Abstract
Centenarians are an outstanding model of successful aging, with genetics and healthy lifestyle certainly being key factors responsible for their longevity. Exercise capacity has been identified to play an important role in healthy aging, but a comprehensive assessment of the limitations to maximal exercise in this population is lacking. Following, health histories, lung function, and anthropometric measures, eight female centenarians (98–102 years old) and eight young females (18–22 years old) performed a series of graded maximal exercise tests on a cycle ergometer that facilitated absolute and relative work rate comparisons. Centenarians revealed a dramatically attenuated lung function, as measured by spirometry (forced expiratory volume in 1 s (FEV1/forced vital capacity (FVC), 55 ± 10%) compared to the young (FEV1/FVC, 77 ± 5%). During exercise, although the centenarians relied heavily on respiratory rate which yielded ∼50% higher dead space/tidal volume, minute ventilation was similar to that of the young at all but maximal exercise, and alveolar PO2 was maintained in both groups. In contrast, peak WR and VO2 were significantly reduced in the centenarians (33 ± 4 vs 179 ± 24 W; 7.5 ± 1.2 vs 39.6 ± 3.5 ml min−1 kg−1). Arterial PO2 of the centenarians fell steadily from the normal range of both groups to yield a large A-a gradient (57 ± 6 mmHg). Metabolic cost of a given absolute work rate was consistently lower, ∼46% less than the young at maximal effort. Centenarians have significant limitations to gas exchange across the lungs during exercise, but this limited oxygen transport is tempered by improved skeletal muscle mechanical efficiency that may play a vital role in maintaining physical function and therefore longevity in this population.
Keywords: Centenarian, Exercise capacity, Lung function
Introduction
Centenarians are perhaps the best example of successful human aging, living ∼50% longer than the world average. Many factors including genetics and health care combine to yield the longevity of this population; however, the components of a healthy lifestyle such as exercise capacity are still certainly important (Perls and Terry 2003a, b; Stessman et al. 2009). Indeed, the capacity to limit age-related diseases has been proposed as one of the mechanisms responsible for successful aging in extremely old subjects (Galioto et al. 2008), and maintaining exercise capacity and subsequently physical function likely play a significant role in this process (Cress et al. 2010). Exercise capacity, defined by peak oxygen consumption (VO2peak) in response to a graded exercise test (Jette et al. 1990) is a strong predictor of health and independence in older adults (Paterson et al. 2004) and has been documented to decline by 10–15% per decade between the ages of 50 and 75 years (Hollenberg et al. 2006; Fleg et al. 2005). However, a comprehensive assessment of limitations to maximal exercise and VO2peak itself in centenarians is currently lacking in the literature.
With progressive age, there is significant decline in lung function, due predominantly to a loss of elastic recoil (Knudson et al. 1983). This increase in lung compliance with age results in reduced maximal expiratory flow rates and an increase in resting functional residual capacity (DeLorey and Babb 1999), which during exercise translates into marked mechanical ventilatory constraints and increased ventilatory requirements in the ninth and tenth decades of life (McClaran et al. 1995). Thus, due to these mechanical constraints, the strategy used to achieve an exercise-induced increase in ventilation differs between the young and the old (Johnson et al. 1994), with the very elderly being likened to patients with chronic obstructive pulmonary disease (McClaran et al. 1995). However, the impact of these age-related differences in lung function on exercise tolerance in centenarians remains unclear.
Skeletal muscle, toward the end of the oxygen cascade, determines oxygen demand and therefore has the potential to tremendously impact exercise capacity. Aging is associated with sarcopenia or the progressive loss of muscle mass. Indeed, thigh muscle volume is typically reduced 24–27% between the second and seventh decade (Janssen et al. 2000), and this decline in muscle mass and lower limb power correlates well with quality of life and activities of daily living (ADL) in the elderly (Narici and Maffulli 2010). Interestingly, there is evidence that sarcopenia is muscle fiber type specific, with type II fibers being more susceptible to atrophy than type I fibers (Lexell et al. 1988). Specifically, Lexell et al. (1988) revealed that the type II fibers of 80-year-old subjects were ∼26% smaller than 20-year-old controls, while type I fibers were not different in size. This age-related loss of fast motor units (type IIA and IIX fibers) causes a progressive shift toward a slower phenotype (Lexell 1995), which, although as of yet not clearly associated with the aging process, could conceivably result in improved mechanical efficiency (Hunter et al. 2001; Gibbs and Gibson 1972).
Consequently, this study was designed to examine the limitations to maximal exercise and VO2peak itself in centenarians compared to their young counterparts. Specifically, the following hypotheses were tested: (1) pulmonary function at rest, as assessed by spirometry, will be diminished in the centenarians compared to the young subjects, 2) this disparate lung function will translate into diminished pulmonary gas exchange in the centenarians during exercise, and (3) limited oxygen availability will compromise skeletal muscle function during exercise in the centenarians compared to their young counterparts.
Methods
Subjects
Eight ambulatory female centenarians (98–102 years old) without overt cardiovascular disease, significant medications, a history of smoking, and severe cognitive impairment (mini-mental state examination (MMSE) > 20) (Folstein et al. 1975) and eight young females (18–22 years old) took part in this research. Three of the centenarians were institutionalized, and five were community dwelling, and although all were capable of independent walking, two centenarians were unable to climb stairs alone. All the young females were physically active, performing some form of endurance-type exercise 3.5 ± 2.0 h per week. Study procedures were approved by University of Verona Institutional Review Board, and both subjects and/or family caregivers gave written informed consent.
