Abstract
Literary data suggest apparently ambiguous interaction between menopausal status and obesity-associated breast cancer risk based on the principle of the carcinogenic capacity of estrogen. Before menopause, breast cancer incidence is relatively low and adiposity is erroneously regarded as a protective factor against this tumor conferred by the obesity associated defective estrogen-synthesis. By contrast, in postmenopausal cases, obesity presents a strong risk factor for breast cancer being mistakenly attributed to the presumed excessive estrogen-production of their adipose-tissue mass. Obesity is associated with dysmetabolism and endangers the healthy equilibrium of sexual hormone-production and regular menstrual cycles in women, which are the prerequisites not only for reproductive capacity but also for somatic health. At the same time, literary data support that anovulatory infertility is a very strong risk for breast cancer in young women either with or without obesity. In the majority of premenopausal women, obesity associated insulin resistance is moderate and may be counteracted by their preserved circulatory estrogen level. Consequently, it is not obesity but rather the still sufficient estrogen-level, which may be protective against breast cancer in young adult females. In obese older women, never using hormone replacement therapy (HRT) the breast cancer risk is high, which is associated with their continuous estrogen loss and increasing insulin-resistance. By contrast, obese postmenopausal women using HRT, have a decreased risk for breast cancer as the protective effect of estrogen-substitution may counteract to their obesity associated systemic alterations. The revealed inverse correlation between circulatory estrogen-level and breast cancer risk in obese women should advance our understanding of breast cancer etiology and promotes primary prevention measures. New patents recommend various methods for the prevention and treatment of obesity-related systemic disorders and the associated breast cancer.
Keywords: Androgen, breast cancer risk, estrogen, insulin resistance, menopause, metabolic syndrome, obesity, type-2 diabetes, visceral adiposity.
INTRODUCTION
Many studies support an apparently Janus-faced ambiguous interaction between obesity and breast cancer risk depending on the menopausal status of patients [1-3]. In young women, before menopause adiposity is erroneously regarded to exhibit a protective effect against breast cancer risk [1, 4, 5]. By contrast, in postmenopausal cases, particularly in the elderly, the association is highly positive, obesity confers a strong risk for breast cancer [2, 6]. Controversial results of clinical studies mistakenly suggest that obesity is advantageous against breast cancer by the defective estrogen synthesis in young women [1]. This assumption seems to be consistent with an inverse relationship between the BMI and serum estradiol levels found in premenopausal cases, particularly in the follicular phase of the cycle [7]. Conversely, postmenopausal obesity is harmful by the erroneously presumed excessive estrogen production of adipose tissue mass in older women [8-10]. Explanations for these supposed ambiguous correlations are in concordance with the preconception that high estrogen levels, either endogenous or exogenous, play crucial role in mammary carcinogenesis [11].
Nevertheless, several authors could find confusing and disturbing associations between obesity and breast cancer risk in postmenopausal women. Obese postmenopausal women, who had never used hormone replacement therapy (HRT) exhibited fairly high breast cancer risk [12]. By contrast, HRT use attenuates or abolishes the increased breast cancer incidence [13-15] suggesting a protective impact of female sexual hormones in aged obese women.
In premenopausal cases, the results of clinical studies justify that obesity induces mild or moderate decrease in circulating estrogen levels reflected by their inclination to anovulatory infertility and long or irregular menstrual cycles [16]. Based on the traditional concept of estrogen induced mammary carcinogenesis, obesity associated defective estrogen production in premenopausal women seems apparently to justify the breast cancer preventive impact of fatness.
Even larger studies, which equivocally strengthen the protective effect of obesity for premenopausal breast cancer risk, confess that the responsible mechanisms are completely obscure. Evidences, provided by clinical endocrinological studies regarding correlations between defective hormonal status of obese women and decreased breast cancer incidence, are inconsistent or fairly contradictory [1]. Considering the whole spectrum of unexplained, apparently ambiguous biological behavior as regards breast cancers arising in premenopausal and postmenopausal women, the existence of two distinct types of breast malignancies was presumed occurring before and after menopause [17, 18].
Obesity is a well-known cancer risk factor associated with different grades of insulin resistance and a disturbance of male to female circulating sexual steroid levels [19, 20]. Hyperinsulinemia, dyslipidemia, elevated fasting glucose level and type-2 diabetes are the well documented concomitants of adiposity associated insulin resistance [21]. Obesity provokes further alterations in the endocrine system as well conferred by an excessive circulatory androgen level at the expense of defective estrogen synthesis [22]. Nevertheless, the health benefit of pathologic states; such as overweight and obesity can hardly be justified at any age of women.
The aim of the present study is to clarify the realistic correlations between breast cancer risk and obesity-associated hormonal alterations during the whole life of women. Moreover, this study tries to reveal the sources of the misleading clinical and epidemiologic results suggesting the breast cancer protective effect of obesity in the young. These associations are examined in an analytical review based on the results of prospective, case-control and meta-analytic studies.
Pathogenetic Mechanisms as Links Between Obesity and Breast Cancer Risk
Clinical and experimental evidences prove that obesity, particularly visceral fatty tissue deposition leads to insulin resistance, associated with diverse immunologic, metabolic and hormonal alterations mediating breast cancer risk (Fig. 1). The main stream of obesity related alterations is a self-generating, progressive insulin resistance in thorough interplay with the dysmetabolism and inflammation of adipose tissue mass and with an imbalance of sexual steroid production in the endocrine organs.
Fig. (1).
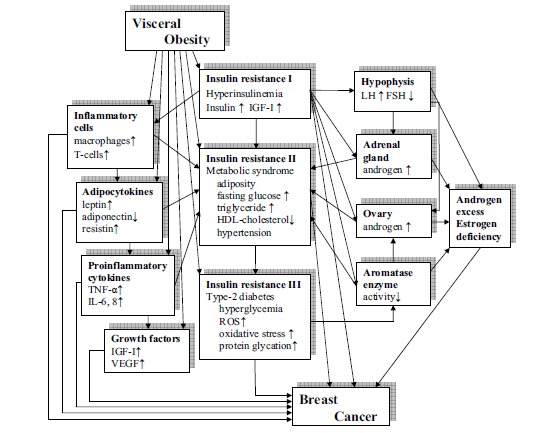
Pathogenetic mechanisms of obesity-related risk for breast cancer.
