Abstract
Exercise has been demonstrated to enhance subsequent insulin-stimulated glucose uptake (GU) by predominantly type II (fast-twitch) muscle of old rats, but previous research has not evaluated exercise effects on GU by type I (slow-twitch) muscle from old rats. Accordingly, we studied male Fischer 344/Brown Norway rats (24 months old) and determined GU (0, 100, 200, and 5,000 μU/ml insulin) of isolated soleus (predominantly type I) and epitrochlearis (predominantly type II) muscles after one exercise session. Epitrochlearis (100, 200, and 5,000 μU/ml insulin) and soleus (100 and 200 μU/ml insulin) GU were greater at 3-h postexercise vs. age-matched sedentary controls. Insulin receptor tyrosine phosphorylation (Tyr1162/1163) was unaltered by exercise in either muscle. Akt phosphorylation (pAkt) was greater for exercised vs. sedentary rats in the epitrochlearis (Ser473 and Thr308 with 100 and 200 μU/ml, respectively) and soleus (Ser473 with 200 μU/ml). AS160 phosphorylation (pAS160) was greater for exercised vs. sedentary rats in the epitrochlearis (Thr642 with 100 μU/ml), but not the soleus. Exercised vs. sedentary rats did not differ for total protein abundance of insulin receptor, Akt, AS160, or GLUT4 in either muscle. These results demonstrate that both predominantly type I and type II muscles from old rats are susceptible to exercise-induced improvement in insulin-mediated GU by mechanisms that are independent of enhanced insulin receptor tyrosine phosphorylation or altered abundance of important signaling proteins or GLUT4. Exercise-induced elevation in pAkt, and possibly pAS160, may contribute to this effect in the epitrochlearis of old rats, but other mechanisms are likely important for the soleus.
Keywords: Glucose transport, Aging, Insulin signaling, Insulin resistance
Introduction
Because whole-body insulin sensitivity has been linked to the occurrence of many age-related diseases (Facchini et al. 2001), it is important to understand the regulation of insulin-mediated glucose disposal with advancing age. Skeletal muscle, the site for most of the insulin-induced blood glucose clearance (DeFronzo et al. 1981), plays a central role in whole-body glucose regulation. In vivo studies using the euglycemic clamp with rats at ~6–14 months old vs. 20–24 months old have reported to have reduced whole-body glucose disposal (Escriva et al. 2007; Gu et al. 2011; Catalano et al. 2005; Nishimura et al. 1988). Moreover, there is evidence for a greater age-related decrement in insulin-stimulated glucose uptake by predominantly type I (slow-twitch) compared to predominantly type II (fast-twitch) skeletal muscles (Escriva et al. 2007; Sharma et al. 2010). In this context, it is valuable to identify interventions to improve insulin sensitivity in both type I and type II muscles during old age.
Many studies have demonstrated that a single bout of exercise can lead to a persistent increase in insulin-stimulated glucose uptake of skeletal muscle in young (~1–3-month-old) rats (Cartee and Holloszy 1990; Cartee et al. 1989a, b; Richter et al. 1982), (~2-month-old ) mice (Hamada et al. 2006), and (~20–30-year-old) humans (Perseghin et al. 1996; Wojtaszewski et al. 2000). In young rats and mice, prior exercise increases insulin-stimulated glucose uptake in both muscles composed of predominantly type I fibers and muscles composed mainly of type II fibers. Acute exercise can also increase insulin-mediated glucose transport in isolated epitrochlearis muscles (which is composed of predominantly type II fibers) from 25-month-old rats (Cartee et al. 1993), but no previous studies have evaluated the effects of a single exercise bout on glucose uptake by a predominantly type I muscle in old rats.
The underlying mechanisms for the exercise-induced enhancement of insulin-stimulated glucose transport remain to be elucidated. Many studies in young individuals have reported little or no effects of acute exercise on proximal insulin signaling steps (including the activation of the insulin receptor, insulin receptor substrate-1, phosphatidylinositol-3-kinase, and Akt) in skeletal muscle (Bonen et al. 1985; Bonen et al. 1984; Zorzano et al. 1985; Hansen et al. 1998; Howlett et al. 2002; Treadway et al. 1989; Wojtaszewski et al. 1999; Thong et al. 2002; Wojtaszewski et al. 2000; Arias et al. 2007; Fisher et al. 2002; Hamada et al. 2006). Although proximal insulin signaling in young rats is not enhanced by acute exercise, there is evidence that acute exercise can lead to a sustained phosphorylation of a distal insulin signaling protein known as Akt substrate of 160 kDa (Arias et al. 2007; Funai et al. 2009; Funai et al. 2010). AS160 is the most distal signaling protein implicated in insulin-mediated GLUT4 translocation in skeletal muscle (Cartee and Wojtaszewski 2007; Kramer et al. 2006; Sakamoto and Holman 2008; Sano et al. 2003; Cartee and Funai 2009; Bruss et al. 2005). In contrast to the well-established exercise effects on AS160 in young rats, the influence of exercise on AS160 phosphorylation in skeletal muscle from old rats has not been previously reported.
