Abstract
Cardiorespiratory fitness has often been interpreted as a surrogate measurement of physical activity rather than an independent coronary heart disease (CHD) risk factor per se. Fitness is also known to be highly heritable, however, and rats bred selectively for treadmill endurance have low CHD risk phenotypes even in the absence of physical activity. Therefore, I assessed whether cardiorespiratory fitness predicted CHD independent of physical activity in 29,721 men followed prospectively for 7.7 years as part of the National Runners' Health Study. Specifically, CHD deaths and incident participant-reported physician-diagnosed myocardial infarction, revascularization procedures (coronary artery bypass grafting and percutaneous coronary intervention), and angina pectoris during follow-up were compared to baseline cardiorespiratory fitness (10-km footrace performance, meters/second). Nonfatal end points for the 80% of these men who provided follow-up questionnaires included 121 nonfatal myocardial infarctions, 317 revascularization procedures, and 81 angina pectora. The National Death Index identified 44 CHD deaths. Per meter/second increment in baseline fitness, men's risks decreased 54% for nonfatal myocardial infarction (p <0.0001), 44% for combined CHD deaths and nonfatal myocardial infarction (p = 0.0003), 53% for angina pectoris (p = 0.001), and 32% for revascularizations (p = 0.002). Adjustment for physical activity (kilometer/day run) had little effect on the per meter/second risk decreases for nonfatal myocardial infarction (from 64% to 63%), combined CHD deaths and nonfatal myocardial infarction (from 34% to 33%), angina pectoris (from 53% to 47%) or revascularizations (from 32% to 26%). In conclusion, the results suggest that cardiorespiratory fitness is a CHD risk factor, largely independent of physical activity, which warrants clinical screening.
Cardiorespiratory fitness has been shown to predict cardiovascular mortality.1 In 1989, this conclusion was reaffirmed in the seminal report by Blair et al,2 but with a twist. They suggested that cardiorespiratory fitness may be a more objective measurement of physical activity than self-reported activity, and they postulated that the substantial cardiovascular disease decreases they observed with greater cardiorespiratory fitness may be achievable by becoming physically more active. However, interpreting cardiorespiratory fitness as only a physical activity surrogate discounts studies that show up to 70% of the variation in aerobic capacity is inherited in humans,3 and selective breeding for fitness produces substantial inherited differences in coronary heart disease (CHD) risk factors in rats even in the absence of training.4 If cardiorespiratory fitness affects incident CHD due to physical activity, then there will be greater statistical power for demonstrating this in the National Runners' Health Study than in other population cohorts because (1) 80% of men provided 10-km footrace performance times, a measurement of cardiorespiratory fitness; (2) vigorous physical activity is recognized as being most effective in increasing maximum aerobic consumption (VO2max)5; and (3) the range of energy expenditures is substantially greater than that represented in other cohort studies. Demonstrating that cardiorespiratory fitness predicts lower incident CHD independent of physical activity would argue for its recognition as an independent CHD risk factor that warrants clinical screening and the need for public health recommendations that target improved fitness.
Methods
The National Runners' Health Study cohort was recruited from 1991 to 1993 by nationwide distribution of a 2-page questionnaire to runners identified through subscription lists to running magazine subscribers and participation in foot race events.6–8 The questionnaire solicited information on demographics, running history, weight history, smoking habit, previous heart attacks and cancer, and medication use. To be eligible for the study, participants were required to provide signed informed consent, be ≥18 years old, and provide, at a minimum, their name, contact information, gender, birth date, weekly running distance, body weight, and height. Follow-up questionnaires were mailed from 1998 to 2001. The University of California, Berkeley, committee for the protection of human subjects approved the study protocol and all participants provided written informed consent.
Baseline cardiorespiratory fitness was assessed as speed in meters per second of the participant's best 10-km race during the previous 5 years (reported as finish time in minutes). Published data support the use of running performance to estimate VO2max.9 Baseline running distances were reported in usual miles run per week, which correlates strongly between repeated questionnaires (r = 0.89),6 and has been shown to be related to biomarkers traditionally associated with physical activity.8 Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was added to the survey) show running represents (mean ± SD) 91.5 ± 19.1% of all vigorously intense activity, and 73.5 ± 23.7% of total leisure-time physical activity.