Obesity Related Insulin Resistant States
Insulin resistance (IR) is a defect of insulin-mediated cellular glucose uptake, which may elicit many disorders in the gene regulation of cellular metabolism, growth, differentiation and mitotic activity. The progression of this disorder predisposes patients to a variety of diseases, such as metabolic syndrome, type-2 diabetes, cardiovascular lesions and malignancies at different sites [23].
Reactive hyperinsulinemia
in the first, compensated phase of insulin resistance maintains serum glucose level within the normal range by means of an increased secretory capacity of insular β-cells [24]. Insulin has diverse metabolic effects and at the same time functions as a growth factor with strong mitogenic capacity. High insulin level in itself may be regarded as cancer risk by the excessive stimulation of multiple cellular signaling cascades [25]. Hyperinsulinemia increases the hepatic synthesis and mitogenic activity of other, insulin-like growth factors, such as IGF-I. High levels of insulin and IGF-I have important role in the alterations of cell proliferation and in tumor induction, particularly in breast and prostate [26]. In a case-cohort study insulin and IGF-I levels were positively associated with breast cancer risk suggesting that hyperinsulinemia may be a substantial link between obesity and mammary carcinogenesis [27]. A recent patent introduced somatostatin analogues and IGF-I inhibition for the prevention and treatment of preneoplastic lesions and cancers of the breast [28].
Metabolic syndrome (prediabetes)
develops in the second, partially uncompensated phase of insulin resistance when the enhanced insulin synthesis of pancreatic islet cells is not enough to maintain euglycemia. This is a quartet of elevated fasting glucose, high serum triglyceride, low HDL cholesterol level and hypertension, being characteristic of viscerally obese patients [29]. Each of these symptoms alone is risk factor for cancer, and together they mean a multiple risk [30, 31].
Hyperglycemia is advantageous for the unrestrained DNA synthesis of tumor cells. It provokes deliberation of free radicals causing derangements in both DNA and enzymes having important role in repair mechanisms [32, 33]. High serum glucose level leads to a non-enzymatic glycation of protein structures, and the glycated products enhance the deliberation of free radicals, cytokines and growth factors [34]. A prospective study on the role of glucose metabolism in breast cancer incidence justified that hyperglycemia among insulin resistant women exhibits direct correlation with mammary cancer risk [35].
Dyslipidemia is a complex disturbance of the lipid metabolism associated with insulin resistance and hyperinsulinemia [36]. Serum level of triglycerides shows close parallelism with insulin resistance, serum insulin level and BMI, whereas being inversely correlated with the high density lipoprotein (HDL) level. Correlation of dyslipidemia and malignant tumors was thoroughly studied in colorectal and breast cancer cases [37, 38]. Results of clinical examinations justified the close association between hypertriglyceridemia and breast cancer [39].
Hypertension usually shows close positive correlation with obesity, insulin resistance and hyperinsulinemia [40, 41]. Elevated insulin level is closely associated with increased activity of the sympathetic nervous system resulting in adrenergic vasoconstriction and hypertension [24, 42]. There are further crossroads between obesity and hypertension by means of hormonal regulation. Activation of the renin-angiotensin-aldosterone system and subsequent elevations in angiotensin II and aldosterone levels are frequently seen in metabolic syndrome [43]. In postmenopausal hypertension, changes in androgen/estrogen ratios favoring for androgens have been implicated to play an important causal role. After menopause, hypertension proved to be a strong risk factor for hormone dependent tumors, separately from obesity [44].
Metabolic syndrome is associated with both increased breast cancer risk and high mortality rates in postmenopausal cases [45, 46]. Time-dependent covariate analyses indicated a positive association between the metabolic syndrome and breast cancer, due primarily to positive associations with serum glucose, serum triglycerides, and diastolic blood pressure [47].
Type-2 diabetes
is the uncompensated phase of insulin resistance when the secretory capacity of insular β-cells becomes exhausted and the decreased serum insulin level results in hyperglycemia. The disrupted glucose homeostasis, the excessive formation of free radicals and the protein glycation depress the activities of the antioxidant scavengers and enzymes. These noxious processes cause serious damages in all biological structures even at a molecular level [32, 48]. The role of free radicals and oxidative stress in the process of carcinogenesis is a well-known fact [49, 50]. Type-2 diabetes seems to be an independent risk for cancer development [51], particularly for breast cancer [39, 52, 53]. A recent patent reports on the invention of a pharmaceutical combination of an insulin sensitivity enhancer and a phosphoinositide 3-kinase inhibitor for the treatment of solid tumors, including breast cancer [54].
Obesity Related Alterations in the Sexual Steroid Production
Obesity related insulin resistance and excessive insulin synthesis in women may contribute to hyperandrogenism and anovulatory infertility through several pathways as insulin is a potent regulator of sexual steroid production in the endocrine organs [55]. Hyperinsulinemia and excessive IGF-I supply stimulates ovarian androgen production at the expense of reduced estrogen synthesis [56]. High insulin level was found to increase the testosterone biosynthesis of human ovarian thecal cells deriving from insulin resistant, infertile women with polycystic ovarian syndrome (PCOS) [57]. Therapeutic improvement of insulin sensitivity and effective reduction of insulin secretion decreased ovarian cytochrome P450c17 alpha activity and normalized serum free testosterone level [58, 59]. A recent patent introduced a new metformin derivative as a therapeutic agent for treating metabolic syndrome, type-2 diabetes, PCOS and cancers depleted of gene P53 [60].
Hyperinsulinemia promotes adrenal androgen synthesis as well by means of increased adrenal sensitivity to adrenocorticotropin. At the same time, high insulin level may favor the luteinizing hormone (LH) secretion of pituitary. Increased LH level also stimulates androgen biosynthesis by the activation of adrenal gland and ovarian theca cells [61, 62]. According to earlier hypotheses, breast cancer risk is increased not only in hyperandrogenic postmenopausal women, but also in premenopausal cases with mild hyperandrogenism and apparently normal (ovulatory) menstrual cycles [63]. In centrally obese, either premenopausal or postmenopausal women, excessive ovarian production of testosterone seems to be a genetically determined risk for breast cancer [64, 65].
Insulin resistance, hyperinsulinemia and excessive IGF-I activity mediate defective estrogen synthesis by counteraction to aromatase enzyme gene expression at cellular level. The aromatase enzyme complex catalyzes the conversion of androgens to estrogens in a wide variety of tissues. In premenopausal women, the ovaries are the principle sources of estradiol; by contrast, in postmenopausal women when the ovaries cease to produce estrogen, it is synthesized in a number of extragonadal sites [66]. These sites include the adipose tissue, breast, endometrium, bone, endothelium, aortic smooth muscle cells, and numerous locations in the brain. All these tissues, particularly breast and endometrium, have high estrogen demand and a local hormone synthesis helps to preserve their structural integrity and functional activity in spite of low circulatory estrogen levels [67].