To address some of the gaps in knowledge about exercise effects on insulin sensitivity in older individuals, we studied the influence of a single exercise session on skeletal muscle glucose uptake and insulin signaling in 24-month-old rats. Important aspects of the experimental design included the following: (1) studying both a predominantly type I muscle (soleus) and a predominantly type II muscle (epitrochlearis); (2) using a range of insulin concentrations (0, 100, 200, and 5,000 μU/ml); (3) assessing the activation of key insulin signaling steps (insulin receptor tyrosine phosphorylation, Akt Ser473 and Thr308 phosphorylation, and AS160 Ser588 and Thr642 phosphorylation); and (4) measuring total abundance of the insulin-regulated glucose transporter protein (GLUT4). We hypothesized that the 24-month-old exercised rats compared to the age-matched sedentary rats would be characterized by elevated insulin-mediated glucose uptake in both the soleus and the epitrochlearis muscles concomitant with a sustained increase in AS160 phosphorylation, but without exercise-related increases in proximal insulin signaling steps (insulin receptor phosphorylation, Akt phosphorylation) or GLUT4 protein abundance.
Experimental procedures
Materials
Unless otherwise noted, all chemicals were purchased from Sigma Chemical (St. Louis, MO) or Fisher Scientific (Hanover Park, IL). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Anti-Akt (#9272), anti-phospho-AktSer473 (pAktSer473, #9271), anti-phospho-AktThr308 (pAktThr308, #9275), and anti-rabbit IgG horseradish peroxidase (#7074) were from Cell Signaling Technology (Danvers, MA). Anti-phospho-AS160Thr642 (pAS160Thr642, #07-802) and anti-AS160 (#07-741), anti-GLUT4 (#CBL-243), anti-mouse IgG horseradish peroxidase conjugate (#12-349), and anti-sheep IgG horseradish peroxidase conjugate (#12-342) were from Millipore (Billerica, MA). Anti-phospho-AS160Ser588 (pAS160 Ser588, #3028P2) was from B-Bridge International Biosciences (San Jose, CA). Anti-phospho-IRTyr1162/1163 (pIRTyr1162/1163, #44-504 G) and anti-IR (#AHR0271) were from Invitrogen (Camarillo, CA). 2-Deoxy-d-[3H]glucose ([3H]-2-DG) and [14 C]mannitol were from Perkin Elmer (Boston, MA).
Animal care
The animal protocol for this study was approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer 344 × Brown Norway, F1 generation rats of 24 months old were obtained from Harlan (Indianapolis, IN) and were allowed to equilibrate to their new environment for 1–2 weeks prior to experimentation. Animals were individually housed in specific pathogen-free conditions in micro-isolation filter top cages, maintained on a 12-12 h light-dark cycle (lights out at 1700 hours), and provided with standard rat chow (Lab Diet #5001; PMI Nutritional International, Brentwood, MO) and water ad libitum.
Exercise protocol
Rats were restricted to about 5 g of chow the night before the experimental day. Exercise started at about 0800 hours. Rats were randomly assigned to exercise and sedentary groups. Rats swam in groups of four in barrels (52 cm in diameter) filled to a depth of ~45 cm with water maintained at 35°C. The exercise protocol consisted of up to eight bouts of swim exercise (the initial bout had 10-min duration, with the subsequent bout durations equaling 10–20 min with 10-min rest intervals separating each bout) with the goal for the total duration of the exercise equaling 90 min. Rats were anesthetized (intraperitoneal injection of sodium pentobarbital, 50 mg/kg) at 3–4 h after the exercising group had completed the protocol.
