Abstract
Here we present a case series from a primate research facility. The index case, a 4-year-old pig-tailed macaque (Macaca nemestrina) experimentally infected with chimeric simian-human immunodeficiency virus (SHIVSF162 P4), developed weight loss and was euthanized. Based on necropsy results the animal was diagnosed with opportunistic atypical mycobacteriosis associated with simian AIDS (SAIDS). Subsequently, tissues from the index animal, as well as tissues and oral mucosal swabs from six SHIV-infected contacts, were analyzed using molecular methods and found to contain nucleic acid sequences characteristic of Mycobacterium tuberculosis complex (MTBC). These data suggest that existing protocols fail to reliably detect MTBC infection in laboratory primates used as experimental models.
Keywords: atypical mycobacteriosis; coinfection; IS6110; Macaca; MTBC; pig-tailed macaque; SHIVSF162 P4
Case series
In June 2010, a 4-year-old male pig-tailed macaque (Macaca nemestrina) that had been experimentally infected with chimeric simian-human immunodeficiency virus (SHIVSF162 P4) and had developed chronic diarrhea and weight loss of 14% of its body weight over a 5-month period was euthanized with a presumptive diagnosis of stage 3 simian AIDS (SAIDS). Over the previous months diagnostic work up revealed depletion of CD4 cells and hypoalbuminemia with consistently negative stool cultures and negative stool examinations for gastro-intestinal parasites. Thoraco-abdominal radiography and abdominal ultrasound 2 weeks prior to euthanasia were normal, with the exception of mild splenomegaly and a circular hyperechoic area visualized in the liver. All routine tuberculin skin testing (TST) performed via intradermal eyelid injection of 1500 units of mammalian old tuberculin (Synbiotics, USA) prior to SHIVSF162 P4 inoculation in March 2009 was negative. No other laboratory abnormalities were noted. On necropsy, numerous multifocal fibrous-encapsulated mesenteric lymph node granulomas ranging in size from millimeters to 2.0 cm in diameter were found. Other organ systems were grossly unremarkable. Histologic analysis revealed mild to extensive multifocal histiocytic infiltration of the jejunum, ileum, colon and cecum. Mesenteric granulomas consisted of fibrous walls surrounding necrotic debris. The liver contained microgranulomas with some giant cells. Although no acid-fast bacteria (AFB) were seen in the mesenteric node granulomas, large numbers of AFB were found in foamy macrophages in the small and large intestine, as well as in the liver, mesenteric, inguinal and axillary lymph nodes. Tissues were not sent for AFB culture. The pathologist's final diagnosis was opportunistic atypical mycobacteriosis associated with SAIDS immunosuppression.
As atypical mycobacteriosis is not regarded as readily transmissible between primates, no quarantine measures were implemented among animals housed with the index case (animal A). However, recognizing that a primary hepatic case of Mycobacterium tuberculosis complex (MTBC) had been recently detected in a pig-tailed macaque in this same colony,1 we requested oral swab samples from contact animals on the same study (animals B, C, D and E) that had been housed adjacent to animal A and who had intermittent grooming contact with animal A subsequent to their experimental infection with SHIV in March 2009. We have previously shown that MTBC DNA can be recovered from the mouths of free-ranging primates in areas where MTBC prevalence in humans is high.2
In November and December 2010, animals B–E were euthanized at study's end per experimental protocol. One of the animals, (animal B), was noted to have 10% weight loss over the preceding 6 months and had, as a result, been given nutritional supplementation. Other than weight loss, no signs or symptoms of disease were noted. Animals C, D and E did not exhibit significant weight loss or other clinical signs or symptoms of disease (Table 1).
Table 1. History and pathology characteristics of seven pig-tailed macaques on a SHIV study where MTBC went undetected.