General procedures
The centenarians were assessed on three separate days. On day 1, a health history physical examination, an evaluation of ADLs (Mahoney and Barthel 1965), a cognitive performance assessment (MMSE) (Folstein et al. 1975), familiarization with exercise procedure/devises, and spirometry were performed. On the second day, muscle mass, maximal handgrip, and blood pressure were measured, and a blood sample taken. On the third and final day, subjects performed a graded maximal cycle exercise test, with indirect calorimetry. Young control subjects were assessed on two separate days. On day 1, assessments of muscle mass, blood pressure, maximal handgrip, and spirometry were performed, and subjects performed a graded maximal cycle exercise test, with indirect calorimetry. On the second day, subjects repeated the graded maximal cycle exercise test, but this time, work rates were matched with that of the relative and absolute capacities of the centenarians.
Spirometry
Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were assessed using a metabolic cart (Quark b2 Cosmed, Rome, Italy), according to the American Thoracic Society standards (Levy et al. 2009).
Muscle mass
The anthropometric assessment of thigh volume was assessed by determining a series of length and circumference measurements and subcutaneous fat, as previously described (Jones and Pearson 1969).
Blood analyses
A fasted venous blood sample was analyzed for glucose, red blood cell content, hemoglobin concentration, high- and low-density lipoprotein, and iron by standard techniques.
Incremental exercise tests and gas exchange assessments
In the centenarians, workload was progressively increased by 5 W at 1-min intervals with the revolutions per minute maintained at 50, until voluntary exhaustion (Monark 881e, Sweden). Peak pulmonary ventilation and gas exchange were calculated as the average of the last 30 s of the highest completed work rate. The use of 1-min workload intervals for the incremental exercise test was selected to maximize the metabolic responses in the periphery while minimizing fatigue, as recommended for Clinical Exercise Testing (Jones 1997). In the young controls, the first graded exercise test was performed with 15-W increments and the second with increments of 15% of maximum work, allowing an absolute and relative work rate comparison between groups. Heart rate and rhythm were monitored by a three-lead ECG and automated external biphasic defibrillator (LIFEPAK20 Medtronic Emergency Response Systems, Inc., USA). A pulse oximeter (Vital test, Edan, China) was used to measure arterial oxygen saturation. Ventilation and pulmonary gas exchange (Quark b2 metabolic cart Cosmed, Rome Italy) were measured continuously at rest and during the graded cycle exercise test. Dead space (Vd), was calculated as:
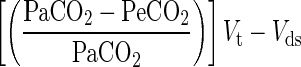 |
where Vt is tidal volume, PaCO2 is the estimated partial pressure of carbon dioxide in the arterial blood (5.5 + 0.90·PetCO2 − 0.0021·Vt), and PeCO2 is the partial pressure of carbon dioxide in the expired air (mean expired CO2) calculated as FeCO2·(PB − 47)/100. FeCO2 is the fraction of expired CO2, PB the barometric pressure, and Vds is the system dead space (50 ml) (Jones 1997).
Arterial partial pressure of oxygen (PaO2) was estimated from arterial oxygen saturation by the Hill equation, assuming a normal HbO2 dissociation curve during graded exercise at sea level, as previously reported by Haseler et al. (2004). In this previous study, a high correlation (r2 = 0.9) between arterial O2 saturation calculated from end-tidal O2 gas measurement and that measured with such an oximeter system was documented. The exercise efficiency was calculated as the relationship between oxygen consumption (VO2) and work rate (WR), taking resting VO2 into account.
Handgrip strength: While lying in a supine position with the arm resting at 90°of abduction and the elbow joint extended at heart level, both the young controls and centenarians performed three maximal voluntary contractions (MVC) using a commercially available handgrip dynamometer (JAMAR BK-7498, Fred Sammons Inc., Brookfield, IL). The average of the three MVCs was recorded as grip strength.
Statistical analyses
Subject characteristics and resting data were analyzed with paired t tests. Repeated measures ANOVA was used to determine differences between groups during exercise, and Tukey post hoc tests were performed where appropriate. Statistical significance was set at P < 0.05, and all data are expressed as mean ± SD.
Results
Subject characteristics
In addition to the 80-year age difference between the centenarians and the young controls, a comparison of subject characteristics is presented in Table 1. An important component of this research was the achievement of this assessment with relative ease and no adverse events, including a lack of arrhythmias or ST-segment changes on the ECG, in this unique population. Thus, a precedent has been set with respect to the feasibility of successful maximal exercise testing in people of such great age.
Table 1.