Muscle dissection and incubation
When rats were deeply anesthetized, soleus and epitrochlearis muscles were removed and rapidly rinsed in warm (35°C) Krebs–Henseleit buffer (KHB). Muscles were longitudinally split into strips of similar size for each muscle (two strips for each epitrochlearis and four strips for each soleus). Muscle strips were subsequently placed in vials containing the appropriate media shaking (45 rpm) and continuous gassing (95% O2/5% CO2) in a heated (35°C) water bath. In the first incubation step, all muscles were incubated in vials containing 2 ml KHB supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, 6 mM mannitol, and either 0, 100, 200, or 5,000 μU/ml insulin for 30 min. All muscles were then transferred to a second vial containing 2 ml of KHB/BSA solution, the same insulin concentration as the previous step, 1 mM 2-DG (including a final specific activity of 2.25 mCi/mmol [3H]-2-DG), and 9 mM mannitol (including a final specific activity of 0.022 mCi/mmol [14 C]mannitol) for 20 min. Following the final incubation step, muscles were rapidly blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80°C for later processing and analysis.
Muscle lysate preparation
Frozen muscles were weighed, homogenized in ice-cold lysis buffer (1 ml/muscle strip) using a TissueLyser II homogenizer (Qiagen Inc, Valencia, CA). The lysis buffer containing Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL; #78510) supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM ß-glycerophosphate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Homogenates were transferred to microfuge tubes, rotated for 1 h at 4°C, and then centrifuged (15,000 × g) for 15 min (4°C) to remove insoluble material. Protein concentration was measured using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL; #23225).
2-Deoxy-d-glucose uptake
Aliquots (200 μl) of the supernatants from centrifuged muscle lysates were combined in a vial with 10 ml of scintillation cocktail (Research Products International, Mount Prospect, IL), and a scintillation counter (Perkin Elmer) was used to determine 3H and 14C disintegrations per minute. These values were used to determine [3H]-2-DG uptake as previously described (Cartee and Bohn 1995; Hansen et al. 1994).
Immunoblotting
An equal amount of protein of each sample was mixed with 6X Laemmli buffer, boiled for 5 min and separated using SDS-PAGE (7% resolving gel) before being transferred to nitrocellulose membranes. Membranes were blocked in 5% BSA in TBST for 30 min at room temperature and transferred to 5% BSA-TBST with the appropriate primary antibody for 2 h at room temperature or for overnight at 4°C. Membranes were washed three times for 10 min in TBST and incubated with secondary antibody (1:2,000) in 5% nonfat milk for 1 h at room temperature. Blots were washed three times for 10 min in TBST and then incubated with West Dura Extended Duration Substrate (Pierce, #34075) to visualize protein bands. Immunoreactive proteins were quantified by densitometry (AlphaEase FC; Alpha Innotech, San Leandro, CA). Values are normalized to the average values of the basal (no insulin) samples on each blot.
Statistical analysis
Student’s t test was used for all comparisons between the sedentary (SED) and exercised (EX) groups. Data are presented as mean ± SEM. A P value ≤0.05 was considered statistically significant.
Results
Exercise
Six of the eight exercising rats were unable to complete the goal for total exercise of 90 min. The mean ± SEM for total exercise duration was 75.8 ± 5.6 min.
2-Deoxy-d-glucose uptake
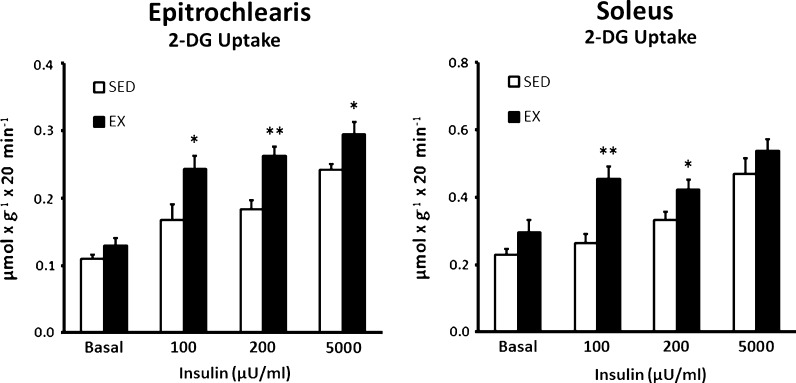
Basal 2-DG uptake of the epitrochlearis was not significantly different between SED and EX rats (Fig. 1). Epitrochlearis 2-DG uptake was significantly increased for EX vs. SED rats with 100 (P < 0.05), 200 (P < 0.01), or 5,000 (P < 0.05) μU/ml insulin. Similar to the results for the epitrochlearis, the basal 2-DG uptake of the soleus was not significantly different between SED and EX rats (Fig. 1). Soleus 2-DG uptake was significantly greater for EX vs. SED rats with 100 (P < 0.01) or 200 (P < 0.05) μU/ml insulin, but not with 5,000 μU/ml.
