J, Malavolta M. Zinc deficiency and IL-6 −174G/C polymorphism in old people from different European countries: effect of zinc supplementation. ZINCAGE study. Exp Gerontol. 2008;43:433–444. doi: 10.1016/j.exger.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Malavolta M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med. 2008;14:419–428. doi: 10.1016/j.molmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Malavolta M, Muti E, Costarelli L, Cipriano C, Piacenza F, Tesei S, Giacconi R, Lattanzio F. Zinc, metallothioneins and longevity: interrelationships with niacin and selenium. Curr Pharm Des. 2008;14:2719–2732. doi: 10.2174/138161208786264188. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity: role of zinc and metallothioneins. J Clin Immunol. 2009;29:416–425. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E, Malavolta M, Lattanzio F, Piacenza F, Basso A, Abbatecola AM, Russo A, Giovannini S, Capoluongo E, Bustacchini S, Guffanti EE, Bernabei R, Landi F (2011) Cu to Zn ratio, physical function, disability, and mortality risk in older elderly (ilSIRENTE study). AGE (in press) [DOI] [PMC free article] [PubMed]
- Moroni F, Di Paolo ML, Rigo A, Cipriano C, Giacconi R, Recchioni R, Marcheselli F, Malavolta M, Mocchegiani E. Interrelationship among neutrophil efficiency, inflammation, antioxidant activity and zinc pool in very old age. Biogerontology. 2005;6:271–281. doi: 10.1007/s10522-005-2625-0. [DOI] [PubMed] [Google Scholar]
- Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med. 2011;171:1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzioli M, Stecconi R, Moresi R, Provinciali M. Zinc improves the development of human CD34+ cell progenitors towards NK cells and increases the expression of GATA-3 transcription factor in young and old ages. Biogerontology. 2009;10:593–604. doi: 10.1007/s10522-008-9201-3. [DOI] [PubMed] [Google Scholar]
- Nasi M, Troiano L, Lugli E, Pinti M, Ferraresi R, Monterastelli E, Mussi C, Salvioli G, Franceschi C, Cossarizza A. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;5:167–175. doi: 10.1111/j.1474-9726.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- Nova E, Lopez-Vidriero I, Varela P, Toro O, Casas JJ, Marcos AA. Indicators of nutritional status in restricting-type anorexia nervosa patients: a 1-year follow-up study. Clin Nutr. 2004;23:1353–1359. doi: 10.1016/j.clnu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Oberleas D, Muhrer ME, O’Dell BL. Dietary metal-complexing agents and zinc availability in the rat. J Nutr. 1966;90:56–62. doi: 10.1093/jn/90.1.56. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Quinti I, Fagiolo U, Cossarizza A, Ortolani C, Guerra E, Sansoni P, Pucillo LP, Scala E, Cozzi E, Bertollo L, Monti D, Franceschi C. Changes in circulating B cells and immunoglobulin classes and subclasses in healthy aged population. Clin Exp Immunol. 1992;90:351–354. doi: 10.1111/j.1365-2249.1992.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Remarque E, Barnett Y, Solana R. T cells and aging. Front Biosci. 1998;3:d59–d99. doi: 10.2741/a266. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142(Suppl 1):S39–S44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin Endocrinol Metab. 1985;14:567–589. doi: 10.1016/s0300-595x(85)80007-4. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc and immunity. Mol Cell Biochem. 1998;188:63–69. [PubMed] [Google Scholar]
- Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis. 2000;182:62–68. doi: 10.1086/315916. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc: Mechanisms of Host Defense. J Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009;28:257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition. 1993;9:218–224. [PubMed] [Google Scholar]
- Prasad AS, Bao B, Beck FW, Sarkar FH. Correction of interleukin-2 gene expression by in vitro zinc addition to mononuclear cells from zinc-deficient human subjects: a specific test for zinc deficiency in humans. Transl Res. 2006;148:325–333. doi: 10.1016/j.trsl.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Provinciali M, Di Stefano G, Stronati S. Flow cytometric analysis of CD3/TCR complex, zinc, and glucocorticoid-mediated regulation of apoptosis and cell cycle distribution in thymocytes from old mice. Cytometry. 1998;32:1–8. [PubMed] [Google Scholar]
- Provinciali M, Montenovo A, Di Stefano G, Colombo M, Daghetta L, Cairati M, Veroni C, Cassino R, Della Torre F, Fabris N. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998;27:715–722. doi: 10.1093/ageing/27.6.715. [DOI] [PubMed] [Google Scholar]
- Rea IM, Ross OA, Armstrong M, McNerlan S, Alexander DH, Curran MD, Middleton D. Interleukin-6-gene C/G 174 polymorphism in nonagenarian and octogenarian subjects in the BELFAST study. Reciprocal effects on IL-6, soluble IL-6 receptor and for IL-10 in serum and monocyte supernatants. Mech Ageing Dev. 2003;124:555–561. doi: 10.1016/s0047-6374(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Saba I, Kosan C, Vassen L, Möröy T. IL-7R-dependent survival and differentiation of early T-lineage progenitors is regulated by the BTB/POZ domain transcription factor Miz-1. Blood. 2011;117:3370–3381. doi: 10.1182/blood-2010-09-310680. [DOI] [PubMed] [Google Scholar]
- Sandstead HH. Understanding zinc: recent observations and interpretations. J Lab Clin Med. 1994;124:322–327. [PubMed] [Google Scholar]
- Sandstead HH. Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am J Clin Nutr. 1995;61:S621–S624. doi: 10.1093/ajcn/61.3.621S. [DOI] [PubMed] [Google Scholar]
- Sandstead HH. Causes of iron and zinc deficiencies and their effects on brain. J Nutr. 2000;130(Suppl 2S):347S–349S. doi: 10.1093/jn/130.2.347S. [DOI] [PubMed] [Google Scholar]
- Sandström B. Dose dependence of zinc and manganese absorption in man. Proc Nutr Soc. 1992;51:211–218. doi: 10.1079/pns19920031. [DOI] [PubMed] [Google Scholar]
- Sandström B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85(Suppl 2):S181–S185. [PubMed] [Google Scholar]
- Sandström B, Cederblad Å. Zinc absorption from composite meals. II. Influence of the main protein source. Am J Clin Nutr. 1980;33:1778–1783. doi: 10.1093/ajcn/33.8.1778. [DOI] [PubMed] [Google Scholar]
- Sandstrom B, Davidsson L, Cederblad L, Lonnerdal B. Oral iron, dietary ligands and zinc absorption. J Nutr. 1985;115:411–414. doi: 10.1093/jn/115.3.411. [DOI] [PubMed] [Google Scholar]
- Sato M, Yanagisawa H, Nojima Y, Tamura J, Wada O. Zn deficiency aggravates hypertension in spontaneously hypertensive rats: possible role of Cu/Zn-superoxide dismutase. Clin Exp Hypertens. 2002;24:355–370. doi: 10.1081/ceh-120004797. [DOI] [PubMed] [Google Scholar]
- Sayeg Porto MA, Oliveira HP, Cunha AJ, Miranda G, Guimarães MM, Oliveira WA, dos Santos DM. Linear growth and zinc supplementation in children with short stature. J Pediatr Endocrinol Metab. 2000;13:1121–1128. doi: 10.1515/jpem.2000.13.8.1121. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Mocchegiani E, Marzola P, Tibaldi A, Mannucci R, Nicolato E, Osculati F. Effect of dietary supplementation with zinc sulphate on the aging process: a study using high field intensity MRI and chemical shift imaging. Biomed Pharmacother. 1998;52:454–458. doi: 10.1016/S0753-3322(99)80024-9. [DOI] [PubMed] [Google Scholar]