Subject characteristics
| Centenarians (N = 8) | Young (N = 8) | P value | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Age (years) | 100 ± 1 | (98–101) | 21 ± 1 | (20–22) | <0.001 |
| Body mass (kg) | 47 ± 5 | (42–54) | 56 ± 4 | (50–64) | 0.001 |
| Height (m) | 1.56 ± 0.02 | (1.53–1.61) | 1.66 ± 0∙05 | (1.54–1.70) | <0.001 |
| BMI (kg m−2) | 19 ± 2 | (17–22) | 21 ± 1 | (19–22) | 0.123 |
| Thigh volume (l) | 4.2 ± 0.2 | (3.9–4.4) | 5.7 ± 0.7 | (4.9–7.0) | <0.001 |
| SBP (mmHg) | 136 ± 5 | (127–141) | 118 ± 5 | (110–125) | <0.001 |
| DBP (mmHg) | 89 ± 4 | (83–94) | 78 ± 5 | (70–85) | <0.001 |
| Glucose (mg dl−1) | 88 ± 4 | (84–96) | 86 ± 3 | (82–90) | 0.067 |
| RBC (106 μl−1) | 3.5 ± 0∙3 | (3.0–3.9) | 4.2 ± 0.2 | (3.9–4.5) | <0.001 |
| Hb (g dl−1) | 11.1 ± 1.2 | (9.0–13.0) | 13.6 ± 0.5 | (13.0–14.0) | <0.001 |
| Fe (μg dl−1) | 23 ± 1 | (21–24) | 83 ± 10 | (70–100) | <0.001 |
| HDL (mg dl−1) | 53 ± 8 | (40–62) | 51 ± 7 | (45–61) | 0.539 |
| LDL (mg dl−1) | 109 ± 12 | (90–125) | 95 ± 8 | (85–110) | 0.014 |
| FEV1 (l) | 0.68 ± 0∙12 | (0.52–0.93) | 3.21 ± 0.51 | (2.32–3.80) | <0.001 |
| FVC (l) | 1.23 ± 0∙09 | (1.14–1.44) | 4.16 ± 0.59 | (3.20–4.90) | <0.001 |
| FEV1·FVC−1 (%) | 55 ± 10 | (44–76) | 77 ± 5 | (72–87) | <0.001 |
| Handgrip (kg) | 9 ± 1 | (7–10) | 32 ± 4 | (25–36) | <0.001 |
| ADL | 72 ± 8 | (60–85) | – | – | – |
| MMSE | 26 ± 2 | (24–28) | – | – | – |