In their follow-up questionnaires, participants reported whether they had percutaneous transluminal coronary angioplasty or coronary artery bypass grafting, if they had been diagnosed by a physician as having had a heart attack or angina pectoris since baseline, and the year of the procedure or diagnosis. Participants reporting any of these procedures or conditions in the year of their baseline survey or before were excluded from these analyses. Nonfatal CHD was defined as the earliest myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, or angina pectoris. Fatal CHD was defined as an International Classification of Disease, Ninth Revision, diagnosis of codes 410 to 414 and 429.2 or an International Classification of Disease, 10th Revision, diagnosis of codes I20 to I25 as provided by the National Death Index. The end point “all fatal and nonfatal CHD” includes fatal and non-fatal CHD as described.
Cox proportional hazard model (JMP 5.0, SAS Institute, Cary, North Carolina) was used with cardiorespiratory fitness (meters per second) as the independent variable and weekly intakes of alcohol, meat, fish, fruit, and aspirin, pack-years of cigarette use, education, and age (age and age squared) as covariates. Results were then adjusted for physical activity (kilometre per day run) to determine whether activity dose explained the association between fitness and incident disease. In addition, effects of adjusting for participant's recalled weight when they began running ≥12 km/week (body mass index [BMI] before exercise) were used to assess possible effects of self-selection7 and adjusted for their baseline BMI to assess whether BMI mediated the relation between cardiorespiratory fitness and CHD. Analyses of nonfatal events included only those participants with follow-up questionnaires, whereas analyses of all fatal and nonfatal CHD invoked the assumption that subjects lost to follow-up were free of CHD at the end of follow-up (a conservative approach because it counted nonfatal CHDs as event-free survival in those lost to follow-up).
Results
There were 30,604 men who had complete data on age, education, diet, aspirin use, BMI, smoking history, running distance, and 10-km performance times, of whom 424 were excluded for baseline cigarette use, 159 for baseline diabetes, and 300 for pre-existing heart disease. Of those remaining, follow-up questionnaires were obtained for 23,939 men (80.6%), and causes of death were obtained from the National Death Index in an additional 243 men with complete baseline data who were nonsmokers, nondiabetic, and without pre-existing heart disease at baseline. Men reported physician diagnoses of 125 myocardial infarctions, 317 revascularization procedures (percutaneous transluminal coronary angioplasty or coronary artery bypass grafting), and 81 incident angina pectoris. The National Death Index identified an additional 44 CHD deaths. Women were excluded because only 35 reported CHD, which were too few for meaningful analyses.
Table 1 presents men's baseline characteristics by 10-km performance. Their participation in follow-up was unrelated to 10-km performance. The fittest men were generally younger, exercised more, consumed somewhat less meat, fish, and alcohol and more fruit, were leaner before starting to exercise and at baseline, and smoked less before baseline. The 10-km footrace performance did not differ between those providing and not providing follow-up questionnaires (p = 0.58). However, those lacking follow-up questionnaires were significantly younger (41.7 ± 0.13 vs 44.6 ± 0.7 years, p <0.0001), less educated (15.92 ± 0.03 vs 16.52 ± 0.02 years, p <0.0001), ran somewhat more (5.80 ± 0.04 vs 5.65 ± 0.02 km/day, p = 0.001), smoked more previously (6.46 ± 0.17 vs 5.76 ± 0.09 pack-years, p = 0.0003), and ate less beef (2.66 ± 0.03 vs 2.82 ± 0.02 serving/week, p <0.0001) at baseline.
Table 1. Baseline characteristics (mean ± SD) and follow-up results for nondiabetic, nonsmoking runners without a history of coronary heart disease by cardiorespiratory fitness.