Fig. 1.
2-Deoxy-d-glucose (2-DG) uptake in epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. Single asterisk indicates P < 0.05 and double asterisk indicates P < 0.01 on SED vs. EX in the same insulin treatment group. Data are means ± SEM. n = 8–10 muscles per exercise group and insulin concentration
Abundance of insulin signaling proteins and GLUT4
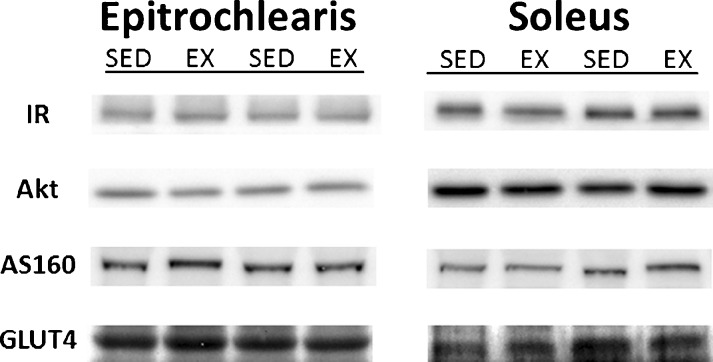
There were no significant differences for SED vs. EX groups in the total abundance of any of the signaling proteins that were evaluated (insulin receptor, Akt, AS160, and GLUT4) for either the epitrochlearis or soleus (Fig. 2).
Fig. 2.
Total protein abundance of key insulin signaling steps (IR, Akt, AS160, and GLUT4) in epitrochlearis and soleus muscles. There were no statistically significant differences for SED vs. EX groups. Data are means ± SEM. n = 8 muscles per exercise group
Insulin receptor phosphorylation
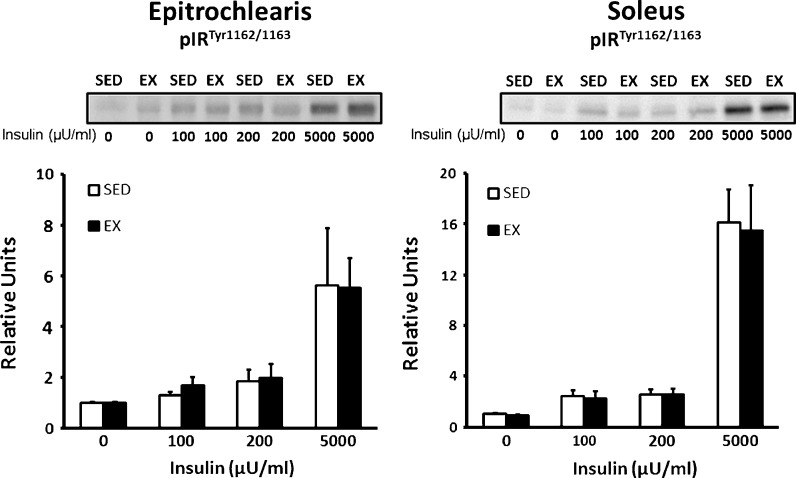
Insulin receptor phosphorylation (Tyr1162/1163) was not significantly different for SED vs. EX groups in either the epitrochlearis or soleus regardless of the insulin concentration (Fig. 3).
Fig. 3.