Data expressed as mean ± SD
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, RBC red blood cells, Hb hemoglobin, Fe serum iron, HDL high-density lipoprotein, LDL low-density lipoprotein, FEV 1 forced expiratory volume in the first second, FVC forced vital capacity, ADL activities of daily living (Barthel index), MMSE mini-mental state examination
Pulmonary ventilation, gas exchange, and metabolism at rest
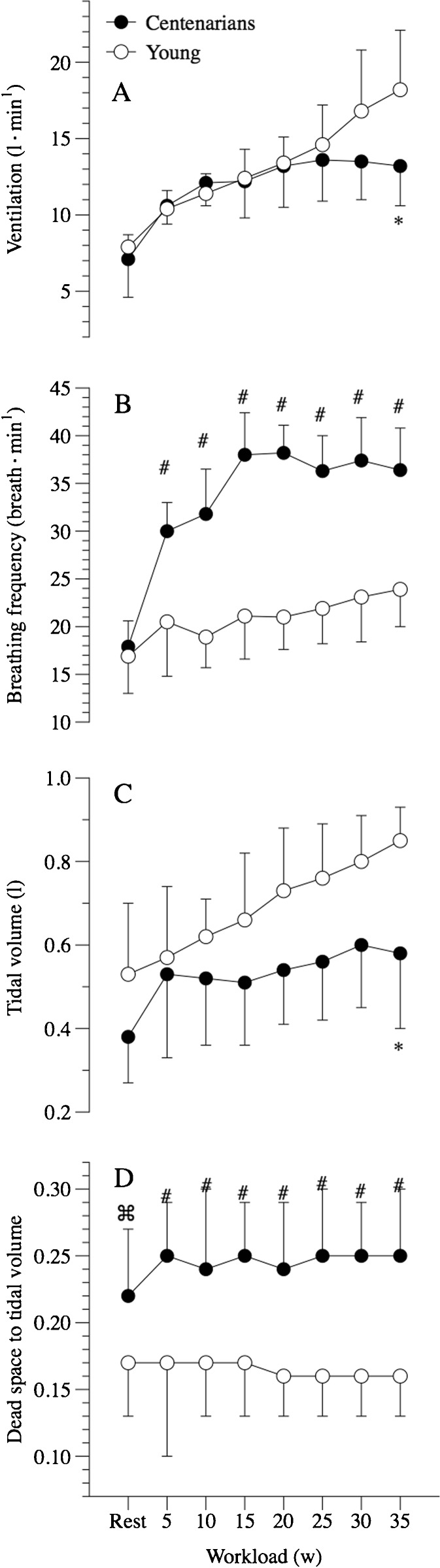
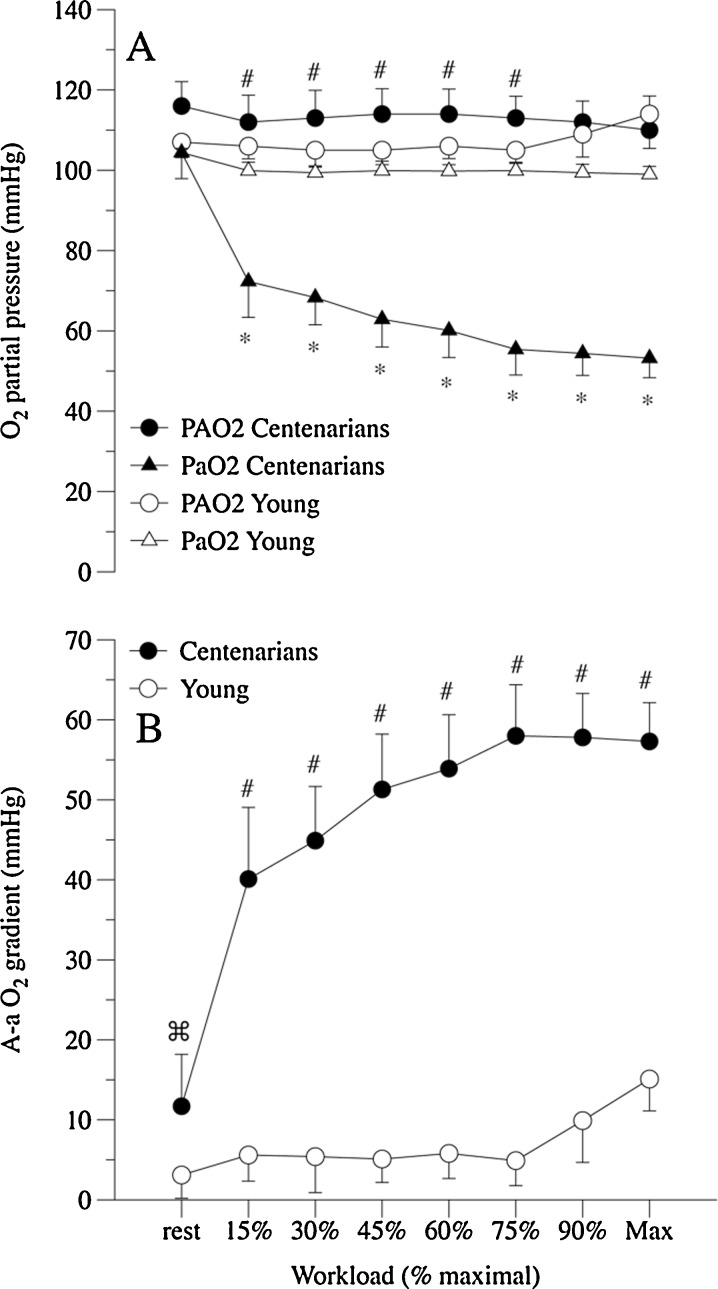
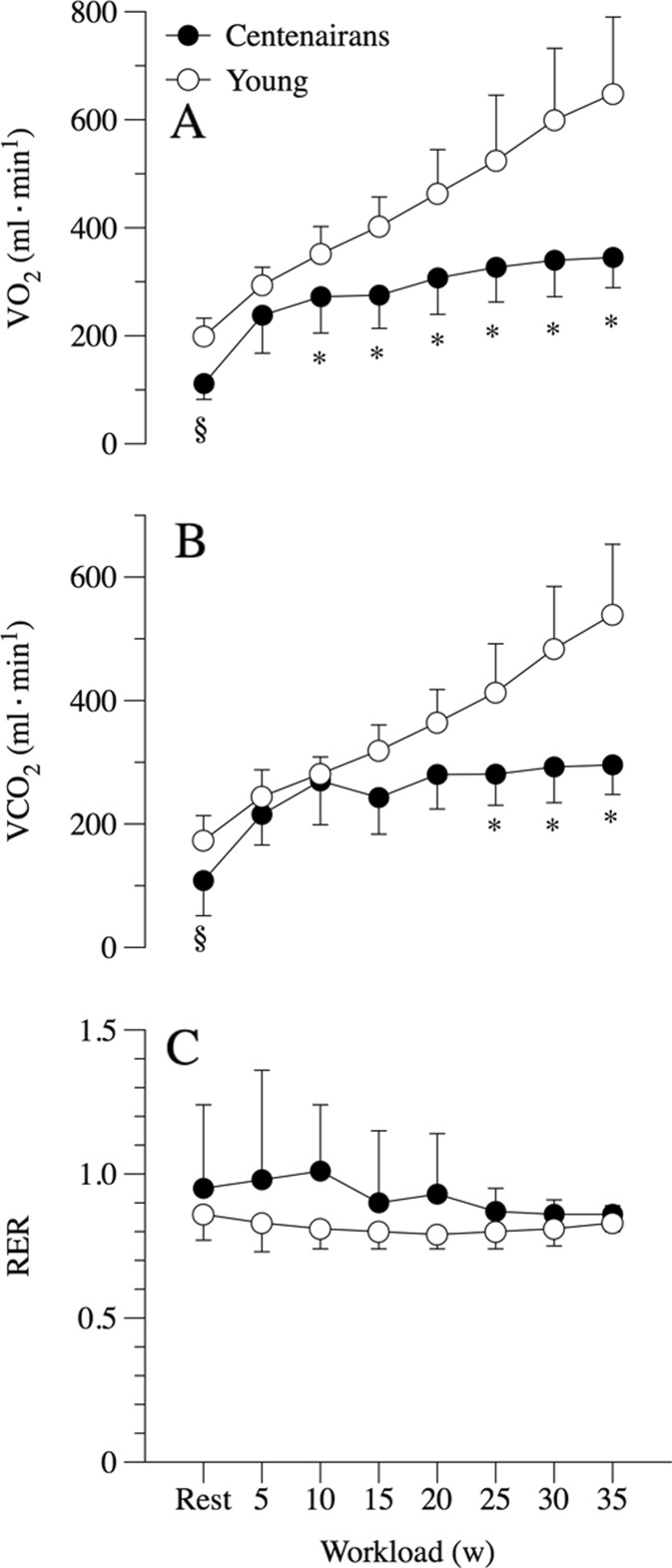
At rest, pulmonary ventilation and its components, breathing frequency and tidal volume, were similar between the centenarians and the young controls (Fig. 1). Calculated dead space was, on the other hand, significantly greater in the centenarians than their young counterparts (Fig. 1). The centenarians tended to have a greater alveolar PO2 (PAO2), but a similar arterial PO2 (PaO2) to the young controls, which yielded a significantly greater resting A-a gradient in the centenarians (Fig. 2). Resting oxygen uptake (VO2) and carbon dioxide production (VCO2) were significantly and equally lower in the centenarians compared to the young controls, and thus, the respiratory exchange ratio (RER) at rest was not different between the two groups (Fig. 3). Additionally, the ratio of dead space to tidal volume (VD∙VT−1) was significantly elevated in the centenarians.