| Cardiorespiratory Fitness (10-km footrace performance, m/s) | |||||
|---|---|---|---|---|---|
|
|
|||||
| <3.25 | 3.25–3.74 | 3.75–4.24 | 4.25–4.74 | ≥4.75 | |
| Total sample size | 2,711 | 7,924 | 10,396 | 6,227 | 2,463 |
| Ischemic heart disease death | 10 | 20 | 8 | 3 | 3 |
| Follow-up questionnaires | |||||
| Number | 2,154 | 6,370 | 8,436 | 5,015 | 1964 |
| Percentage | 79.5 | 80.4 | 81.1 | 80.5 | 79.7 |
| Nonfatal myocardial infarction | 32 | 45 | 39 | 8 | 1 |
| Angina pectoris | 19 | 32 | 22 | 8 | 0 |
| Revascularization procedure | 58 | 127 | 99 | 28 | 5 |
| Running (km/day)*§ | 3.7 ± 2.3 | 4.5 ± 2.3 | 5.6 ± 2.8 | 7.0 ± 3.4 | 9.0 ± 4.4 |
| Age (years)* | 51.0 ± 11.3 | 47.5 ± 9.7 | 44.0 ± 9.0 | 40.4 ± 8.7 | 34.7 ± 8.6 |
| Education (years)*† | 16.5 ± 2.5 | 16.4 ± 2.5 | 16.4 ± 2.5 | 16.4 ± 2.4 | 16.2 ± 2.4 |
| Meat intake (servings/week)*§ | 2.9 ± 2.6 | 2.9 ± 2.6 | 2.8 ± 2.6 | 2.6 ± 2.6 | 2.7 ± 2.9 |
| Fish intake (servings/week)*‡ | 1.7 ± 1.5 | 1.6 ± 1.5 | 1.5 ± 1.4 | 1.4 ± 1.4 | 1.3 ± 1.4 |
| Fruit intake (pieces/week)*§ | 10.6 ± 8.9 | 10.6 ± 8.7 | 11.0 ± 8.4 | 11.7 ± 9.5 | 11.5 ± 9.4 |
| Alcohol intake (ml/day)* | 11.7 ± 16.2 | 12.5 ± 17.2 | 11.9 ± 16.3 | 11.0 ± 15.5 | 9.2 ± 15.0 |
| Aspirin (tablets/day)* | 0.4 ± 0.8 | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.3 ± 0.7 | 0.3 ± 0.6 |
| Cigarettes (pack-years)†§ | 11.0 ± 18.9 | 8.2 ± 15.6 | 5.4 ± 11.9 | 3.4 ± 9.0 | 1.3 ± 5.4 |
| Body mass index (kg/m2) | |||||
| Before exercise*§ | 25.9 ± 3.7 | 25.2 ± 3.3 | 24.3 ±3.4 | 23.1 ± 3.7 | 20.6 ±4.1 |
| Baseline*§ | 25.5 ± 3.1 | 24.6 ± 2.6 | 23.8 ± 2.3 | 22.9 ±2.1 | 22.0 ±1.9 |
Body mass index before exercise is calculated from participants' body weight when they began running ≥12 miles per week.
p ≤0.0001 without adjustment for age.
p <0.01;
p <0.001;
p <0.0001 when adjusted for age.
Table 2 presents the hazard ratios for CHD by baseline cardiorespiratory fitness levels. Each meter/second increment in running performance was associated with a 44% lower risk for CHD death and nonfatal myocardial infarction, a 54% lower risk for nonfatal myocardial infarction, a 53% lower risk for angina pectoris, and 32% lower risk for revascularization procedures (percutaneous transluminal coronary angioplasty or coronary artery bypass grafting). Adjustment for physical activity had little effect on the hazard ratios. Fatal CHDs were too few for separate analyses. Adjustment for men's pre-exercise BMI also had little effect on the hazard ratios, and except for angina pectoris, all remained significant when adjusted for baseline BMI.
Table 2. Hazard ratios for coronary heart disease and angina by baseline cardiorespiratory fitness (10-km race performance, per meter/seconds) from Cox proportional hazard model.
| Hazard ratio per Meter/Second (95% confidence interval) and Statistical Significance | ||||
|---|---|---|---|---|
|
| ||||
| Unadjusted for km/day or BMI | Adjusted for Physical Activity (km/day) | Adjusted for BMI Before Exercise | Adjusted for BMI at Baseline | |
| Coronary heart disease death and nonfatal myocardial infarction | 0.56 (0.42–0.77) | 0.57 (0.41–0.80) | 0.59 (0.43–0.82) | 0.68 (0.49–0.94) |
| p value | 0.0003 | 0.001 | 0.002 | 0.02 |
| Nonfatal myocardial infarction | 0.46 (0.32–0.66) | 0.46 (0.32–0.69) | 0.49 (0.33–0.72) | 0.58 (0.39–0.86) |
| p value | <0.0001 | 0.0002 | 0.0003 | 0.008 |
| Angina pectoris | 0.47 (0.30–0.74) | 0.53 (0.33–0.89) | 0.45 (0.28–0.74) | 0.52 (0.32–0.85) |
| p value | 0.001 | 0.02 | 0.002 | 0.01 |
| Percutaneous transluminal coronary angioplasty and coronary artery bypass grafting | 0.68 (0.54–0.86) | 0.74 (0.57–0.96) | 0.69 (0.54–0.88) | 0.83 (0.64–1.06) |
| p value | 0.002 | 0.02 | 0.003 | 0.14 |
Adjusted for age (age and age squared), education, aspirin use, pack-years of cigarette use, and intakes of meat, fruit, and alcohol. Additional adjustment for physical activity (kilometers per day), BMI when first began running ≥12 km/day (BMI before exercise) or BMI at baseline where indicated. Samples consist of 29,721 men with fatal and nonfatal end points combined and 23,939 men with nonfatal end points alone.