- Schroder AK, Rink L. Neutrophil immunity of the elderly. Mech Ageing Dev. 2003;124:419–425. doi: 10.1016/s0047-6374(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Food (SCF) (2002) Opinion of the scientific committee on food on the tolerable upper intake level of zinc. Brussels, Belgium, pp. 15–25
- Shankar AH, Prasad AS. Zn and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Smith JC., Jr The vitamin A-zinc connection: a review. Ann NY Acad Sci. 1980;355:62–75. doi: 10.1111/j.1749-6632.1980.tb21328.x. [DOI] [PubMed] [Google Scholar]
- Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–1620. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- Song MK, Adham NF. Role of prostaglandin E2 in zinc absorption in the rat. Am J Physiol. 1978;234:E99–E105. doi: 10.1152/ajpendo.1978.234.2.E99. [DOI] [PubMed] [Google Scholar]
- Starling RD, Poehlman ET. Assessment of energy requirements in elderly populations. Eur J Clin Nutr. 2000;54(Suppl 3):S104–S111. doi: 10.1038/sj.ejcn.1601031. [DOI] [PubMed] [Google Scholar]
- Steel L, Cousins RJ. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol. 1985;248:G46–G53. doi: 10.1152/ajpgi.1985.248.1.G46. [DOI] [PubMed] [Google Scholar]
- Stewart-Knox BJ, Simpson EE, Parr H, Parr H, Rae G, Polito A, Intorre F, Meunier N, Andriollo-Sanchez M, O’Connor JM, Coudray C, Strain JJ. Zinc status and taste acuity in older Europeans: the ZENITH study. Eur J Clin Nutr. 2005;59:S31–S36. doi: 10.1038/sj.ejcn.1602295. [DOI] [PubMed] [Google Scholar]
- Su JC, Birmingham CL. Zn supplementation in the treatment of anorexia nervosa. Eat Weight Disord. 2002;7:20–22. doi: 10.1007/BF03354425. [DOI] [PubMed] [Google Scholar]
- Sweeney MP, Bagg J, Fell GS, Yip B. The relationship between micronutrient depletion and oral health in geriatrics. J Oral Pathol Med. 1994;23:168–171. doi: 10.1111/j.1600-0714.1994.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Swoboda JR, Kiyak HA, Darveau R, Persson GR. Correlates of periodontal decline and biologic markers in older adults. J Periodontol. 2008;79:1920–1926. doi: 10.1902/jop.2008.080005. [DOI] [PubMed] [Google Scholar]
- Tallkvist J, Bowlus CL, Lönnerdal B. Functional and molecular responses of human intestinal Caco-2 cells to iron treatment. Am J Clin Nutr. 2000;72:770–775. doi: 10.1093/ajcn/72.3.770. [DOI] [PubMed] [Google Scholar]
- Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Taysi S, Cikman O, Kaya A, Demircan B, Gumustekin K, Yilmaz A, Boyuk A, Keles M, Akyuz M, Turkeli M. Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with zinc-deficient diet. Biol Trace Elem Res. 2008;123:161–167. doi: 10.1007/s12011-008-8095-x. [DOI] [PubMed] [Google Scholar]
- Thomson AB. Small intestinal disorders in the elderly. Best Pract Res Clin Gastroenterol. 2009;23:861–874. doi: 10.1016/j.bpg.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Tinker D, Rucker RB. Role of selected nutrients in synthesis, accumulation, and chemical modification of connective tissue proteins. Physiol Rev. 1985;65:607–657. doi: 10.1152/physrev.1985.65.3.607. [DOI] [PubMed] [Google Scholar]
- Toniato E, Chen XP, Losman J, Flati V, Donahue L, Rothman P. TRIM8/GERP RING finger protein interacts with SOCS-1. J Biol Chem. 2002;277:37315–37322. doi: 10.1074/jbc.M205900200. [DOI] [PubMed] [Google Scholar]