In premenopausal insulin resistant women with type-2 diabetes, the ovaries exhibit decreased capacity to convert androgen to estrogen, probably due to a reduction of ovarian aromatase activity [68]. Conversely, in patients with either estrogen deficiency (aromatase deficiency) or estrogen resistance (estrogen receptor mutation) glucose intolerance, hyperinsulinemia and lipid abnormalities are concomittant alterations associated with excessive gonadotropin and androgen levels [69].
Estrogens decrease low grade inflammatory reactions and may in parallel reduce the glucocorticoid responses. Low estrogen levels after menopause allow the predominance of glucocorticoids and the manifestation of the metabolic syndrome. These observations suggest that the disturbed equilibrium between sex hormones and glucocorticoids may be a critical element in the manifestation of metabolic syndrome-related pathologies [70].
Inflammatory and Metabolic Dysfunctions of Adipose Tissue in Visceral Obesity
Visceral adipose tissue has important functions in energy homeostasis, metabolic equilibrium, immune responses and in the regulation of cell proliferation. Secretion of signaling molecules; adipokines regulates the cellular microenvironment both locally and systemically [71-73].
The chronic low grade inflammation associated with obesity is an important player in tumor development and progression. Increased IGF-I level may mediate the inflammation of adipose tissue via its effects on immunologically active cells including macrophages and T cells [74]. IGF-I may provoke macrophage migration and invasion and increased production of proinflammatory cytokines.
Adipokines include unique products of fatty tissue known as adipocytokines; such as leptin, adiponectin resistin, ghrelin etc. [75]. Leptin biosynthesis is in close direct correlation with insulin level and this may explain the increased leptin levels observed in obesity [76]. High leptin concentrations may constitute a possible link relating obesity and breast cancer promoting the invasion and migration of tumor cells [77]. Conversely, obesity may downregulate the secretion of adiponectin, an adipokine with anti-inflammatory, insulin sensitizing and anti-tumor properties [78]. The balance of leptin as well as the adiponectin concentrations are the critical factors in breast cancer risk and in other obesity related cancer genesis [79].
Proinflammatory cytokines (TNF-α, IL-6, IL-8) produced in excessive adipose tissue mass increase nitric oxide (NO) production, a substrate for reactive oxygen species (ROS). Accumulation of cytokines and ROS further contribute to insulin resistance resulting in elevated circulating glucose and free fatty acid levels. All signaling molecules of fatty tissue including cytokines, hormones and growth factors are involved in the regulation of the proliferation, invasion and metastatic spread of tumor cells [80]. Proinflammatory cytokines within the tumor microenvironment correlate with growth, increased invasiveness and poor prognosis in many types of cancer, including breast cancer [81].
Protective Effects of Estrogen Against Obesity and Obesity Associated Alterations
Out of several hormones affecting body mass and adipose tissue deposition, estrogens promote, maintain and control the ideal distribution of body fat [82]. These steroids are known to regulate differentiation and metabolism of adipocytes as well. Estrogen deficiency and defective estrogen signaling results in obesity with increasing adipose tissue mass, preferentially in visceral location [19, 82].
Anti-Obesity Effects of Estrogen
Estrogen regulates the metabolism, differentiation, growth and cell kinetic mechanisms of adipocytes. In healthy premenopausal women central adipocytes show higher insulin sensitivity and exhibit a higher turnover rate despite similar fat content as compared with male cases [83]. Conversely, after menopause, decreased ovarian estrogen synthesis results in increasing insulin resistance in central adipocytes and higher fasting insulin levels conferring increased risk for metabolic and cardiovascular diseases [84, 85]. In experiments, estradiol is able to inhibit the glucocorticoid production in rodent adipocytes of mesenteric origin, providing novel insight into the anti-obesity mechanism of estrogen effect [86].
Obesity and overweight are important concomitants of insulin resistance and they are strongly associated with disturbed equilibrium of male to female sexual hormone concentrations in women [87]. Healthy estrogen predominance in women induces subcutaneous gluteofemoral adipose tissue deposition [84]. Conversely, androgen excess and deficient estrogen synthesis exhibits close correlation with visceral obesity both in pre- and postmenopausal women [22]. In young women with PCOS, there are several advantages of treatment with oral contraceptives, including protection from the development of endometrial carcinoma, regularization of menses, amelioration of hirsutism, acne and obesity [88]. Similarly, in obese postmenopausal women with or without type-2 diabetes, HRT reduces abdominal adiposity and insulin resistance [89] and decreases the risk for obesity associated breast cancer [15].
Anti-Atherogenic and Anti-Hypertensive Effects of Estrogen
Healthy premenopausal women are typically protected from cardiovascular diseases and hypertension as compared with men and this has been hypothesized to be because of the protective effects of estrogens. Conversely, obese, diabetic young women, and postmenopausal cases may lose this protected state as their bioavailable estradiol levels are strongly reduced [90].
Estrogen may have crucial role in the maintenance of normal serum lipid levels. Postmenopausal women have higher total cholesterol, LDL cholesterol and triglyceride levels, whereas lower HDL cholesterol levels as compared with premenopausal cases. These changes may be regarded as a shift toward a more atherogenic lipid profile in estrogen deficient milieu [91]. Postmenopausal estrogen therapy may reduce the risk of cardiovascular disease and this beneficial effect may be mediated in part by favorable changes in plasma lipid levels [92]. Estrogens have important regulatory role in the hepatic lipid metabolism as well. In aged rats, estradiol administration lowered the level of lipid peroxidation and improved the dysfunction parameters of the liver [93].
Estradiol has cardiovascular protective impact by its anti-hypertensive activity as well. Estrogens can downregulate components of the renin-angiotensin system (RAS) and reduce the expression and activity of angiotensin I-converting enzyme [94, 95]. Estradiol inhibits the excessive synthesis of vasoconstrictor endothelin and improves endothelial dysfunction in ovariectomized female spontaneously hypertensive rats [96]. In the pathogenesis of postmenopausal hypertension increased androgen to estrogen ratio may be associated with the activation of renin-angiotensin and endothelin systems [97].
Clinical and experimental studies support that estradiol has also antioxidant activities [98, 99] that may be effective against inflammatory lesions, atherosclerotic vessel injuries and malignancies.