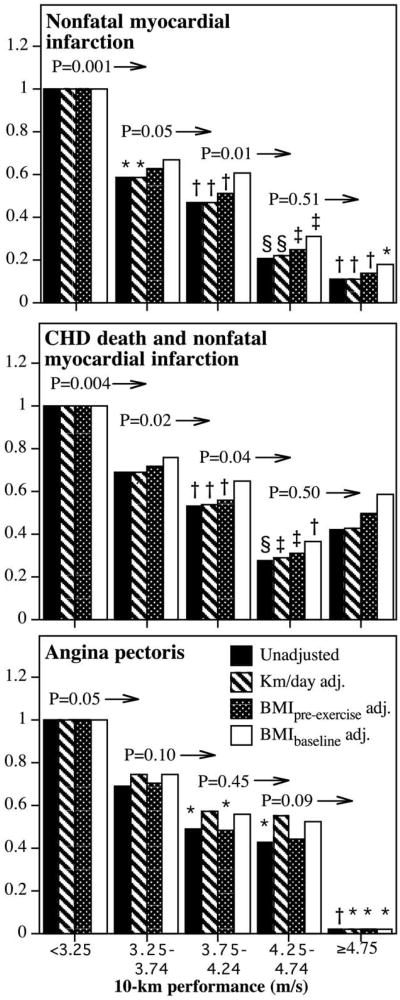
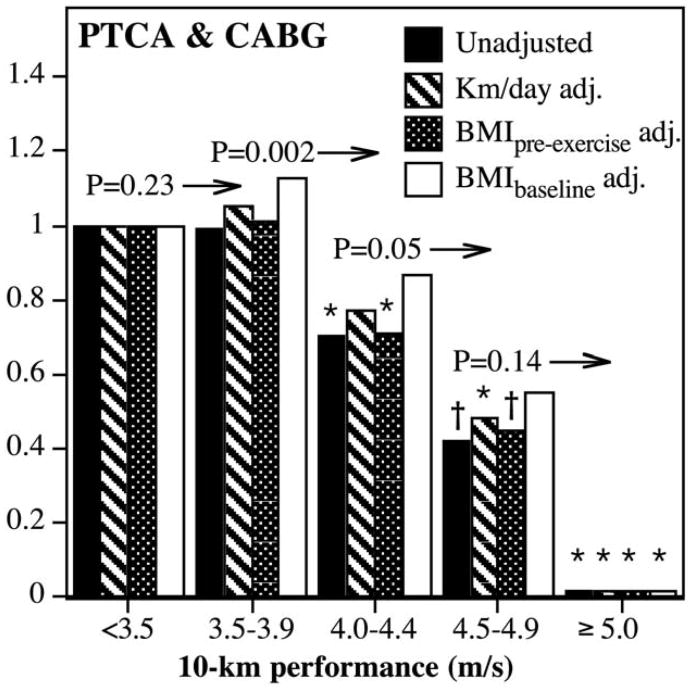
Figure 1 shows that risk for nonfatal myocardial infarction decreased linearly with increasing fitness. Those runners able to run faster than 4.75 m/s had an 89% lower risk than the least fit (i.e., slowest) men. There was significantly less risk in those running ≥3.25 compared to <3.25 m/s (p = 0.001), ≥3.75 compared to 3.25 to 3.74 m/s (p = 0.05), and ≥4.25 compared to 3.75 to 4.24 m/s (p = 0.01). Adjustment for physical activity had no effect on risk decrease associated with baseline fitness. Adjustment for the men's BMI when they began running (i.e., self-selection) also had little effect on the risk decrease, as did adjustment for baseline BMI, although adjustment for the latter did eliminate the significance of all fitness intervals <4.25 m/s but not those intervals ≥4.25 m/s. Including fatal CHD with nonfatal myocardial infarctions yielded similar results except for a loss of significance at the highest fitness category. Angina pectoris risk also decreased linearly with cardiorespiratory fitness, and the risk was essentially nonexistent in the fittest men. Risk for revascularization procedures decreased primarily at >4 m/s in race performance (Figure 2) and was only slightly attenuated by adjustment for physical activity. Those who ran 4.5 to 4.9 m/s were at 58% lower risk for revascularization than those who ran <4.0 m/s, and those who ran ≥5.0 m/s had no detectable risk.
