Abstract
Aging is accompanied by a progressive decline in almost all functions of the immune system. To investigate a possible impact of age on IgE production, this study evaluated total and allergen-specific serum IgE levels in a large cohort of allergic patients. This study included 6,370 allergic patients (2,961 females, 3,409 males; mean age, 21.7 years; age range, 0-96 years). Total and allergen-specific serum IgE levels were measured by immunoenzymatic assay. The analysis of variance showed a significant difference (P<0.0001) in the mean value of total IgE among the different age groups of patients. Moreover, specific IgE levels for all allergens examined differed significantly among the age groups of patients (P<0.0001), with a specific trend pattern for each allergen. Total IgE increased with age, but allergen-specific IgE levels significantly decreased with age, with a trend specific for each allergen tested.
Keywords: Age, allergy, serum, specific IgE and total IgE
INTRODUCTION
Aging is characterized by a progressive decline in almost all system and organ functions.1 In particular, aging significantly affects the immune system in a process defined as "immunosenescence".2-4 Functional hematopoietic potential is maintained under basal conditions; however, stem cell renewal gradually declines during stress with aging. Secondary lymphoid tissues show age-dependent architectural modifications. Generally, reduced antibody production and impaired dendritic cell activity are present in elderly subjects.
Allergy is defined as an inflammatory reaction that involves IgE binding to the allergen. Thus, IgE may be considered the hallmark of allergic disorders. IgE is easily detected in serum and can be measured as both total and allergen-specific values. In fact, the serum IgE assay is used to diagnose allergy.
Allergy prevalence tends to decline with age. A Dutch general population study documented that serum IgE levels were lower in the oldest subpopulation, 45-70 years.5 This result was confirmed in a stratified population sample with an age range of 8-73 years.6 Another study conducted on a general population with an age range of 19-99 years again confirmed that the elderly subjects had the lowest IgE levels.7 Several other studies on allergic patients reported consistent findings: older patients had lower IgE levels.8-11 However, these surveys on allergic patients were characterized by relatively limited sample size or age range. Therefore, the present study evaluated a large cohort of allergic patients with a wide age range to investigate a possible impact of age on IgE production.
MATERIALS AND METHODS
Patients
This study enrolled 6,370 allergic patients (2,961 females, 3,409 males; mean age, 21.7 years; age range, 0-96 years). All patients suffered from allergic rhinitis and/or asthma and were evaluated at the IRCCS San Matteo Hospital of Pavia (Italy) from January 2006 to June 2012. All patients gave written informed consent, and the Institutional Review Board approved the study.
Assay
Serum levels of specific IgE were detected by immunofluorometric assay (IFMA) (ImmunoCAP; Phadia, Uppsala, Sweden) in peripheral blood samples from patients. Serum was separated from each blood sample within 4 hours and stored at -20℃ until used to evaluate allergen-specific IgE against the following allergens: Dermatophagoides pteronyssinus, Alternaria, cat, and dog dander, rye grass, birch, ragweed, and Artemisia. The allergen of interest, which was covalently coupled to ImmunoCAP, was allowed to react with the specific IgE in the patient sample. After washing away non-specific IgE, enzyme-labeled antibodies against IgE were added to form a complex, unbound enzyme-anti-IgE was washed away, and the bound complex was then incubated with a developing agent. After stopping the reaction, the fluorescence of the sample was measured. Higher fluorescence intensity indicated a greater amount of specific IgE present in the sample. To evaluate the test results, fluorescence intensity was converted to concentration with the use of a calibration curve. Quantitative specific IgE concentrations are expressed in kU/L according to the traceable calibration using the World Health Organization's second international reference preparation for human IgE. Specific IgE levels higher than 0.35 kU/L were considered positive.