| Animal A | Animal B | Animal C | Animal D | Animal E | Animal F | Animal G | |
|---|---|---|---|---|---|---|---|
| Date challenged with SHIV | 27 Mar 2009 | 26 Mar 2009 | 27 Mar 2009 | 27 Mar 2009 | 26 Mar 2009 | 26 Mar 2009 | 26 Mar 2009 |
| Date of death | 8 Jun 2010 | 29 Nov 2010 | 9 Dec 2010 | 16 Dec 2010 | 20 Dec 2010 | 1 Apr 2010 | 17 May 2010 |
| %Weight loss | 14% | 10% | No | No | No | 22% | 13% |
| Diarrhea | Yes | No | No | No | No | Yes | Yes |
| TST | NEG 08/2008 | NEG 08/2008 | NEG 08/2008 | NEG 08/2008 | NEG 08/2008 | NEG 08/2008 | NEG 08/2008 |
| Findings on gross pathology | Emaciation, extensive, chronic, multifocal mesenteric lymph node granulomas | Emaciation; mild, multifocal atelectasis; lymphadenopathy | No significant disease | No significant disease | No significant disease | Emaciation | Emaciation |
| Histological findings | Histiocytic infiltration of the jejunum, ileum, colon and cecum; mesenteric granulomas; hepatic microgranulomas with giant cells | Lymphohistiocytic, plasmacytic, eosinophilic gastro-entero-colitis; enteric villar blunting and fusion | Lymphohistiocytic and eosinophilic gastro-entero-colitis;enteric villar blunting and fusion | Lymphohistiocytic, plasmacytic, eosinophilic gastro-entero colitis; enteric villar blunting and fusion; colonic spirochetosis | Lymphohistiocytic, plasmacytic, eosinophilic gastro-entero colitis; enteric villar blunting and fusion; colonic spirochetosis | Plasmacytic and eosinophilic gastro-entero-colitis; lymphoid depletion; hemosiderin deposition in lamina propria of small intestine | Lymphohistiocytic, plasmacytic and eosinophilic gastro-entero-colitis; enterocolonic amyloidosis |
| AFB detected | + intestinal, mesenteric, inguinal and axillary lymph nodes | Not performed | Not performed | Not performed | Not performed | Not performed | Not performed |
| Cause of death on pathology report | SAIDS; opportunistic atypical mycobacteriosis | End of Study | End of study | End of Study | End of Study | SAIDS; IBD, food allergy, hypersensitivity, dietary intolerance | IBD food allergy, hypersensitivity, dietary intolerance; secondary amyloidosis from GI tract inflammation |
Abbreviations: AFB, acid fast bacteria; IBD, inflammatory bowel disease; SAIDS, simian autoimmune deficiency syndrome; SHIV, simian-human immunodeficiency virus; TST, tuberculin skin test results prior to inoculation with SHIV.
On necropsy, gross pathology of animals B, C, D and E was essentially normal, but histological analysis revealed lymphohistiocytic, plasmacytic and eosinophilic gastro-entero-colitis with enteric villar blunting and fusion in all four animals. In addition, extensive colonic spirochetosis was found in colonic specimens of animals D and E (Table 1). At necropsy, swabs of the mouth, liver and lung tissue from animals A–E were provided for nucleic acid analysis.
Also, oral, liver and lung swabs obtained at necropsy from two additional M. nemestrina (animals F and G) that had been experimentally infected with SHIV, housed in the same room as the index animal and that were euthanized after developing diarrhea and weight loss were included in the polymerase chain reaction (PCR) analysis. Histological analysis of animal F's tissues found plasmacytic and eosinophilic gastro-entero-colitis, lymphoid depletion and hemosiderin deposition in lamina propria of the small intestine. Histologic analysis of animal G's tissues showed lymphohistiocytic, plasmacytic and eosinophilic gastro-entero-colitis and enterocolonic amyloidosis (Table 1). Staining and culture of tissue for AFB were not performed on animals B–G.
IS6110, a nucleic acid sequence diagnostic for mycobacteria of the MTBC and not present in atypical mycobacteria, was amplified from tissue samples from all seven animals (Table 2).3 For all seven IS6110 amplifications, nucleic acid sequences were identical to the target IS6110 sequence of NCBI reference sequence NC_000962.2 (Figure 1). Importantly, IS6110 was detected in oral swabs acquired from animals B, C, D and E while they were alive.
Table 2. IS6110 amplification of swabsa collected at live and/or necropsy time points from SHIV-infected pigtailed macaques.
| Animal ID | A | B | C | D | E | F | G | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mtDNAb | IS6110 | mtDNA | IS6110 | mtDNA | IS6110 | mtDNA | IS6110 | mtDNA | IS6110 | mtDNA | IS6110 | mtDNA | IS6110 | |
| Necropsy oral swab | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Necropsy liver swab | + | + | − | − | + | + | + | + | + | + | + | + | + | + |
| Necropsy lung swab | + | + | − | − | + | + | + | + | + | − | + | + | + | + |
| Necropsy granuloma swabc | + | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Oral swab collected while livingd | NC | NC | + | + | + | + | + | + | + | + | NC | NC | NC | NC |
Abbreviations: NA, not applicable (no granulomas detected); NC, not collected.