Figure 1.
Hazard ratio from survival analyses of men's reported physician-diagnosed myocardial infarction (top), nonfatal infarction plus CHD death (International Classification of Disease, Ninth Revision, codes 410 to 414 and 429.2; International Classification of Disease, 10th Revision, codes I20 to I25) (middle), and angina pectoris (bottom) by cardiorespiratory fitness (10-km footrace performance). Hazard ratios were adjusted for age, education, intakes of meat, fish, fruit and alcohol, aspirin use, and pack-years of cigarette use. Significance levels provided (above bars and to left of arrows) are relative to all greater fitness levels (e.g., men who exceed 4.24 m/s had significantly lower risk of CHD death and nonfatal myocardial infarction than men who ran 3.75 to 4.24 m/s at p = 0.04, middle panel). Significant differences relative to the least fit (slowest) runners are coded (*p <0.05; †p <0.01; ‡p <0.001; §p <0.0001). Sample sizes are provided in Table 1. Fitness categories were defined a priori to be of constant width and symmetrically distributed (Table 1) while providing adequate sample sizes within each category.
Figure 2.
Hazard ratio from survival analyses of participant-reported revascularization procedures (percutaneous transluminal coronary angioplasty [PTCA] and coronary artery bypass grafting [CABG]) by cardiorespiratory fitness adjusted (adj.) for age, education, intakes of meat, fish, fruit and alcohol, aspirin use, and pack-years of cigarette use. Significance levels provided (above bars and to left of arrows) are relative to all faster 10-km performances (Figure 1). Significant differences relative to the least fit (slowest) runners are coded (*p <0.05; †p <0.01). Fitness categories were chosen to illustrate that risk decrease for revascularization procedures was due primarily to decreasing risk in the fittest men.
Discussion
These analyses demonstrate significant inverse associations between incident CHD and cardiorespiratory fitness as measured by 10-km performance times that could not be explained by physical activity. The somewhat weaker associations for revascularization procedures may reflect their occurrence at a younger age with less disease progression than myocardial infarctions. Results are consistent with these runners' fitness-related decreases in BMI, hypertension, hypercholesterolemia, diabetes, blood pressures, and plasma triglyceride concentrations when adjusted for activity.6,8 Table 2 suggests the CHD–fitness relation was mediated in part by BMI, but not because leaner men and women chose to run further, because adjusting for pre-exercise BMI did not affect the risk decrease. These results are also consistent with meta-analyses that distinguished CHD–risk decreases associated with fitness from those associated with physical activity.10
When assessed in the laboratory, cardiorespiratory fitness is generally assessed by VO2max, which is the highest rate of oxygen uptake and use during exercise.11 In 1923, Hill and Lupton12 hypothesized that success in middle- and long-distance running required a high VO2max, which varied across runners. In humans, the ability of the cardiorespiratory system to deliver oxygen to the muscle is the primary determinant of VO2max (70% to 85%13), with trained subjects able to produce 60% greater maximum cardiac output than the untrained.14 The muscle's mitochondrial enzyme activities and increased fat oxidation are the primary determinants of submaximal exercise performance relative to VO2max.11 The correlation of VO2max with 10-km performance time (r = −0.9115) is comparable to its correlation with treadmill test duration as employed by Blair et al2 (r = 0.92 to 0.94), although in trained subjects running economy may play a larger role in setting the lactate threshold determining performance than in the untrained.11
Although caution is warranted in extrapolating rodent findings to humans, selective breeding in rats suggests genetic differences could strongly influence interindividual differences in cardiorespiratory fitness and their relation to CHD. Untrained high-capacity running (HCR) rats are able to run an 8.4-fold greater distance and 2.5-fold faster than those selected for low-capacity running (LCR) and have 39% lower percent visceral fat, 16% lower fasting glucose, 57% lower insulin, 63% lower triglycerides, and 48% lower free fatty acid concentrations in plasma and 13% lower 24-hour blood pressure.4 HCR rats have 30% greater VO2max because of their improved oxygen supply, extraction ratio, and tissue diffusion capacity than LCR rats.16 Their muscles have 46% lower triglyceride and 56% diacylglycerol content and 73% higher triglyceride lipase activity than those of LCR rats.17 In humans, training-induced increases in VO2max are inherited.18 Similarly, HCR rats show greater training response19 and greater training-induced increases in skeletal muscle expression of genes associated with lipid metabolism and fatty acid elongation than LCR rats.19