Fig. 1.
Pulmonary ventilation at rest and during incremental cycle exercise to maximum in centenarians and submaximum in young controls. Ventilation (a), breathing frequency (b), tidal volume (c), and dead space to tidal volume ratio (d). Data expressed as mean ± SD. § significantly elevated in the centenarians at rest. * significantly elevated in the centenarians during exercise
Fig. 2.
Pulmonary gas exchange at rest and during incremental exercise to maximal effort in both centenarians and young controls. Alveolar (PAO2) and arterial (PaO2) oxygen partial pressure (a) and alveolar to arterial oxygen partial pressure gradient (A-a O2) (b). Vertical lines indicate standard deviations. Data expressed as mean ± SD. § significantly reduced in the centenarians at rest. * significantly reduced in the centenarians during exercise. ⌘ significantly elevated in the centenarians at rest. # significantly elevated in the centenarians during exercise
Fig. 3.
Metabolism at rest and during incremental cycle exercise to maximum in centenarians and submaximum in young controls. Oxygen uptake (a), CO2 production (b), and respiratory exchange ratio (c). Data expressed as mean ± SD. § significantly reduced in the centenarians at rest. * significantly reduced in the centenarians during exercise
Pulmonary ventilation, gas exchange, and metabolism during incremental exercise
Pulmonary ventilation (Ve) was similar in the centenarians and young controls from 5 to 25 W; however, at 30 W, there was a divergence in Ve that achieved significance at 35 W. This equated to maximal exercise for the majority of the centenarians but was still very light exercise for the young controls (Fig. 1). The approach to attaining this maximal exercise-induced Ve also differed between the centenarians and the young controls, with the older subjects exhibiting a significantly elevated breathing frequency and a tendency for a reduced tidal volume in all but the final 35-W work rate, where this too was significantly attenuated in this group (Fig. 1). As documented at rest, VD∙VT−1 remained consistently higher in centenarians than their young counterparts during exercise.
Across all work rates achieved by the centenarians, PAO2 remained constant and elevated compared to the young controls until the young subjects reached 90% and 100% effort and their PAO2 rose to a similar level to the centenarians (Fig. 2). At the onset of exercise (15% of WRmax), PaO2 of the centenarians initially fell to below 80 mmHg and then continued to decline with progressively more intense exercise, falling below 60 mmHg at maximal exercise. This was in stark contrast to the PaO2 of the young controls, which remained relatively invariant from submaximal to maximal exercise (Fig. 2). Consequently, the A-a O2 gradient in the centenarians rose rapidly during progressive exercise, approaching 60 mmHg at maximal exercise, while the young controls revealed a small increase in this gradient, apparent only at 90% and 100% of WRmax (Fig. 2).
Both the centenarians and young controls exhibited a significant increase in VO2 and VCO2 as the cycle work rate was incremented. One centenarian achieved a maximal work rate of 25 W, two achieved 30 W, and the remaining five attained 35 W (Table 2). All centenarians reported stopping the exercise due to leg discomfort and the inability to maintain 50 rpm on the cycle ergometer. The young controls achieved a maximal work rate of 179 ± 24 W (range, 140–215 W); the reason for the cessation of exercise in the young controls was also the inability to maintain 50 rpm on the cycle ergometer due to both whole body and leg discomfort. Centenarians achieved a significantly lower maximal work rate of 33 ± 4 W (range, 25–35 W). Thus, the VO2peak achieved by the centenarians was significantly lower (7.5 ± 1.2 ml min−1 kg−1) than the young controls (39.6 ± 3.5 ml min−1 kg−1) (Table 2). This was also the case for maximal HR (102 ± 4 and 178 ± 10 bpm, in the centenarians and young, respectively); however, when both groups were compared at the same absolute work rate (30–35 W), the young group exhibited a similar HR to the old (99 ± 9 bpm). Again, when compared at the same absolute work rates (Fig. 3), the centenarians exhibited a significantly attenuated VO2 and VCO2, but as these were both proportionately reduced (below 1), there was no difference in respiratory exchange ratio (RER) between the groups.
Table 2.
Maximal work rate, oxygen consumption, and heart rate in centenarians and young controls at the end of a graded exercise test to maximum effort
| Centenarians (N = 8) | Young (N = 8) | P value | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Maximal work rate (watts) | 33 ± 4 | (25–35) | 179 ± 24 | (215–140) | <0.001 |
| VO2peak (ml min−1 kg−1) | 7.5 ± 1.1 | (6.1–9.3) | 39.6 ± 3.5 | (36.5–46.7) | <0.001 |
| HRpeak (beats min−1) | 102 ± 4 | (97–109) | 178 ± 10 | (166–190) | <0.001 |
VO2peak represents peak of oxygen consumption, and HRpeak represents peak heart rate. Data expressed as mean ± SD
Discussion
The principal finding of this study was that, as hypothesized, centenarians have significantly attenuated pulmonary function that translates into severe gas exchange limitations across the lungs during exercise. However, contrary to our hypotheses, this limited oxygen transport was tempered by profound improvements in skeletal muscle metabolic efficiency. Therefore, the centenarians were successfully able to perform submaximal efforts that, when performed by their younger counterparts, exceeded the maximal VO2 of the older group. Thus, it is quite possible that in the face of failing lungs, this until now unrecognized skeletal muscle metabolic efficiency plays a vital role in maintaining physical function and therefore longevity in this highly select and successful population.