Insulin receptor tyrosine phosphorylation (Tyr1162/1163) in the epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. There were no statistically significant differences for SED vs. EX groups. Data are means ± SEM. n = 8 muscles per exercise group and insulin concentration
Akt phosphorylation
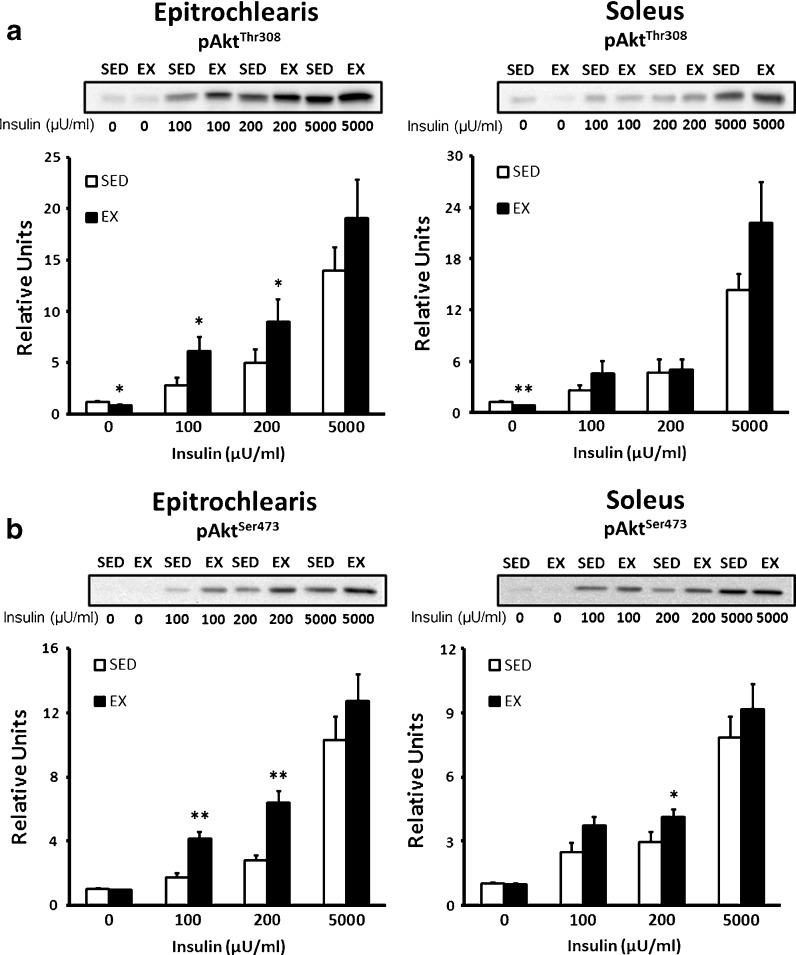
In the epitrochlearis, without insulin, there was a small but statistically and significantly greater (P < 0.05) value for Akt Thr308 phosphorylation of SED vs. EX rats. The epitrochlearis of EX vs. SED rats had significantly greater phosphorylation on both Akt Thr308 (Fig. 4a) and Akt Ser 473 (Fig. 4b) with 100 (P < 0.05) or 200 (P < 0.05) μU/ml insulin, but not with 5,000 μU/ml insulin (Fig. 4a, b). There was no significant exercise effect on Akt Ser473 phosphorylation in the epitrochlearis without insulin. In the soleus, without insulin, Akt Thr308 phosphorylation was slightly but significantly greater (P < 0.01) for SED vs. EX rats. There were no significant exercise effects on soleus Akt Thr308 phosphorylation with 100, 200, or 5,000 μU/ml insulin (Fig. 4a). The soleus of EX vs. SED rats had significantly greater Akt Ser473 phosphorylation only with 200 μU/ml insulin (P < 0.05) (Fig. 4b).
Fig. 4.
Akt phosphorylation in epitrochlearis and soleus muscles. a pAktThr308 in epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. Muscle lysate was immunobloted with the pAktThr308 antibody. b pAktSer473 in epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. Muscle lysate was immunobloted with the pAktSer473 antibody. Single asterisk indicates P < 0.05 and double asterisks indicates P < 0.01 on SED vs. EX in the same insulin treatment group. Data are means ± SEM. n = 8 muscles per exercise group and insulin concentration
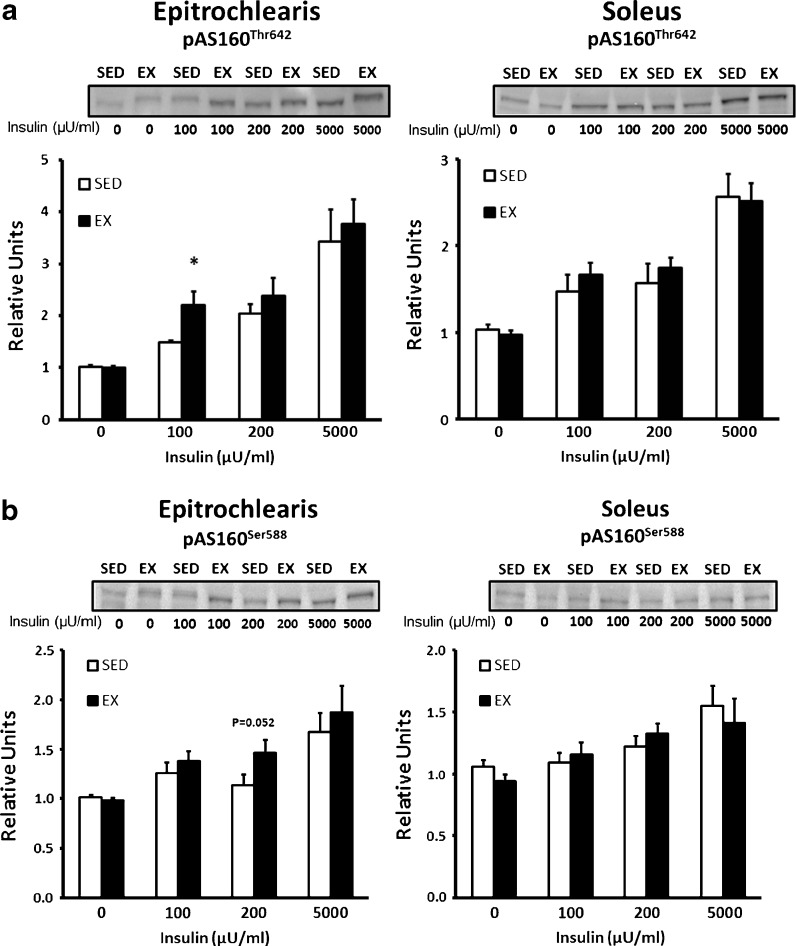
AS160 phosphorylation