Antidiabetogenic Impacts of Estrogen
Estrogens have beneficial effects on the energy metabolism and glucose homeostasis by means of several pathways [100]. Estrogens advantageously regulate the insulin production capacity of the pancreatic islet cells [85]. Estrogen receptor alpha (ER-α) activation promotes β-cell mass survival and insulin biosynthesis in diabetic and obese cases, whereas ERβ activation improves glucose stimulated insulin secretion [101]. Estrogen administration seem to be a therapeutic avenue to preserve functional β-cell mass in patients with diabetes mellitus.
In the liver, estrogen regulates insulin sensitivity by the balanced activation of glycogen synthase and glycolytic enzymes to maintain the equilibrium of glycogen synthesis and glycogenolysis. In ER-α knockout mice, hepatic insulin resistance was associated with decreased glucose uptake in skeletal muscles [102].
In the peripheral tissues, ERs advantageously modulate the insulin stimulated glucose uptake through regulation of the phosphorylation of insulin receptor protein. In hyperinsulinemia, high concentrations of estradiol can inhibit the excessive insulin signaling in adipocytes [103], which may be a safety impact both against pathological glucose uptake and dangerous mitogenic activity.
ERs have crucial roles in cellular glucose uptake by regulation of intracellular glucose transporters (GLUTs) and enhancing both GLUT4 expression and translocation [104]. In human adipocytes GLUT4 abundance is highly correlated with insulin responsiveness. In women with PCOS, decrease in insulin stimulated glucose uptake was associated with reduced amount of GLUT4 on adipocyte membrane [105].
Estrogens and ER signals have pivotal role in the regulation of growth hormone (GH) activity by means of modulation of its secretion and cellular GH receptor function. Estrogens play a major and positive role in the regulation of GH-IGF-I axis in both genders [106], which may be in close correlation with their antidiabetogenic and anticancer capacities.
Postmenopausal hormone replacement therapy (HRT) reduced abdominal obesity, insulin resistance, new-onset diabetes, hyperlipidemia, blood pressure, adhesion molecules and procoagulant factors in women without diabetes and reduced insulin resistance and fasting glucose in women with diabetes [89].
Regulation of Adipokine Secretion, Inflammatory Reactions and Growth Factor Activity by Estrogen
Low grade systemic inflammation may be associated with estrogen deficient states, namely menopause and ovariectomy. At early stages of estrogen deficiency estrogen administration decreased the inflammation associated risk of developing cardiovascular disease [107]. Nevertheless, estradiol may contribute even to the vascular healing process and to the prevention of lumen restenosis in atherogenic arteries. It improves reendothelialization through ER-α activation and decreases smooth muscle cell migration and proliferation through ER-β stimulation [108].
Phytoestrogens may advantageously influence the level of adipokines in insulin resistant women. In postmenopausal cases, diet, physical exercise and daily oral intake of soy isoflavones had a beneficial lowering effect on serum leptin, and TNF-α levels and showed a significant increase in mean serum levels of adiponectin [109]. Epidemiologic studies support that phytoestrogen rich foods may be beneficial consumed before or during adolescence for the prevention of breast cancer [110].
Estrogen advantageously regulates serum levels of available growth factors. In clinical studies estradiol lowered, whereas testosteron increased total IGF-I level and estradiol specifically suppressed unbound, free IGF-I level [111]. Crosstalk between estrogen receptors and growth factor (IGF-I, EGF, VEGF) receptor signaling pathways is well-known both in healthy tissues and malignancies [112, 113]. Nevertheless, estradiol may induce both growth stimulation and growth inhibition depending on the ratio and activity of ERs and GFRs [114].
The presumed synergistic contribution of ERs and GFRs to cancer development and progression would be a permanent danger without contraregulatory impact. Inhibition of growth factor signaling in apparently ER-negative breast cancer cells successfully restored ER expression suggesting a dynamic, inverse relationship between the two receptor systems [115]. Moreover, excessive EGF predominance decreased both ER-α protein concentration and gene transcription activity in the human breast cancer cell line MCF-7 [116]. These results suggest rather an alternative role of estrogen and growth factor actions in tumor cell proliferation.
Correlations Between Grade and Distribution of Adiposity and Breast Cancer Risk
Fat deposition is thoroughly affected by male to female sexual steroid level [84]. Circulating estrogen level may be the key regulator in mediating differences in adipose tissue distribution between pre- and postmenopausal women [117]. In obese premenopausal women, peripheral, subcutaneous adiposity is typical and there is a lower incidence of obesity associated dysmetabolism. By contrast, in obese postmenopausal cases, circulating levels of estrogen are dramatically decreased and adipose tissue distribution becomes more male-like. Predominance of visceral adiposity and the associated metabolic and hormonal disorders mean high risk for obesity related diseases, included breast cancer [71].
The most important data characterizing the grade of obesity are body mass index (BMI) and weight in kilogram [4, 5, 118] reflecting general adiposity. Knowing the hormonal and metabolic background of the diverse distribution of adipose tissue, BMI or body weight in kilogram may not correctly reflect the correlations among obesity, hormonal disturbances and breast cancer risk in young women [14]. Body circumference measurements; such as hip (HC), waist (WC) and waist to hip ratio (WHR) inform about the regional distribution of fatty tissue deposition [119-121] so as to quantify the mass of visceral abdominal fat and the metabolic risk of patients.
Some authors reported on the value of WC, HC and WHC ratio in the prediction of premenopausal breast cancer occurrence [119, 122]. Conversely, in other studies, body fat distribution, such as WC had no defining role in the establishment of insulin resistance or premenopausal breast cancer risk [2, 119].
Magnetic resonance imaging (MRI) was used to quantify separately the mass of visceral and subcutaneous abdominal fat depositions in obese adolescent girls [123]. Mass of visceral fat was highly correlated with insulin secretion and insulin resistance. Conversely, abdominal subcutaneous fat mass measured by MRI did not show close correlation with the quantified indicators of insulin resistance.
In conclusion, neither BMI nor circumference measurement may exactly separate the metabolically indifferent, subcutaneous fat and the dangerous visceral fat, which may partially explain the controversial correlations between obesity grade and breast cancer risk.
Lifelong Changes in the Estrogen Level of Women and their Relation to Obesity and Breast Cancer Risk
Circulating female sexual steroid levels and fertility continuously decrease during the life of women. The ability to conceive is at its peak in young women under 30 years of age with a continuous decline from the fourth decade, which suggests decreasing fertility even in the premenopausal phase [124]. Menopause at 50-51 years of age means a sudden break in ovarian estrogen synthesis and confers further decline in the circulating hormone level.