Maximal oxygen uptake in centenarians
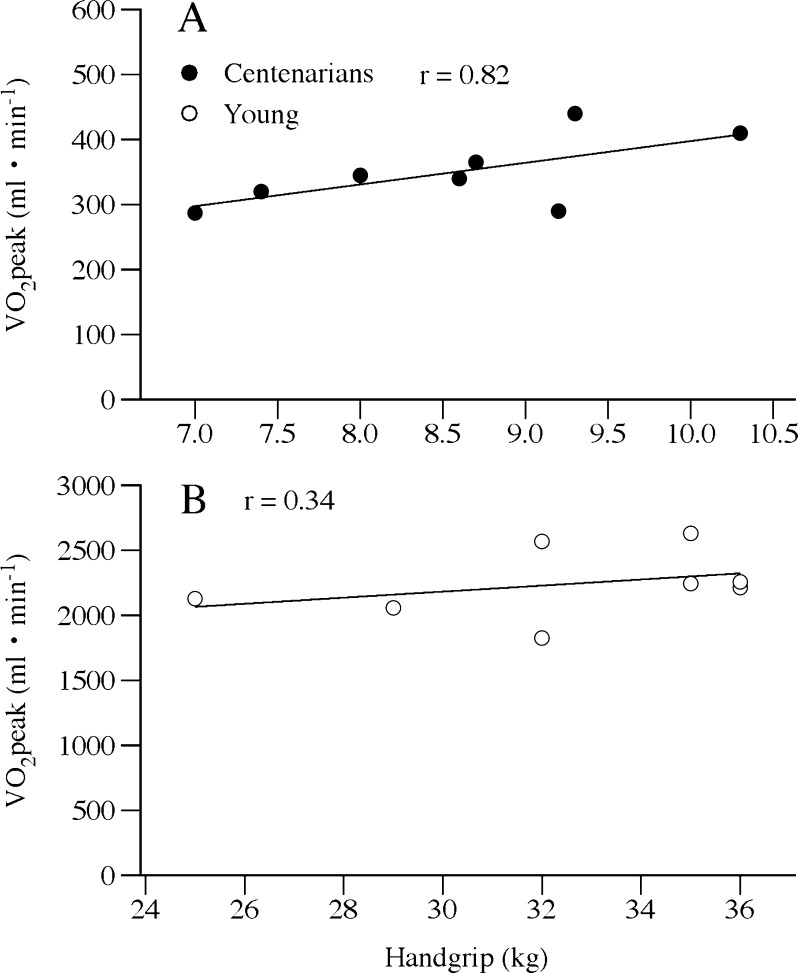
To our knowledge, this is the first investigation to directly measure VO2max in a cohort of centenarians. There are two intertwined physiological and methodological concerns regarding such data collection and interpretation in this population: first, the recognized slowing of VO2 onset kinetics with age (Murias et al. 2011; Bell et al. 1999; DeLorey et al. 2004) and, second, the need to balance this phenomenon with the goal of an attainable and valid incremental test to maximal effort, while avoiding the confounding issue of peripheral fatigue (Jones 1997). Although the 5-W increments were significantly more challenging increases in effort for the centenarians than the young, these increments were still only increases of ∼15% of the maximum work rate. A previous study utilizing 10%, 15%, and 20% of maximal work rate increments revealed that, although subjects achieved a greater work rate with the smaller increments, VO2peak was unaltered (Davis et al. 1982), supporting both the validity of the current data and the methodological approach. Additionally, the current direct assessment of VO2peak in this population (2.1 ± 0.3 metabolic equivalents, METS) is in excellent agreement with the extrapolation, beyond the upper age limit (85 years), of the Gulati nomogram (1.96 ± 0.15 METS) (Gulati et al. 2005). It is also interesting to note that VO2peak and maximal handgrip strength, both recognized to be strong predictors of health and independence in older adults (Fleg et al. 2005; Paterson et al. 2004; Cress et al. 2010; Rantanen et al. 1999), were well correlated in the centenarians studied here (r = 0.8, P = 0.02) (Fig. 4a), but not in the young (r = 0.3, P = 0.4) (Fig. 4b). This observation not only adds credence to the maximal exercise testing results but also highlights the potential use of handgrip strength assessment as a potentially useful alternative to such whole body testing in this population.
Fig. 4.