The specificity of PCR using IS6110 primers to detect MTBC infection and disease has been measured in several human populations to be in the range of 98% and higher.16 Measurement of PCR test specificity in human populations is facilitated by the availability and routine use of multiple diagnostic modalities, including history and physical exam, radiography, PPD and sputum culture, which constitute a ‘gold standard' against which new tests can be compared. Similar population data for primates are unavailable, in part because diagnostic tests for MTBC in primates are unwieldy to apply, inaccurate and lack a reliable gold standard for detecting MTBC.5 However, indirect evidence for high specificity of IS6110 PCR in primates comes from studies in free-ranging macaques showing that IS6110 is not detected (0/39) in macaques living alongside human populations with very low levels of MTBC infection, while IS6110 in macaques living alongside human populations with high MTBC prevalence, such as Indonesia, are much higher (68%).2
Swabs of buccal mucosa and necropsy tissues were individually placed in sterile lysis buffer and DNA was subsequently extracted following a standard phenol-chloroform procedure. To avoid cross-contamination of samples, or contamination from positive controls, all pre-PCR work was performed in a dedicated laboratory with dedicated instruments, consumables, and reagents. Work flow was unidirectional from pre-PCR to post-PCR laboratories. Extraction and PCR-setup hoods were UV irradiated for at least 20 min between each extraction and PCR preparation. Hoods were cleaned with 10% commercial bleach solution and RNase between work sessions. Alongside each DNA extraction, we ran at least one negative extraction blank. All PCR amplification procedures were performed in a separate post-PCR laboratory, and multiple PCR blanks were included in each PCR run. Additionally, tissue was obtained as a negative control from an infant pigtailed macaque that was never in contact with any of the animals reported in this study. DNA extracted from this negative control was consistently negative for IS6110.
Following DNA extraction, we assessed recoverability of DNA from samples by amplifying a 101 base-pair fragment of macaque mitochondrial DNA using the primers MT12Sa′ 5′CTG GGA TTA GAT ACC CAC TAT3′ and MT12So 5′GTC GAT TAT AGG ACA GGT TCC3′.17 In samples that contained DNA, a 123 base-pair fragment of the MTBC-specific repetitive element, IS61103 was targeted using the primers TB130-F and TB130-R.18 Real-time PCR was performed with the SsoFast EvaGreen supermix (Bio-Rad Laboratories, Inc.), in a 20 μl reaction containing each primer at a final concentration of 0.2 μM and 2 μl DNA template for 40 cycles. Positive controls for M. tuberculosis H37Rv and for M. bovis BCG were added immediately prior to thermal cycling and without opening the tubes containing swab sample DNA or blanks. Product high resolution melt peaks were assessed relative to the positive and negative controls. Samples with positive melt curves matching IS6110 were then directly sequenced to ensure that the amplicon was indeed from IS6110.
Gross pathological examination identified granulomas only in animal A.
Buccal swabs of the oral mucosa were collected from live animals B–D 3 days after the death of animal A.
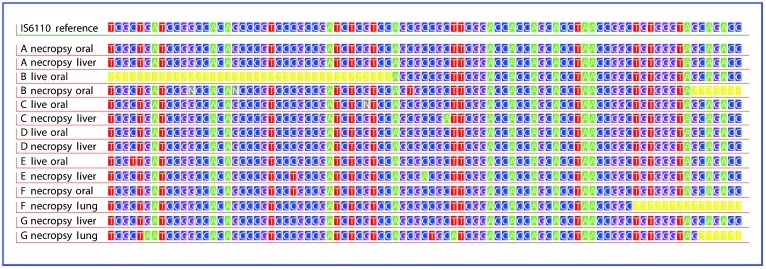
Figure 1.
PCR sequence data for IS6110-positive tissues of each animal, compared to IS6110 reference sequence from M. tuberculosis H37Rv. IS6110 reference sequence from NC_00962.2 and direct PCR sequencing results from swabs of oral mucosa and necropsied liver and lung tissues. IS6110 is diagnostic of the MTBC and does not occur in atypical mycobacteria. Each animal had at least two different PCR-positive swab samples that gave readable sequence. The ‘yellow ?' symbol indicates absence of sequence data, while the ‘n' symbol indicates unreadable bases. Low initial concentration of starting DNA targets led to single nucleotide amplification errors at loci in Samples B necropsy oral, C necropsy liver, E live oral, E necropsy liver, and F necropsy oral, but BLAST of sequences in NCBI database indicates significant match only to IS6110.