In premenopausal women, the good equilibrium of sex steroid synthesis defines health and reproductive capacity, whereas good, symptom-free adaptation to the estrogen deficient environment is a prerequisite of postmenopausal health. Changes in the hormonal equilibrium during women’s lives strongly influence the obesity associated breast cancer risk (Table 1).
Table 1.
Lifelong Changes in the Hormonal Status of Obese Women and their Breast Cancer Risk.
| Life Periods of Obese Women | Estrogen Level | Insulin Resistance | Breast Cancer Risk |
|---|---|---|---|
| Children | ↓ | ↑ | ↑ |
| Adolescent girls | ↓↓ | ↑↑ | ↑↑ |
| Premenopausal women | |||
| Type-2 diabetes | ↓ | ↑ | ↑ |
| Anovulation | ↓ | ↑ | ↑ |
| Nulliparity | ↓ | ↑ | ↑ |
| Contraceptive use | ↑ | ↓ | ↓ |
| Multiparity | ↑ | ↓ | ↓ |
| In vitro fertililization | ↑ | ↓ | ↓ |
| Postmenopausal women | |||
| HRT user | ↑ | ↓ | ↓ |
| Non HRT user | ↓ | ↑ | ↑ |
| Type-2 diabetes | ↓↓ | ↑↑ | ↑↑ |
| Hysterectomy | ↓↓ | ↑↑ | ↑↑ |
Obesity Associated Hormonal Alterations in Childhood and Adolescence and their Prediction for Breast Cancer Risk
Obesity is a detrimental disorder and may not be protective against malignancies even in children. Childhood obesity is associated with insulin resistance and hyperinsulinemia mediating risks for chronic diseases and cancer in the adult life [125]. Many factors of metabolic syndrome, such as elevated glucose level, hypertension, high triglyceride and low HDL-cholesterol level even type-2 diabetes might occur in these young prepubertal obese cases [126].
Some studies suppose a definite key age; for example 10 years when breast cancer “protective” obesity turns to harmful between the ages of 10 and 20 years as a prediction of elevated breast cancer risk in the premenopausal life [127]. Others found fatness in childhood to be associated with decreased breast cancer risk both for pre- and postmenopausal women [128]. Further authors postulate that only teenager obesity is protective against premenopausal breast cancer initiation but if it is persistent after teenage years than it may confer an increased risk for postmenopausal breast cancer [129]. Nevertheless, finding a key age at which obesity in young girls turns from a cancer protective to a cancer promoting agent seemed to be unsuccessful.
Obesity in puberty with extreme somatic growth and explosion-like sexual development means a great challenge for the entire metabolic and hormonal systems. In obese children, puberty becomes a more serious danger for insulin resistant states as compared with normal weight cases [130, 131]. In obese adolescent patients, increased insulin resistance does not return to prepubertal values at the end of puberty and represents high risk for adult metabolic syndrome, type-2 diabetes and for their complications [126, 132].
In adolescent girls, obesity associated metabolic storms are related to abnormal ovarian sexual steroidgenesis as well, resulting in excessive androgen and defective estrogen production and greater frequency of irregular, anovulatory cycles [129, 133, 134]. High serum androgen concentrations developing in puberty are preserved into adulthood and are reflected by defective fertility patterns at least until 30 years of age [133, 134]. This observation may be in concordance with an increased premenopausal breast cancer risk of delayed first childbearing [18, 135].
Equilibrium of sexual hormone production and the development of regular cycles are prerequisites not only for reproduction but also for somatic health in women [19, 20, 136]. Pathological alterations in the critical period of puberty, such as obesity endangering both the fertility and survival of women might not be protective against cancer initiation in either pre- or postmenopausal cases. In conclusion, obesity related endocrine alterations in children, adolescents and at young age might really be defining factors for breast cancer risk, being not protective, but rather highly dangerous.
Controversial Associations Between Obesity Related Anovulatory, Irregular Menstrual Cycles and Breast Cancer Risk in Premenopausal Women
In premenopausal cases, obesity is associated with defective estrogen synthesis and decreased circulatory estrogen levels, particularly in the follicular phase [7]. These obesity-related hormonal alterations may usually confer irregular menstrual cycles, anovulation, infertility and reduced response to fertility treatment [16]. Theory of estrogen induced mammary carcinogenesis supports the hypothesis that in obese young women, anovulation and the associated menstrual cycle disorders may be protective against breast cancer risk [137].
Results of cohort and case-control studies exhibit inconsistent data concerning the associations of menstrual disorders and breast cancer incidence. Some investigators found an increased breast cancer incidence in women with long menstrual cycles [138, 139] others found no associations [140-142] or conversely, a decreased risk [137, 143, 144]. In a large study, the results were quite controversial, in women having long cycles the risk of breast cancer was increased, whereas, in those whose cycle was estimated to be irregular, the risk was reduced [145]! In a prospective study, anovulatory infertility was associated with decreased breast cancer incidence [146]. By contrast, a further study of the same working group in the same year established that other factors than anovulatory disorders may be protective against breast cancer risk in obese young women [116].
Ambiguous correlations between obesity related hormonal disturbances and breast cancer risk may be explained by the fact that anovulation and menstrual irregularities are strong risk factors for breast cancer both in obese cases and non-obese controls. Among symptom-free lean control girls, laboratory findings of hyperinsulinemia and excessive androgen concentration may reveal the insulin resistance and occult anovulatory cycles [23].
In premenopausal women with polycystic ovarian syndrome (PCOS) the coexistence of anovulatory infertility and insulin resistance represents common risk for the cancers of highly hormone dependent breast, endometrium and ovary [147, 148]. Mortality data of infertile women with PCOS were examined in a long-term follow up study in the UK and breast cancer was the most common cause of death among these patients [149]. In a Brazilian study, anovulatory disorders with or without PCOS were promoters of endometrial and breast cancer by hyperinsulinemia and hyperandrogenism in infertile women [150]. In an American study, nulliparity, irregular menstrual cycles, obesity, diabetes and hypertension were strongly associated with endometrial cancer risk in premenopausal women [151]. In young, nulliparous women, endometrial carcinomas are frequently associated with synchronous primary cancers of the ovary or breast [151, 152].