In the epitrochlearis without insulin, there were no significant differences for EX vs. SED rats for phosphorylation of AS160 on Thr642 or Ser588 (Fig. 5a, b). AS160 Thr642 phosphorylation in the epitrochlearis was significantly greater (P < 0.05) for EX vs. SED rats with 100 μU/ml insulin, but not with 200 or 5,000 μU/ml insulin (Fig. 5a). AS160 Ser588 phosphorylation in the epitrochlearis tended to be greater (P = 0.052) for EX vs. SED rats with 200 μU/ml insulin, but there were no exercise effects with the other insulin concentrations (Fig. 5b). In the soleus, there were no significant differences between SED and EX rats for AS160 phosphorylation on either phosphosite regardless of the insulin dose (Fig. 5a, b).
Fig. 5.
AS160 phosphorylation in epitrochlearis and soleus muscles. a pAS160Thr642 in epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. b pAS160Ser588 in epitrochlearis and soleus muscles with 0, 100, 200, or 5,000 μU/ml insulin. Single asterisk indicates P < 0.05 on SED vs. EX in the same insulin treatment group. Data are means ± SEM. n = 8 muscles per exercise group and insulin concentration
Discussion
Because skeletal muscle is a heterogenous tissue composed of muscle fibers that can vary markedly with regard to their contractile or metabolic phenotype, it is valuable to assess the influence of exercise on muscles with differing fiber type profiles. The current results are the first to demonstrate that acute exercise by old rats can lead to improved insulin-stimulated glucose uptake by skeletal muscles composed mainly of either type I (soleus) or type II (epitrochlearis) fibers. Earlier research using young rats (~1.5–2 months old) had also found that exercise can lead to increased insulin-stimulated glucose uptake by skeletal muscles with various muscle fiber type compositions, including muscles composed of predominantly type II (Cartee et al. 1989a; Cartee and Holloszy 1990) or predominantly type I (Tanaka et al. 2007) fibers. An improvement in insulin sensitivity is indicated by a reduction in the insulin dose required for half-maximal stimulation (Kahn 1978), and the results from this experiment are consistent with improved insulin sensitivity for both the soleus and the epitrochlearis. Glucose uptake was also increased with a maximally effective insulin dose in the epitrochlearis, but not in the soleus after exercise.