Comment
IS6110 is known to occur only in mycobacteria of the MTBC, a group of closely related bacteria that includes Mycobacterium tuberculosis, which infects a third of the world's human population.4 Atypical mycobacteria do not contain the IS6110 sequence.3 Thus, the mycobacteria detected in this study are not atypical mycobacteria. It should be emphasized that, in order to prevent false positive results stemming from contamination, stringent anti-contamination protocols were followed and appropriate negative controls were performed (see footnote to Table 2).
The MTBC has been implicated in epizootic infection of laboratory primates over the past decades.2,5 Primate research facilities have gone to significant lengths to create populations (known as specific pathogen-free, or SPF) that are free of mycobacteria and a select group of enzootic simian viruses.6 SPF colonies are designed to protect laboratory workers from enzootic primate-borne pathogens and to ensure that results obtained using primate biomedical models are not confounded by the presence of known pathogenic agents. The results described here suggest that present protocols in place at primate facilities sometimes fail to detect MTBC infection. Our results do not establish the timing of these animals' infections; both reactivation of latent infection and newly acquired MTBC infection could have occurred. Notwithstanding, all animals in this study were born in the United States; thus, infection occurred at a domestic primate facility.
These data raise several questions. First, what role, if any, did MTBC play in the wasting disease observed in four of the animals, three of which had diarrhea? Are the histologic findings of intestinal leukocytic infiltration present in all animals related to MTBC and/or SHIV? Does MTBC act as a cofactor in causing disease in SHIV-infected pig-tailed macaques in an analogous manner to the synergistic action of HIV and MTBC in humans? Are there other infectious agents (such as the colonic spirochetes detected in two animals) or noninfectious factors involved? Further research, employing prospective and/or experimental designs, is needed to shed light on these issues.
Second, how prevalent is MTBC infection in laboratory primate populations?7 Are particular primate species or those that are on immunosuppressive studies at greater risk than others? Evidence shows that both within and among primate species variation in host response to infection with MTBC is much greater than previously understood.8,9,10,11 Additionally, functional differences in response to SIV or SHIV infection are also broad,12,13 and the host response to SIV influences the course of MTBC coinfection.14 Currently, TST is used to screen for and diagnose infection with MTBC in laboratory primates.5 TST relies on an animal's ability to mount an immune response. Because SHIV suppresses immune function by depleting the host's T lymphocytes, TST is not used to screen for mycobacterial infection in SHIV-infected laboratory animals. This is ironic and problematic, as infection with immunodeficiency viruses may increase macaques' susceptibility to mycobacteria, as occurs in humans.15 SHIV-infected macaques being used to model HIV are precisely those in whom detection of mycobacterial infection is most critical. Our findings reemphasize the importance of more rigorous protocols for the detection of MTBC in primate research laboratories.
Failure to reliably detect MTBC in laboratory primates raises a fundamental question about scientific experiments that use nonhuman primates as models of human pathophysiology. Could undetected infection with MTBC confound the results of research? Can we assume that the outcomes we observe in animals used to model host responses to a broad range of disease processes are governed by known independent variables, or is there another unknown, the presence of MTBC, which could interfere with the processes we are attempting to understand? After all, the nonhuman primate model is the raison d'etre of primate research facilities. It is this question that argues most strongly for the development of new protocols for the screening and diagnosis of MTBC in primate colonies. We agree with Sizemore and colleagues16 that ‘quickly and accurately identifying the presence of Mtb in… both latent and active TB is a critical first step to prevention of additional cases.' The oral swab PCR used in this case series relies upon direct detection of the infectious agent rather than on a host immune response, and was able to identify the presence of MTBC DNA in four animals while they were still alive. Adoption of direct pathogen identification methods such as oral swab PCR could allow researchers the opportunity to monitor the presence of MTBC infections in their biomedical models. Further testing of this method in controlled trials is warranted in order to determine whether oral swab PCR can play a role in widespread screening and diagnosis for MTBC infection in the primate biomedical model.