Hyperprolactinemia is also associated with cycle disorders, reproductive dysfunction and hyperandrogenism in women, however, it may be sharply discerned from PCOS and other disorders related to androgen excess. An increased overall cancer risk was found in hyperprolactinemia patients but further investigations are necessary to confirm these results [153]. Data of recent studies suggest positive association between elevated prolactin level and breast cancer risk, predominantly among young, premenopausal women [154].
How can we explain the increased prevalence of breast, endometrial and ovarian cancers in women with fertility disorders, even without obesity? In premenopausal cases, slight or moderate decrease in circulating female sexual steroid levels may be enough to block the delicate mechanism of ovulation. At the same time, a slightly estrogen deficient milieu confers preferential cancer risk for the female organ triad having high estrogen demand [19, 136].
Correlations Between Reproductive Data and Obesity-Associated Breast Cancer Risk in Premenopausal Women
In women, multiparity and risk for malignancies at several sites exhibit an inverse relationship [155-157]. High parity shows tumor protective effect even against female breast, endometrial and ovarian cancers [158, 159]. This may be attributed to the good equilibrium of female sexual steroid production associated with good fertility. Moreover, pregnancy equivalent, high female sexual hormone treatment could prevent the chemically induced mammary carcinogenesis in female Lewis rats [160].
Nulliparity may be associated with ovulatory disorders in the majority of cases and there are studies, which equivocally exhibited increased prevalence of breast cancer in nulliparous women [161, 162]. Furthermore, coexistence of nulliparity and overweight may amplify each other’s effect on increasing breast cancer risk having strong synergism [161]. Delayed first childbearing proved to be a risk factor of breast cancer among older premenopausal women as well [18, 135], which may also be associated with long term sex hormone imbalance and fertility disorders. High breast cancer risk in correlation with nulliparity and delayed first birth suggests an important role of defective estrogen synthesis and anovulatory disorders in mammary carcinogenesis.
Possible role of fertility medications in the increased risk of breast cancer has been hypothesized. However, large studies were not able to find any associated risk of breast cancer after ovulation provocation and in vitro fertilization (IVF) in anovulatory cases [163]. Moreover, an American case-cohort study established that use of clomiphene as treatment for infertility lowers the increased risk of breast cancer in women with ovulatory abnormalities [164]. Recently, overall cancer risk among infertile women before IVF was found to be markedly increased in a large study in Sweden [165]. However, after IVF assisted childbirth, cancer risk was significantly decreased mainly due to a lower than expected risk for breast and cervical cancers. Similarly, a conspicuous great reduction in breast cancer incidence was found among those infertile women who underwent to high dose estrogen treatment as ovulation induction therapy [146]. These recent literary data support the breast cancer lowering value of IVF assisted childbirth among endangered women with anovulatory infertility.
Correlations Between Postmenopausal Obesity and Breast Cancer Risk in Women
In postmenopausal estrogen deficient, obese cases the regional distribution of fat deposition near uniformly affects the visceral region in close correlation with their high breast cancer risk [6].
Obese postmenopausal women never using HRT are obviously insulin resistant. Moreover, ageing after menopause is a further factor increasing the cancer risk as it is associated with a continuous estrogen loss and parallel advancing insulin resistance [166]. In old obese women, struggle between the harmful sequels of insulin resistance and the low level of protective female sexual steroids will be frequently finished by cancer initiation. These correlations explain the higher breast cancer risk of obese postmenopausal women who do not receive hormone treatment as compared with HRT users.
In obese HRT user postmenopausal women, the incidence of breast cancer is equivocally reduced [15]. The protective effect of estrogen substitution may counteract to the obesity associated insulin resistance and their breast cancer risk decreases [19]. In HRT user women the hormonal and metabolic equilibrium becomes similar to that of obese young women with relatively preserved circulatory estrogen levels. Estradiol substitution increases insulin sensitivity [100] yields favorable changes in plasma lipid levels [92] and by its anti-obesity effect decreases the accumulation of visceral fat deposition [167]. All these impacts justify the beneficial anticancer capacity of HRT in obese women after menopause.
A great challenge is to explain the beneficial anticancer impact of HRT in obese postmenopausal women based on the misbelief that they have excessive circulatory estrogen level. To solve this contradiction, some authors assumed that mediators other than estrogen, such as insulin and insulin-like growth factors might confer the obesity associated breast cancer risk after menopause [27].
Effects of Exogenous Hormone Treatment and Environmental Endocrine Disruptors on Breast Cancer Risk
Oral contraceptive (OC) use replaces the natural menstrual cycle with relatively steady levels and fluctuations of artificial sex hormones.
Recent epidemiological studies have confirmed that combined oral contraceptives provide substantial protection against endometrial and ovarian cancer in the endangered anovulatory women [168, 169]. Effect of OCs on breast cancer risk seems to be controversial. Increased risk was confined to women who have begun pill use as teenagers or those with very long term use [168]. Nevertheless, endometrial, ovarian and breast cancers exhibit conspicuously similar epidemiology and they frequently synchronously appear in anovulatory young women [151, 152]. These observations may suggest that a proper selection of patients and controls would lead to the realistic evaluation of beneficial OC effect even on breast cancer risk.
In PCOS cases, treated with OCs, the volume of cystic ovaries is reduced, ovarian testosterone secretion is decreased and there are favorable effects on carbohydrate and lipid metabolism as well [169]. A recent patent disclosed a method for treating hyperandrogenism and associated conditions, including PCOS by a fatty acid ester of estrogen or an estrogen derivative compound [170]. This therapy seems to be more advantageous against the dangerous dysmetabolism of PCOS cases than OC administration.
Use of HRT has highly controversial associations with breast cancer risk. Till now, the carcinogenic capacity of postmenopausal estrogen therapy was a prevailing concept [171]. By contrast, recent literary data support the cancer protective capacity of postmenopausal estrogen treatment both for the moderately and highly hormone responsive organs of women [136, 172]. Results of these studies prophesied a breakthrough regarding the traditional concept of estrogen induced mammary carcinogenesis.
In 2011, the WHI Randomized Controlled Trial strengthened that the estrogen treatment in women with prior hysterectomy resulted in a significantly lower risk for breast cancer than in untreated controls [173]. Breast cancer risk of women with hysterectomy may be near uniformly high because of their abrupt, shocking hormone deprivation. HRT studies on these homogenously selected cases seem to be methodologically fairly strong, yielding unexpectedly correct results [136, 172].
Today, increasing numbers of evidence from the clinical trials suggest that unopposed estrogen as hormone treatment does not increase the risk of breast cancer, and may even reduce it. The earlier concepts regarding the breast cancer risk of hormone replacement therapy are completely changing [174-178].