We probed potential mechanisms for the exercise-induced improvement in insulin action by evaluating the activation of key insulin signaling proteins, including the insulin receptor, Akt and AS160. Prior exercise did not alter tyrosine phosphorylation of the insulin receptor in either muscle. However, Akt phosphorylation (pThr308 and pSer473) was significantly increased for the exercised vs. the sedentary group in the epitrochlearis with 100 μU/ml and 200 μU/ml. Akt phosphorylation was also significantly greater for exercised vs. sedentary rats for soleus muscles with 200 μU/ml (pSer473). Several previous studies of young or adult rats (Arias et al. 2007; Koshinaka et al. 2009; Tanaka et al. 2007), mice (Hamada et al. 2006), or humans (Thong et al. 2002; Wojtaszewski et al. 2000) have not found prior exercise effects on insulin-stimulated pSer473 in muscle. However, earlier studies reported that acute exercise could result in greater insulin-stimulated pThr308 for skeletal muscles from young rats (Arias et al. 2007; Funai et al. 2009; Koshinaka et al. 2009). In the current study, we also detected small but statistically significant exercise-related decrements in pThr308 in both muscles from old rats in the absence of insulin. Previous studies have not reported altered pThr308 or pSer473 in muscles without insulin 3–4 hours after acute exercise by young rats. The mechanisms accounting for the small changes in basal Akt phosphorylation in old rats are uncertain. However, there was no evidence for a functional consequence given that there were no detectable exercise effects on either basal AS160 phosphorylation or basal glucose uptake.
AS160 is the most distal insulin signaling protein that has been clearly linked to enhanced glucose transport in skeletal muscle (Cartee and Funai 2009; Cartee and Wojtaszewski 2007; Sakamoto and Holman 2008). There was a significantly greater pThr642 for the epitrochlearis with 100 μU/ml insulin and a non-significant trend for higher pSer588 in the epitrochlearis with 200 μU/ml insulin. It seems reasonable to suspect that the observed effects of exercise on Akt phosphorylation in the epitrochlearis may have played a role in the greater AS160 phosphorylation in this muscle after exercise. It is notable that an earlier research on young rats (Arias et al. 2007; Funai et al. 2009; Funai et al. 2010) found that acute exercise can result in a sustained increase in epitrochlearis AS160 phosphorylation in the absence of insulin (i.e., increased baseline AS160 phosphorylation). AS160 phosphorylation in the presence of insulin was also increased in epitrochlearis muscles from young rats, but the increase in young rats appeared to be entirely attributable to the greater baseline values (without insulin) rather than an insulin-induced increase above basal levels of AS160 phosphorylation. Prior exercise did not alter AS160 phosphorylation on either site in the soleus of old rats regardless of the insulin concentration. The lack of uniformly elevated insulin-stimulated Akt phosphorylation in the soleus of exercised rats is consistent with the absence of significant exercise effects on AS160 phosphorylation in this muscle. The current data are apparently the first to be published with regard to AS160 phosphorylation in the soleus after acute exercise by rats of any age.
GLUT4 abundance was unaltered by exercise in either muscle. These results are consistent with previous data for young (3.5-month-old) rats 4 h after acute exercise (Cartee et al. 1993). The elevated insulin-stimulated glucose transport after a single exercise session by young rats (Hansen et al. 1998) or humans (Thorell et al. 1999) is attributable to greater GLUT4 translocation to cell surface membranes rather than to increased GLUT4 expression.
In conclusion, the current results have demonstrated that both predominantly type I and type II skeletal muscles from old rats remain susceptible to exercise-induced improvement in insulin-mediated glucose uptake. Furthermore, these benefits of acute exercise can be attained without alterations in key proximal insulin signaling events (receptor tyrosine phosphorylation), changes in abundance of several key signaling proteins (insulin receptor, Akt and AS160), or elevated GLUT4 protein expression. It seems possible that the increased insulin-induced phosphorylation of Akt contributes to greater insulin-stimulated glucose uptake in the epitrochlearis muscle of old rats after a single exercise session, but other mechanisms are likely to contribute to the postexercise benefits in the soleus. Atypical protein kinase C, an insulin signaling protein that has been reported to be essential for the full insulin effect on skeletal muscle glucose uptake (Farese et al. 2007), is a potential candidate for an Akt-independent mechanism to explain the increased glucose uptake by the soleus. The exercise-induced increase in AS160 phosphorylation in the epitrochlearis was not found with all insulin doses, suggesting that other mechanisms might also contribute to the elevated glucose uptake that was found with each insulin concentration. In this context, it would be valuable to study the effects of exercise by old rats on other Akt substrates that have been implicated as regulators of GLUT4 translocation, e.g., TBC1D1, myosin 5A, and CDP138 (Roach et al. 2007; Yoshizaki et al. 2007; Xie et al. 2011). Finally, it would also be useful to use genetic or chemical approaches to modulate Akt and/or AS160 phosphorylation in muscles from old animals to test the role of these signaling proteins in the improved insulin-stimulated glucose uptake after exercise.