Acknowledgments
The authors are grateful to Nicholas Lerche, DVM, MPVM who strongly believed that an interdisciplinary approach to infectious disease research was critical for a complete and nuanced understanding of our biomedical models and research outcomes. Shiu-Lok Hu, PhD provided useful discussions and critical reading of the manuscript. This research was supported by funding from NIH-NCRR grant P51 RR000166 and RR 02S014, NIH-NIAID grant R01 AI078229 and N66001-02-C-8072 DARPA.
References
- Stockinger DE, Roellich KM, Vogel KW, et al. Primary hepatic Mycobacterium tuberculosis complex infection with terminal dissemination in a pig-tailed macaque (Macaca nemestrina) J Amer Assoc Lab Anim Sci. 2011;50:258–262. [PMC free article] [PubMed] [Google Scholar]
- Wilbur A, Engel G, Rompis A, et al. From the mouths of monkeys: Detection of Mycobacterium tuberculosis complex DNA from buccal swabs of synanthropic macaques. Am J Primatol. 2012;74:676–686. doi: 10.1002/ajp.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach KD. Use of an insertion sequence for laboratory diagnosis and epidemiologic studies of tuberculosis. Ann Emerg Med. 1994;24:450–453. doi: 10.1016/s0196-0644(94)70182-2. [DOI] [PubMed] [Google Scholar]
- WHO . Global tuberculosis control—epidemiology, strategy, financing. Geneva; World Health Organization; 2009. [Google Scholar]
- Lerche NW, Yee JL, Capuano SV, Flynn JL. New approaches to tuberculosis surveillance in nonhuman primates. Inst Lab Anim Res J. 2008;49:170–178. doi: 10.1093/ilar.49.2.170. [DOI] [PubMed] [Google Scholar]
- Morton WR, Agy MB, Capuano SV, Grant RF. Specific pathogen-free macaques: definition, history, and current production. Inst Lab Anim Res J. 2008;49:137–144. doi: 10.1093/ilar.49.2.137. [DOI] [PubMed] [Google Scholar]
- Wilbur A, Engel GA, Jones-Engel L. TB infection in the nonhuman primate biomedical model: tip of the iceberg. Med Hypotheses. 2012;79:365–367. doi: 10.1016/j.mehy.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Rodgers M, Smith L, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immunol. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Capuano SV, Croix D, et al. Non-human primates: a model for tuberculosis research. Tuberculosis. 2003;83:116–118. doi: 10.1016/s1472-9792(02)00059-8. [DOI] [PubMed] [Google Scholar]
- Mehra S, Golden NA, Dutta NK, et al. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol. 2011;40:233–243. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormus B, Blanchard J, Alvarez X, Didier P. Evidence for a rhesus monkey model of asymptomatic tuberculosis. J Med Primatol. 2004;33:134–145. doi: 10.1111/j.1600-0684.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- Smith M, Dale C, DeRose R, et al. Analysis of pigtail macaque major histocompatibility complex class I molecules presenting immunodominant simian immunodeficiency virus epitopes. J Virol. 2005;79:684–695. doi: 10.1128/JVI.79.2.684-695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt BF, O'Connor DH, Lafont BA, et al. MHC class I allele frequencies in pigtail macaques of diverse origin. Immunogenetics. 2006;58:995–1001. doi: 10.1007/s00251-006-0164-8. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhou D, Chalifoux L, et al. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus- mycobacterium coinfection. Infect Immunol. 2002;70:869–877. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski A, Jansson M, Sköld M, Rottenberg M, Källenius G. Tuberculosis and HIV Co-Infection. PLoS Pathogens. 2012;8:e1002464. doi: 10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore CF, Schleif AC, Bernstein JB, Heilman CA. The role of biomedical research in global tuberculosis control: gaps and challenges. Emerg Microbes Infect. 2012;1:e9. doi: 10.1038/emi.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R, Tang WY, Wee SY, Barkham T. Evaluation of a real-time probe-based PCR assay with internal control for the direct detection of Mycobacterium tuberculosis complex. Eur J Clin Microbiol Infect Dis. 2011;30:131–135. doi: 10.1007/s10096-010-1059-z. [DOI] [PubMed] [Google Scholar]
- Poinar H, Kuch M, Pääbo S. Molecular analyses of oral polio vaccine samples. Science. 2001;292:743–744. doi: 10.1126/science.1058463. [DOI] [PubMed] [Google Scholar]