HRT decreases the accumulation of central fatty tissue in obese postmenopausal women and provides them long term metabolic and health benefits [27, 167]. HRT in older women equivocally attenuates the obesity-associated breast cancer risk [14, 15, 176] similarly to the preserved circulatory estrogen level in case of fat premenopausal women. In conclusion, estrogen treatment in postmenopausal women decreases the obesity and insulin resistance associated breast cancer incidence as well.
Exposure to environmental estrogens (xenoestrogens) occurs through diet, household products and cosmetics, but concentrations of single compounds in breast tissue are generally lower than needed for assayable estrogenic responses [179]. Exposure to complex mixtures of estrogenic chemicals in consumer products is regarded as a factor in breast cancer development based on the principle of estrogen induced mammary carcinogenesis.
Endocrine disruptors (EDs) are environmental chemicals that are able to bind hormonal receptors. These compounds may disrupt the endocrine regulation of reproduction and adaptive mechanisms and promote the development of endocrine disorders. Since several EDs have a structure similar to that of endogenous steroid hormones such as estrogens, they have an affinity for steroid hormone receptors and may alter the normal hormone-mediated reactions [180]. Environmental EDs may interfere with the development of healthy menstrual cycles in puberty resulting in anovulatory cycles in women.
Poor natural light exposure mediates deleterious metabolic and hormonal alterations by excessive melatonin secretion; such as insulin resistance, deficiencies of estrogen, thyroxin and vitamin D [181]. Long term exposition to darkness may deteriorate the development of healthy hormonal equilibrium in puberty endangering the somatic health as well. Light deficiency confers increased breast cancer risk among night shift worker women and dark skinned immigrants in northern countries.
Estrogen Therapy of Breast Cancer
Before the introduction of antiestrogens, high dose estrogen was successfully administered as endocrine therapy for postmenopausal women with advanced breast cancer. Nowadays, the ambiguous, unreliable therapeutic effects and high toxicity of antiestrogens suggest the necessity of finding new strategies for breast cancer treatment [182]. Recently, the old-fashioned high-dose estrogen treatment in patients with advanced breast cancer and previous estrogen deprivation was demonstrated to be effective in prospective clinical trials. Anti-tumor mechanism of estrogen therapy is under debate, but one can hypothesize that after a long-term antiestrogen treatment estrogen may become an apoptotic trigger rather than a survival signal for breast cancer cells [183].
Phytoestrogen containing health supplement compositions are recommended by some patents for the prevention or treatment of postmenopausal breast cancer and for the alleviation of menopausal symptoms [184]. Soybean extract, which has phytoestrogen component proved to be useful as a therapeutic agent for solid cancers, such as breast cancer [185]. A recent patent reported on the use of steroid compounds as anticancer drugs. They have anti-obesity, anti-hyperglycemic, anti-autoimmune and anti-hypercholes-terinemic impacts as well, similarly to estrogen [186].
CONCLUSION
Obesity-associated hormonal disorders confer breast cancer risk during the whole life of women without any ambiguous interaction between obesity and menopausal status. Erroneous results regarding the breast cancer protective effect of obesity in young women derive mainly from the deceivingly lower tumor incidence among them. Further misleading factor may be that hormonal disorders related to anovulatory infertility are strong cancer risk factors for the breast both in obese and non-obese young women.
Low incidence rate of breast cancer in young obese women may be a very important misleading finding. In the majority of premenopausal obese cases a predominantly subcutaneous adipose tissue deposition results in milder insulin resistance being counteracted by their preserved hormonal cycle. Consequently, in obese premenopausal women their circulatory estrogen level confers protective effect against breast cancer rather than obesity.
Tumor cell kinetics may also provide an explanation for the low breast cancer incidence rate in obese premenopausal cases. A long term exposition to harmful environmental and systemic factors is necessary for cancer initiation; moreover the tumor growth from the initiation to clinically diagnosable size takes further several years. This double delay of clinical appearance of breast cancers may frequently result in a postmenopausal tumor, though it was initiated in the premenopausal period.
Anovulatory infertility and the associated hormonal defects seem to be stronger breast cancer risk factors than adiposity alone and these alterations are not rare even among control cases with normal weight. Studies on obesity-related breast cancer risk disregarded the random occurrence of these hormonal alterations both among obese cases and lean controls. Moreover, oral contraceptive use is fairly widespread among premenopausal women, which may reverse the mild hormonal defects and attenuates their cancer risk either in obese or in lean cases. Strict selection of healthy, lean control women for obesity associated breast cancer studies and taking into account the OC use among obese cases and controls will justify the health advantage of normal body weight over obesity.
Recognition of the inverse correlation between circulatory estrogen-level and breast cancer risk in obese women should advance our understanding of breast cancer etiology. Moreover, it would promote primary cancer prevention measures and the introduction of causal cancer therapy.
CURRENT & FUTURE DEVELOPMENTS
Changing concepts concerning breast cancer etiology provide completely new strategies against mammary tumors (Tables 2 and 3). Circulatory endogenous estrogen was blamed to provoke cancer initiation and promotion in the highly hormone responsive female organs, however, this misbelief yielded fairly controversial results in the clinical and experimental fields of medicine. Nowadays, overwhelming literary data suggest that estrogens have advantageous impact on dysmetabolism and hyperandrogenism, which are essential sources of tumor development.
Table 2.
Hormonal Risk Factors for Breast Cancer.
| Traditional Concept | Author’s New Concept |
|---|---|
| Excessive circulatory estrogen level | Reduced circulatory estrogen level |
| Increased aromatase activity | Defective aromatase activity |
| Unopposed estrogen level | --------- |
| Ovulatory peak of estrogen level | Anovulatory disorder |
| Contraceptive use | Defective hormonal cycle |
| Cross talk of estrogen and GFs | Missing surveillance of estrogen on GFs |
| Xenoestrogens | --------- |
| Hormone replacement therapy | Missing hormone replacement therapy |
| Excessive light exposure | Light deficiency |
| Androgen excess | Androgen excess |
| Hyperinsulinemia | Hyperinsulinemia |
Table 3.