The relationship between VO2peak and maximal handgrip force in the centenarians (a) and young controls (b)
Impact of lung function at rest and during exercise in centenarians
At rest, although ventilation itself was not different between the young controls and the centenarians (Fig. 1), upon closer inspection of the open circuit calorimetry data, even in this state of relatively low metabolic demand, there was already a very important distinction between these two groups. Specifically, the dead space to tidal volume ratio was significantly elevated in the centenarians, likely explained by an age-induced increase in lung compliance (DeLorey et al. 2007). Such a conclusion is supported by the spirometric assessments that revealed a significantly reduced FEV1, FVC, and the ratio of these two variables in the centenarians compared to the young controls (Table 1). These findings are typical of the increased lung compliance associated with smoking-induced emphysema, although none of these subjects had a smoking history. The practical consequence of this pulmonary dysfunction was that even at rest, there was a clear A-a gradient in the centenarians that was not evident in the young controls (Fig. 2b). Interestingly, due to the limited peripheral metabolic demand, both because of the resting conditions and the smaller body mass of the centenarians (Fig. 1), this finding was more a consequence of a greater PAO2 than a reduced PaO2 (Fig. 2a).
Upon the commencement of exercise, the significantly elevated VD∙VT−1 of the centenarians was maintained, but was now in addition to a significantly elevated respiratory rate (Fig. 1) and a tendency toward lower tidal volumes, both indicative of structurally induced alterations in the approach to achieve adequate ventilation. However, although not consistently attenuated, ventilation tended to be lower at the penultimate level of graded exercise and was significantly lower than the controls at maximal effort (Fig. 1). The assessment of PAO2 indicates that this ventilatory strategy was apparently successful as PAO2 remained elevated compared to the young controls across much of the graded exercise test. However, this observation was undoubtedly assisted by the apparent failure to move oxygen from the lungs to the blood at an adequate rate (Fig. 2a) and the subsequent development of a large A-a gradient across all exercise levels (Fig. 2b).
Based upon both the exercise-induced hypoxemia literature (Dempsey et al. 2008) and the evidence that direct hypoxia itself (Roca et al. 1992) can severely impact exercise performance, there is little doubt that this large A-a gradient had a negative impact on the maximum work rate achieved during cycle exercise in the centenarians. The current findings are certainly in agreement with previously documented decrements in lung function during exercise with advancing age (Johnson et al. 1994), although of greater magnitude which may be explained by the approximate 25-year age difference between the centenarians studied in the current research and the subjects who were considered old in the previous work. Unfortunately, the current data cannot determine the cause of a large A-a gradient which could be a consequence of limited lung diffusing capacity, ventilation/perfusion mismatch, or, less likely, a significant shunt. Thus, it would certainly be of interest to re-assess such a group of subjects with and without adequate supplemental oxygen to determine if such an approach could avoid this arterial oxygen desaturation, and, if this were the case, what the practical consequence would be in terms of exercise capacity.
Metabolism at rest and during exercise in centenarians
Although pulmonary VO2 assesses whole body metabolic demand, it is well recognized that during incremental exercise, an increase in VO2 predominantly reflects an increased metabolic demand at the muscular level (Poole et al. 1992). Thus, the finding that the centenarians had a significantly attenuated oxygen cost when compared to the young controls, at all but the initial work rate, indicates that this phenomenon is likely a consequence of altered skeletal muscle metabolism during exercise. Of course, the marked difference in leg muscle mass between the two groups (36% lower in centenarians) initially evokes concern that this attenuated oxygen cost of work may simply be due to relatively severe sarcopenia (Woo et al. 2009). Specifically, the weight of each leg, which contributes unmeasured work, was significantly lower in the centenarians and may have contributed to the apparently different oxygen cost of cycling between the old and the young. However, the evidence against this being a significant factor are twofold. First, a direct assessment of the oxygen cost of unweighted cycling was performed in both the young controls (200 ± 50 ml/min) and the centenarians (181 ± 90 ml/min) prior to the graded exercise testing, and there was no discernable group difference. Second, if this were a consistent factor which explained the significantly attenuated oxygen cost of work in the centenarians, the VO2/WR relationship of this group would be expected to have the same slope, but a different intercept, which was not the case. In fact, the intercept was similar, but the slope was greatly attenuated (Fig. 3a). Instead, it is possible that the age-related loss of muscle mass, predominantly caused by the loss of fast motor units, may have had a positive effect on mechanical efficiency and therefore the oxygen cost of performing work by the adaptation to a slower muscle fiber phenotype (Hunter et al. 2001; Gibbs and Gibson 1972).
An adaptation and subsequent improvement in mechanical efficiency with age have important implications for the functional capacity of the centenarians. Specifically, the dramatically different slope of the VO2/WR relationship (Fig. 3a) between the centenarians and their young counterparts reveals a scenario in which the young controls, exhibiting a slope typical of the literature within the range of 9–11 ml/O2/W (Gaesser and Brooks 1975; Kamon et al. 1973), is already utilizing the same amount of oxygen as the old group did at their maximum work rate of 35 W at only 15 to 20 W. This difference in the oxygen cost of work may be of great practical significance, as this means that the amount of work that centenarians can achieve within the much attenuated scope of their aerobic capacity, likely due to the limited function of the failing lungs, is greatly enhanced. Such a phenomenon is highly likely to translate into greater physical function and the ability to perform ADLs that otherwise would have been beyond the aerobic capacity of a centenarian. Whether this potential for increased activity is the cause or consequence of achieving such a great age is certainly a very intriguing question, which unfortunately cannot be answered by the current data.