Selective breeding has also identified associations between genetic predisposition to poor cardiorespiratory fitness and CHD. Untrained HCR rats have greater expression of α-myosin heavy chain protein than untrained LCR rats, which have more β-myosin heavy chain protein expression.20 The α-myosin heavy chain protein has a higher adenosine triphosphatase activity21 and higher proportions of the α- to β-myosin heavy chain isoforms correlated with greater cardiomyocyte contractile velocity and more economical force generation.22 Exercise training increases α-myosin heavy chain, whereas aging, heart failure, cardiomyopathy, and pressure overload increase β-myosin heavy chain.23 Greater β-myosin heavy chain protein expression in LCR rats is consistent with their low VO2max16 and their shift in substrate usage from normal mitochondrial fatty acid β oxidation to carbohydrate metabolism.24 Untrained HCR rats have increased susceptibility to cardiac failure leading to fatal intractable irregular contractions during hypoxia, due possibly to cosegregating deficiencies in cardiac calcium homeostasis.21
The present findings are relevant to the formulation of public health guidelines. Current guidelines primarily target the volume of physical activity rather than cardiorespiratory fitness,25 whereas fitness-targeted guidelines would place greater emphasis on vigorous exercise and interval training. Fitness improvements are achieved by training intensities that exceed 50% VO2max except in low fit subjects (VO2max <40 ml/min/kg) who improve with training at ≤40% VO2max.26 Vigorous physical activities (i.e., running and other activities requiring over sixfold the energy expenditure of sitting at rest) are more effective at improving cardiorespiratory fitness than moderate-intensity physical activities (i.e., brisk walking and other activities requiring three- to sixfold the energy expenditure of sitting at rest). Interval training at 90% to 95% of maximum heart rate has also been shown to produce greater increases in VO2max than endurance training at 70% or 85% of maximum heart rate.27 Although current guidelines acknowledge the potential benefit of more vigorous exercise, they primarily treat vigorous exercise as a means for achieving the same total activity dose over a shorter duration. In humans, comparable increases in skeletal muscle carbohydrate metabolism, lipid oxidation, and mitochondrial biogenesis have been reported for 4 to 6 30-second sprints performed 3 times weekly as for 200 to 300 minutes per week of submaximal cycling.28 In LCR rats, high-intensity interval training at 85% to 90% of VO2max produced significant corrections in metabolic syndrome.29
This study's primary limitation is its lack of clinical and medical record verification of CHD. The Nurses' Health Study reported that, although 32% of participant-reported myocardial infarctions could not be confirmed using World Health Organization criteria (symptoms and typical electrocardiographic changes or increase of serum cardiac enzymes), most were nevertheless hospitalizations for cardiac disease.30 Moreover, men who run regularly may differ from others genetically, socioeconomically, psychologically, and with respect to other health behaviors. However, biological processes that relate CHD to fitness will likely be similar in runners and nonrunners. Moreover, significant associations of fitness with multiple CHD end points strongly support their association.
Acknowledgments
The author thanks Kathryn Hoffman, MS, for her help in collecting the data and reviewing the manuscript.
This research was supported in part by grants from the Institute of Aging (AG032004) and the National Heart, Lung, and Blood Institute (HL094717), and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory, Berkeley, California (Department of Energy Grant DE-AC03-76SF00098 to the University of California).