Total IgE was measured by a fluorescence immunoassay (FEIA; Phadia) according to the manufacturer's instructions; a low-range anti-IgE antibody was employed for infants. Anti-IgE covalently coupled to the ImmunoCAP reaction vessel was allowed to react with the total IgE in the patient sample. After washing, enzyme-labeled antibodies against IgE were added to form a complex, unbound enzyme-anti-IgE was washed away, and the bound complex was then incubated with a developing agent. After stopping the reaction, the fluorescence of the sample was measured. The fluorescence intensity was directly proportional to the concentration of IgE in the sample. To evaluate the test results, the response of the patient sample was compared directly to the response using the calibrators.
Statistical analysis
Statistical analysis was performed using the statistical software package Medcalc 9 (Frank Schoonjans, BE). Descriptive statistics are expressed as means and standard error of the mean. The non-parametric Kruskal-Wallis rank test was performed to evaluate the analysis of variance between groups of allergens. Values of P≤0.05 were considered to indicate statistical significance.
RESULTS
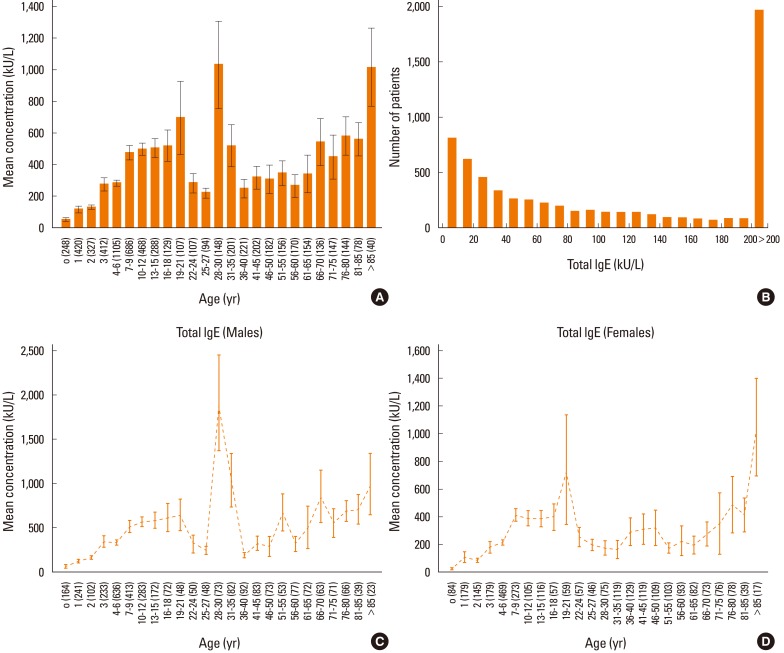
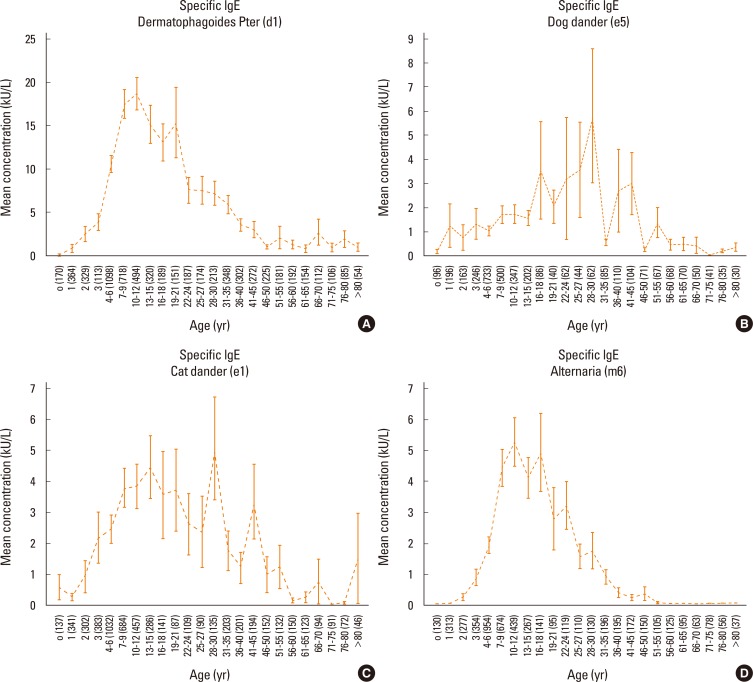
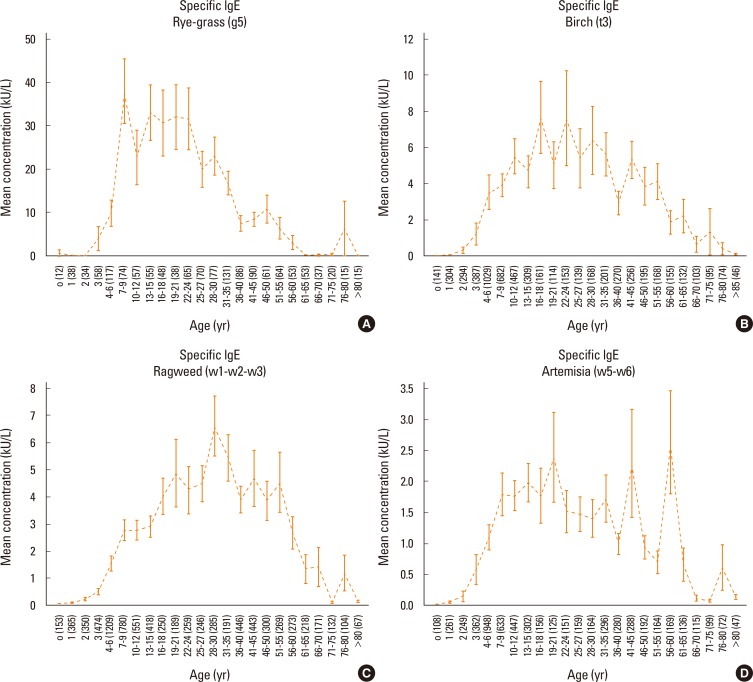
Patients were categorized into several age groups. The analysis of variance showed that the mean values of total IgE differed significantly (P<0.0001) among the groups of patients (as shown in Fig. 1), even when categorized by sex (Fig. 1C and 1D). In our population, almost all patients showed total serum IgE concentrations higher than 200 kU/L (Fig. 1B). Moreover, specific IgE levels for all tested allergens differed significantly among the different age groups of patients (P<0.0001), as shown in Fig. 2 and Fig. 3.