Acknowledgments
This research was supported by the National Institutes of Health Grant AG-010026.
References
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292(4):E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- Bonen A, Tan MH, Watson-Wright WM. Effects of exercise on insulin binding and glucose metabolism in muscle. Can J Physiol Pharmacol. 1984;62(12):1500–1504. doi: 10.1139/y84-248. [DOI] [PubMed] [Google Scholar]
- Bonen A, Tan MH, Clune P, Kirby RL. Effects of exercise on insulin binding to human muscle. Am J Physiol. 1985;248(4 Pt 1):E403–E408. doi: 10.1152/ajpendo.1985.248.4.E403. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54(1):41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;268(5 Pt 1):E902–E909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev. 2009;37(4):188–195. doi: 10.1097/JES.0b013e3181b7b7c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol. 1990;258(2 Pt 1):E390–E393. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32(3):557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256(4 Pt 1):E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256(4):E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol. 1993;75(2):972–978. doi: 10.1152/jappl.1993.75.2.972. [DOI] [PubMed] [Google Scholar]
- Catalano KJ, Bergman RN, Ader M. Increased susceptibility to insulin resistance associated with abdominal obesity in aging rats. Obes Res. 2005;13(1):11–20. doi: 10.1038/oby.2005.4. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Escriva F, Gavete ML, Fermin Y, Perez C, Gallardo N, Alvarez C, Andres A, Ros M, Carrascosa JM. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194(1):131–141. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86(8):3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest. 2007;117(8):2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282(1):E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297(1):E242–E251. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(5):E999–E1010. doi: 10.1152/ajpendo.00758.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Du Y, Liu Y, Ma L, Li L, Gong Y, Tian H, Li C (2011) Effect of aging on islet beta-cell function and its mechanisms in Wistar rats. Age (Dordr). doi:10.1007/s11357-011-9312-7 [DOI] [PMC free article] [PubMed]
- Hamada T, Arias EB, Cartee GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol. 2006;101(5):1368–1376. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76(2):979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol. 1998;85(4):1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes. 2002;51(2):479–483. doi: 10.2337/diabetes.51.2.479. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metab Clin Exp. 1978;27(12 Suppl 2):1893–1902. doi: 10.1016/S0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Koshinaka K, Kawasaki E, Hokari F, Kawanaka K. Effect of acute high-intensity intermittent swimming on postexercise insulin responsiveness in epitrochlearis muscle of fed rats. Metab Clin Exp. 2009;58(2):246–253. doi: 10.1016/j.metabol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281(42):31478–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Kuzuya H, Okamoto M, Yoshimasa Y, Yamada K, Ida T, Kakehi T, Imura H. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol. 1988;254(1 Pt 1):E92–E98. doi: 10.1152/ajpendo.1988.254.1.E92. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335(18):1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403(2):353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295(1):E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278(17):14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sharma N, Arias EB, Sajan MP, MacKrell JG, Bhat AD, Farese RV, Cartee GD. Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J Appl Physiol. 2010;108(6):1631–1640. doi: 10.1152/japplphysiol.01412.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metab Clin Exp. 2007;56(12):1719–1728. doi: 10.1016/j.metabol.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Thong FS, Derave W, Kiens B, Graham TE, Urso B, Wojtaszewski JF, Hansen BF, Richter EA. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51(3):583–590. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol. 1999;277(4 Pt 1):E733–E741. doi: 10.1152/ajpendo.1999.277.4.E733. [DOI] [PubMed] [Google Scholar]
- Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol. 1989;256(1 Pt 1):E138–E144. doi: 10.1152/ajpendo.1989.256.1.E138. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest. 1999;104(9):1257–1264. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Gade KB, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49(3):325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- Xie X, Gong Z, Mansuy-Aubert V, Zhou QL, Tatulian SA, Sehrt D, Gnad F, Brill LM, Motamedchaboki K, Chen Y, Czech MP, Mann M, Kruger M, Jiang ZY. C2 Domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab. 2011;14(3):378–389. doi: 10.1016/j.cmet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol. 2007;27(14):5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Balon TW, Garetto LP, Goodman MN, Ruderman NB. Muscle alpha-aminoisobutyric acid transport after exercise: enhanced stimulation by insulin. Am J Physiol. 1985;248(5 Pt 1):E546–E552. doi: 10.1152/ajpendo.1985.248.5.E546. [DOI] [PubMed] [Google Scholar]