Study limitations
Clear limitations of the current study were the absence of men and the use of cycle exercise, which may have accentuated the age differences due to sarcopenia in the centenarians. Further limitations were the clear differences in habitual physical activity between the young and centenarian groups, the lack of cardiac output measurements, serum lactate assessments, and the direct O2 partial pressure measurement during the exercise. However, with respect to the latter issue, it was not deemed ethically appropriate to subject members of this relatively fragile population to undergo arterial catheterization. Although there are uncertainties associated with the conversion of arterial saturation to partial pressure with the Hill equation, it should be noted that we have successfully utilized this approach in the past (Haseler et al. 2004). Indeed, the large intergroup differences in estimated PaO2 documented in this study are unlikely to be the result of such relatively small inaccuracies associated with this conversion.
Conclusions
In the face of the failing lungs, increased skeletal muscle metabolic efficiency in centenarians may be an important component of maintaining physical function. This phenomenon may contribute to the longevity of this population.
Acknowledgments
The authors greatly appreciate the time and effort of the subjects that participated in this study.
This work was supported in part by the PPG (PO1 HL, 09830) the VA Merit grant, and Mons Mazzali Foundation.
References
- Bell C, Paterson DH, Kowalchuk JM, Cunningham DA. Oxygen uptake kinetics of older humans are slowed with age but are unaffected by hyperoxia. Exp Physiol. 1999;84(4):747–759. doi: 10.1017/S0958067099018631. [DOI] [PubMed] [Google Scholar]
- Cress ME, Gondo Y, Davey A, Anderson S, Kim SH, Poon LW (2010) Assessing physical performance in centenarians: norms and an extended scale from the Georgia centenarian study. Curr Gerontol Geriatr Res. doi:10.1155/2010/310610 [DOI] [PMC free article] [PubMed]
- Davis JA, Whipp BJ, Lamarra N, Huntsman DJ, Frank MH, Wasserman K. Effect of ramp slope on determination of aerobic parameters from the ramp exercise test. Med Sci Sports Exerc. 1982;14(5):339–343. doi: 10.1249/00005768-198205000-00005. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Babb TG. Progressive mechanical ventilatory constraints with aging. Am J Respir Crit Care Med. 1999;160(1):169–177. doi: 10.1164/ajrccm.160.1.9807045. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Effect of age on O(2) uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol. 2004;97(1):165–172. doi: 10.1152/japplphysiol.01179.2003. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metab. 2007;32(6):1251–1262. doi: 10.1139/H07-121. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, McKenzie DC, Haverkamp HC, Eldridge MW. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest. 2008;134(3):613–622. doi: 10.1378/chest.07-2730. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol. 1975;38(6):1132–1139. doi: 10.1152/jappl.1975.38.6.1132. [DOI] [PubMed] [Google Scholar]
- Galioto A, Dominguez LJ, Pineo A, Ferlisi A, Putignano E, Belvedere M, Costanza G, Barbagallo M. Cardiovascular risk factors in centenarians. Exp Gerontol. 2008;43(2):106–113. doi: 10.1016/j.exger.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Gibbs CL, Gibson WR. Energy production of rat soleus muscle. Am J Physiol. 1972;223(4):864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, Marwick TH, Pandey DK, Wicklund RH, Thisted RA. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97(3):1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- Hollenberg M, Yang J, Haight TJ, Tager IB. Longitudinal changes in aerobic capacity: implications for concepts of aging. J Gerontol A Biol Sci Med Sci. 2006;61(8):851–858. doi: 10.1093/gerona/61.8.851. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve. 2001;24(5):654–661. doi: 10.1002/mus.1051. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Badr MS, Dempsey JA. Impact of the aging pulmonary system on the response to exercise. Clin Chest Med. 1994;15(2):229–246. [PubMed] [Google Scholar]
- Jones NL. Clinical exercise testing. 4. Philadelphia: Saunders; 1997. [Google Scholar]
- Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204(2):63P–66P. [PubMed] [Google Scholar]
- Kamon E, Metz KF, Pandolf KB. Climbing and cycling with additional weights on the extremities. J Appl Physiol. 1973;35(3):367–370. doi: 10.1152/jappl.1973.35.3.367. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- Levy ML, Quanjer PH, Booker R, Cooper BG, Holmes S, Small I. Diagnostic spirometry in primary care: proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations. Prim Care Respir J. 2009;18(3):130–147. doi: 10.4104/pcrj.2009.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No:11–16 [DOI] [PubMed]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol. 1995;78(5):1957–1968. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- Murias JM, Spencer MD, Kowalchuk JM, Paterson DH. Influence of phase I duration on phase II VO2 kinetics parameter estimates in older and young adults. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R218–R224. doi: 10.1152/ajpregu.00060.2011. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maffulli N (2010) Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. doi:10.1093/bmb/ldq008 [DOI] [PubMed]
- Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52(10):1632–1638. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- Perls T, Terry D. Genetics of exceptional longevity. Exp Gerontol. 2003;38(7):725–730. doi: 10.1016/S0531-5565(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Perls T, Terry D. Understanding the determinants of exceptional longevity. Ann Intern Med. 2003;139(5 Pt 2):445–449. doi: 10.7326/0003-4819-139-5_Part_2-200309021-00013. [DOI] [PubMed] [Google Scholar]
- Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol. 1992;72(2):805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281(6):558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol. 1992;73(3):1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Arch Intern Med. 2009;169(16):1476–1483. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- Woo J, Leung J, Sham A, Kwok T. Defining sarcopenia in terms of risk of physical limitations: a 5-year follow-up study of 3,153 Chinese men and women. J Am Geriatr Soc. 2009;57(12):2224–2231. doi: 10.1111/j.1532-5415.2009.02566.x. [DOI] [PubMed] [Google Scholar]