Fig. 1.
(A) Total IgE distribution among the groups of patients. Serum concentrations are expressed as means (bars) and standard error of the mean (line). (B) Distribution of the number of patients per each total IgE concentration. (C, D) Total IgE distribution among the male (C) and female patients (D). Serum concentrations are expressed as means (bars) and standard error of the mean (line).
Fig. 2.
Serum-specific IgE levels among the groups of patients were evaluated separately for each allergen: Dermatophagoides pteronyssinus (A), dog dander (B), cat dander (C) and Alternaria (D). Serum concentrations are expressed as means and standard error of the mean.
Fig. 3.
Serum-specific IgE levels among the groups of patients were evaluated separately for each allergen: Dermatophagoides pteronyssinus (A), dog dander (B), cat dander (C) and Alternaria (D). Serum concentrations are expressed as means and standard error of the mean.
DISCUSSION
Serum IgE may be considered a typical biomarker for the allergic phenotype, as allergic disorders are characterized by IgE-mediated inflammation. IgE measurement is commonly used to diagnose allergy. This study investigated the impact of age on total and allergen-specific serum IgE levels in a large cohort of allergic patients.
Total IgE levels did not decrease with age. There was a peak in the group of 19- to 21-year-old patients, followed by a relevant peak in the group of 28- to 30-year-old patients. Interestingly, there was an increasing trend during aging, with a peak in the oldest subgroup (>85 years). This trend was more evident in females. A possible explanation for this surprising observation may be that an impaired regulatory function occurs during senescence. In fact, it is not unusual to detect autoantibodies in elderly subjects.