References
- 1.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Shops DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: the Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW, III, Paffenbarger RS, Clark DG, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, Pérusse L, Thériault G, Leblanc C. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc. 1986;18:639–646. [PubMed] [Google Scholar]
- 4.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 5.Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, Gandrakota R. Effect of intensity of aerobic training on VO2max. Med Sci Sports Exerc. 2008;40:1336–1343. doi: 10.1249/MSS.0b013e31816c4839. [DOI] [PubMed] [Google Scholar]
- 6.Williams PT. Vigorous exercise, fitness, and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40:998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obesity (Silver Spring) 2008;16:102–106. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 8.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Intern Med. 1998;158:237–245. doi: 10.1001/archinte.158.3.237. [DOI] [PubMed] [Google Scholar]
- 9.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–204. [PubMed] [Google Scholar]
- 10.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hill AV, Lupton H. Muscular exercise, lactic acid, and the supply and utilization of oxygen. Q J Med. 1923;16:135–171. [Google Scholar]
- 13.Cerretelli P, Di Prampero PE. Gas exchange in exercise. In: Fishman AP, Farhi LE, Tenney SM, Geiger SR, editors. Handbook of Physiology. Bethesda, MD: American Physiological Society; 1987. pp. 297–339. [Google Scholar]
- 14.Dempsey JA, Hanson P, Henderson K. Exercise-induced arterial hypoxemia in healthy humans at sea-level. J Physiol Lond. 1984;355:161–175. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costill DL, Thomason H, Roberts E. Fractional utilization of the aerobic capacity during distance running. Med Sci Sports Exerc. 1973;5:248–252. [PubMed] [Google Scholar]
- 16.Henderson KK, Wagner H, Favret F, Britton SL, Koch LG, Wagner PD, Gonzalez NC. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. J Appl Physiol. 2002;93:1265–1274. doi: 10.1152/japplphysiol.00809.2001. [DOI] [PubMed] [Google Scholar]
- 17.Spargo FJ, McGee SL, Dzamko N, Watt MJ, Kemp BE, Britton SL, Koch LG, Hargreaves M, Hawley JA. Dysregulation of muscle lipid metabolism in rats selectively bred for low aerobic running capacity. Am J Physiol Endocrinol Metab. 2007;292:E1631–E1636. doi: 10.1152/ajpendo.00702.2006. [DOI] [PubMed] [Google Scholar]
- 18.Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS, Rao DC. Familial aggregation of VO2max response to exercise training: results from the Heritage family study. J Appl Physiol. 1999;87:1003–1008. doi: 10.1152/jappl.1999.87.3.1003. [DOI] [PubMed] [Google Scholar]
- 19.Bye A, Høydal MA, Catalucci D, Langaas M, Kemi OJ, Beisvag V, Koch LG, Britton SL, Ellingsen Ø, Wisløff U. Gene expression profiling of skeletal muscle in exercise-trained and sedentary rats with inborn high and low VO2max. Physiol Genomics. 2008;12:213–221. doi: 10.1152/physiolgenomics.90282.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palpant NJ, Szatkowski ML, Wang W, Townsend D, Bedada FB, Koch LG, Britton SL, Metzger JM. Artificial selection for whole animal low intrinsic aerobic capacity co-segregates with hypoxia-induced cardiac pump failure. PLoS ONE. 2009;4:e6117. doi: 10.1371/journal.pone.0006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pope B, Hoh JFY, Weeds A. The ATPase activities of rat cardiac myosin isozymes. FEBS Lett. 1980;118:205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- 22.Holubarsch CH, Goulette RP, Litten RZ, Martin BJ, Mulieri LA, Alpert NR. The economy force development, myosin isozyme pattern and myofibrillar ATPase activity in normal and hyperthyroid rat myocardium. Circ Res. 1985;56:78–86. doi: 10.1161/01.res.56.1.78. [DOI] [PubMed] [Google Scholar]
- 23.Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986;66:710–730. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- 24.Bye A, Langaas M, Høydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen Ø, Wisløff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics. 2008;33:100–109. doi: 10.1152/physiolgenomics.00269.2007. [DOI] [PubMed] [Google Scholar]
- 25.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. American College of Sports Medicine; American Heart Association. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Med Sci Sports Exerc. 1990;22:265–274. [PubMed] [Google Scholar]
- 27.Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 28.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81:723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]