- Topinková E. Aging, disability and frailty. Ann Nutr Metab. 2008;52(Suppl 1):6–11. doi: 10.1159/000115340. [DOI] [PubMed] [Google Scholar]
- Turnlund JR, Michel MC, Keyes WR, King JC, Margen S. Use of enriched stable isotopes to determine zinc and iron absorption in elderly men. Am J Clin Nutr. 1982;35:1033–1040. doi: 10.1093/ajcn/35.5.1033. [DOI] [PubMed] [Google Scholar]
- CSFII/DHKS 1994–1996 data set and documentation: the 1994 continuing survey of food intakes by individuals and the 1994–1996 diet and health knowledge survey. On: CSFII/DHKS 1994–96 CD-ROM. Riverdale, MD: Agricultural Research Service; 1996. [Google Scholar]
- Uciechowski P, Kahmann L, Plumakers B, Malavolta M, Mocchegiani E, Dedoussis G, Herbein G, Jajte J, Fulop T, Rink L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43:493–498. doi: 10.1016/j.exger.2007.11.006. [DOI] [PubMed] [Google Scholar]
- van der Putten GJ, Vanobbergen J, De Visschere L, Schols J, de Baat C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: a systematic literature review. Nutrition. 2009;25:717–722. doi: 10.1016/j.nut.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Varela P, Marcos A, Navarro MP. Zinc status in anorexia nervosa. Ann Nutr Metab. 1992;36:197–202. doi: 10.1159/000177718. [DOI] [PubMed] [Google Scholar]
- Varin A, Larbi A, Dedoussis GV, Kanoni S, Jajte J, Rink L, Monti D, Malavolta M, Marcellini F, Mocchegiani E, Herbein G, Fulop T., Jr In vitro and in vivo effects of zinc on cytokine signalling in human T cells. Exp Gerontol. 2008;43:472–482. doi: 10.1016/j.exger.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vasto S, Mocchegiani E, Candore G, Listì F, Colonna-Romano G, Lio D, Malavolta M, Giacconi R, Cipriano C, Caruso C. Inflammation, genes and zinc in ageing and age-related diseases. Biogerontology. 2006;7:315–327. doi: 10.1007/s10522-006-9046-6. [DOI] [PubMed] [Google Scholar]
- Villarino Rodríguez A, García-Linares MC, García-Fernández MC, García-Arias MT. Evaluation of diet and biochemical parameters for minerals in a group of elderly subjects in the province of Leon (Spain) Nutr Hosp. 2003;18:39–45. [PubMed] [Google Scholar]
- Volkert D, Kreuel K, Heseker H, Stehle P. Energy and nutrient intake of young-old, old-old and very-old elderly in Germany. Eur J Clin Nutr. 2004;58:1190–1200. doi: 10.1038/sj.ejcn.1601950. [DOI] [PubMed] [Google Scholar]
- von Bulow V, Rink L, Haase H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3′,5′-cyclic monophosphate. J Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou B. Dietary zinc absorption: a play of Zips and ZnTs in the gut. IUBMB Life. 2010;62:176–182. doi: 10.1002/iub.291. [DOI] [PubMed] [Google Scholar]
- Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol. 2000;20:240–249. doi: 10.1023/a:1006659401385. [DOI] [PubMed] [Google Scholar]
- Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci USA. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998;68(2 Suppl):442S–446S. doi: 10.1093/ajcn/68.2.442S. [DOI] [PubMed] [Google Scholar]
- Wood RJ, Zheng JJ. High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997;65:1803–1809. doi: 10.1093/ajcn/65.6.1803. [DOI] [PubMed] [Google Scholar]
- Yu YY, Kirschke CP, Huang L. Immunohistochemical analysis of ZnT1, 4, 5, 6, and 7 in the mouse gastrointestinal tract. J Histochem Cytochem. 2007;55:223–234. doi: 10.1369/jhc.6A7032.2006. [DOI] [PubMed] [Google Scholar]