On the other hand, allergen-specific IgE levels typically decreased with age. The results showed that each allergen used in this study exhibited a particular trend with age. House dust mites induced the earliest IgE response, as clinically relevant IgE levels were detectable during infancy. This early response may result from the ubiquitous presence of mites and their persistence over time throughout the year. The response to cat allergen was more precocious than the response to dog allergen. The response to Alternaria was characteristic of adolescents and young adults, but rapidly declined after 25-27 years. Pollens and grasses induced early IgE production, and this declined after 30 years, even though these allergens caused the highest IgE levels. Birch-specific IgE peaked during adolescence and young adulthood, whereas IgE against ragweed and Artemisia (both members of the Compositae family) peaked later.
Thus, this study demonstrated that there is a discrepancy between the changes in total and specific IgE levels with age. Total IgE production did not decline with aging, whereas allergen-specific IgE production was diminished in elderly patients. In addition, each allergen induced a particular production trend with age.
The large enrolled population is a particular strength of this survey. Nevertheless, this study has several limitations. First, this study did not include patient follow-up. Additionally, we did not measure the relationship between IgE levels and symptom severity or function, and did not consider any diseases or possible confounding factors such as smoking status, parasite infestation, environmental exposures, seasonal variations, and number of sensitizing allergens. Therefore, further cohort studies and long-term follow-up trials are needed to confirm these preliminary findings.
In conclusion, total IgE increased with age. Allergen-specific IgE levels significantly decreased with age, with a particular trend for each allergen tested.
ACKNOWLEDGMENTS
The authors thank Vania Giunta, Serena Buzzacchino, Cristina Torre, and Giuseppe Mantegna (Fondazione IRCCS Policlinico S. Matteo, Pavia) for technical support.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Scichilone N, Callari A, Augugliaro G, Marchese M, Togias A, Bellia V. The impact of age on prevalence of positive skin prick tests and specific IgE tests. Respir Med. 2011;105:651–658. doi: 10.1016/j.rmed.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Agrawal S, Gupta S. Dendritic cells in human aging. Exp Gerontol. 2007;42:421–426. doi: 10.1016/j.exger.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkhof M, Dubois AE, Postma DS, Schouten JP, de Monchy JG. Role and interpretation of total serum IgE measurements in the diagnosis of allergic airway disease in adults. Allergy. 2003;58:905–911. doi: 10.1034/j.1398-9995.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 6.Sapigni T, Biavati P, Simoni M, Viegi G, Baldacci S, Carrozzi L, Modena P, Pedreschi M, Vellutini M, Paoletti P. The Po River Delta Respiratory Epidemiological Survey: an analysis of factors related to level of total serum IgE. Eur Respir J. 1998;11:278–283. doi: 10.1183/09031936.98.11020278. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa T, Houjyo S, Dobashi K, Sato K. Influence of aging and sex on specific IgE antibody production. Intern Med. 1994;33:396–401. doi: 10.2169/internalmedicine.33.396. [DOI] [PubMed] [Google Scholar]
- 8.Hanneuse Y, Delespesse G, Hudson D, de Halleux F, Jacques JM. Influence of ageing on IgE-mediated reactions in allergic patients. Clin Allergy. 1978;8:165–174. doi: 10.1111/j.1365-2222.1978.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 9.Karakaya G, Kalyoncu AF. The natural course of atopy determined by skin prick tests in patients with bronchial asthma and/or rhinitis. Allergol Immunopathol (Madr) 2006;34:257–262. doi: 10.1157/13095874. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JY, King SL, Kuo BI, Chiang CD. Age of onset and the characteristics of asthma. Respirology. 2004;9:369–372. doi: 10.1111/j.1440-1843.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Stoy PJ, Roitman-Johnson B, Walsh G, Gleich GJ, Mendell N, Yunis E, Blumenthal MN. Aging and serum immunoglobulin E levels, immediate skin tests, RAST. J Allergy Clin Immunol. 1981;68:421–426. doi: 10.1016/0091-6749(81)90195-0. [DOI] [PubMed] [Google Scholar]