Protective Hormonal Factors Against Breast Cancer.
| Traditional Concept | Author’s New Concept |
|---|---|
| Obesity (in young) | Normal weight |
| Anovulation | Healthy ovulatory cycles |
| Hysterectomy (preventive) | Preservation of gynecological organs |
| Antiestrogen (preventive) | Estrogen prevention |
Recent patents disclose oral contraceptive and metformin derivative treatment possibilities for breast cancer prevention and therapy. Moreover, a new invention provides special estrogen derivatives being metabolically more advantageous for the treatment of PCOS than oral contraceptives. As anovulatory disorders in young women are strong risk factors for breast cancer, insulin resistance screening of asymptomatic occult cases in the population and their hormone treatment would be effective primary breast cancer prevention.
In postmenopausal cases, obesity, metabolic syndrome, type-2 diabetes and hysterectomy are strong risk factors for mammary malignancies. Hormone replacement therapy in these endangered cases would be advantageous preventive measure.
The present practices of breast cancer therapy have fairly controversial results. Worldwide administration of antiestrogen compounds for breast cancer treatment yielded thorough disappointment. Antiestrogens are cytostatic agents blocking the most important regulatory mechanisms associated with estrogen receptors. They do not have only toxic effects but also strong carcinogenic capacity. Recently, scientists in some centers confess, that one armed estrogen treatment is rather cancer protective than carcinogenic. Certain patents disclose the beneficial effects of phytoestrogens in breast cancer prevention and therapy. Moreover, publications on the high dose estrogen treatment of advanced breast cancer cases are regularly returning and the results are fairly encouraging. Today, we are on the route to regard estrogens as beneficial anti-cancer drugs rather than carcinogenic agents.
ACKNOWLEDGEMENTS
The author thanks her PhD students; Dr. Siamak Eftekhari and Dr. Szilvia Mihályi for their help in the compilation of literary data.
CONFLICT OF INTEREST
The author confirms that this article content has no conflicts of interest.
REFERENCES
- 1.Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66(1 ):33–8. doi: 10.1016/j.maturitas.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi S, Favero A, LaVecchia C, Barón AE, Negri E, DalMaso L, et al. Body size indices and breast cancer risk before and after menopause. Int J Cancer. 1996;67(2 ):181–6. doi: 10.1002/(SICI)1097-0215(19960717)67:2<181::AID-IJC5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Ursin G, Longnecker MP, Haile RW, Greenland S. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 1995;6(2 ):137–41. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki R, Iwasaki M, Inoue M, Sasazuki S, Sawada N, Yamaji T, et al. Body weight at age 20 years subsequent weight change and breast cancer risk defined by estrogen and progesterone receptor status - the Japan public health center-based study. Int J Cancer. 2011;129(5 ):1214–24. doi: 10.1002/ijc.25744. [DOI] [PubMed] [Google Scholar]
- 5.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171(11 ):1183–94. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaVecchia C, Negri E, Franceschi S, Talamini R, Bruzzi P, Palli D, et al. Body mass index and postmenopausal breast cancer. An age specific analysis. Br J Cancer. 1997;75(3 ):441–4. doi: 10.1038/bjc.1997.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potischman N, Swanson CA, Siiteri P, Hoover RN. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1996;88(11 ):756–8. doi: 10.1093/jnci/88.11.756. [DOI] [PubMed] [Google Scholar]
- 8.Rose DP, Vona-Davis I. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9(8 ):1091–1. doi: 10.1586/era.09.71. [DOI] [PubMed] [Google Scholar]
- 9.Vona-Davis I, Howard-McNatt M, Rose DP. Adiposity type-2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5 ):395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 10.Key T, Appleby P, Barnes I, Reeves G Endogenous hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8 ):606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Hormonal contraception and postmenopausal hormone therapy. IARC, vol. 72, Lyon, France. 1999 [Google Scholar]
- 12.Lahmann PH, Hoffmann K, Allen N, vanGils CH, Khaw KT, Tehard B, et al. Body size and breast cancer risk Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2004;111(5 ):762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Chapman M, Harvie M. Energy restriction for breast cancer prevention. Recent Results Cancer Res. 2009;181:97–111. doi: 10.1007/978-3-540-69297-3_11. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference waist hip ratio and risk of breast cancer in the Nurses’ Health Study. Am J Epidemiol. 1999;150(12 ):1316–24. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity body size and risk of postmenopausal breast cancer: The Women s Health Initiative (United States) Cancer Causes Control. 2002;13(8 ):41–51. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 16.Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond Engl) 2008;4(2 ):183–94. doi: 10.2217/17455057.4.2.183. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson GD, Rose DP. Breast cancer and obesity: An update. Nutr Cancer. 2003;45(1 ):1–16. doi: 10.1207/S15327914NC4501_1. [DOI] [PubMed] [Google Scholar]
- 18.Althuis MD, Brogan DD, Coates RJ, Gammon MD, Malone KE, Schoenberg JB, et al. Breast cancers among very young premenopausal women (United States) Cancer Causes Control. 2003;14(2 ):151–60. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 19.Suba Z. Interplay between insulin resistance and estrogen deficiency as co-activators in carcinogenesis. Pathol Oncol Res. 2012;18(2 ):123–33. doi: 10.1007/s12253-011-9466-8. [DOI] [PubMed] [Google Scholar]
- 20.Suba Z. Insulin resistance estrogen deficiency and cancer risk. In: Suba Z, editor. Estrogen versus Cancer. New York: Nova Science Publishers Inc; 2009. pp. 127–46. [Google Scholar]
- 21.Suba Z. In: Obesity and risk for salivary gland tumors. Ramonde Z, Fochas EH, editors. New York: Nova Science Publishers Inc; 2009. pp. 71–86. [Google Scholar]
- 22.Björntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20(4 ):291–302. [PubMed] [Google Scholar]
- 23.Reaven GM. Banting lecture 1988 Role of insulin resistance in human disease. Diabetes. 1988;37(12 ):1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 24.Bloomgarden ZT. Second World Congress on the Insulin Resistance Syndrome Mediators pediatric insulin resistance the polycystic ovary syndrome and malignancy. Diab Care. 2005;28(8 ):1821–30. doi: 10.2337/diacare.28.7.1821. [DOI] [PubMed] [Google Scholar]
- 25.Gupta K, Krishnaswamy G, Karnad A, Peiris A. Insulin A novel factor in carcinogenesis. Am J Med Sci. 2002;323(3 ):140–5. doi: 10.1097/00000441-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Rohan T. Role of insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92(18 ):1472–89. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 27.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin insulin-like growth factor and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1 ):48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinberg DL. Somatostatin analogs and IGF-I inhibition for breast cancer prevention. US20090325863. 2009
- 29.Reaven GM. Insulin resistance cardiovascular disease and the metabolic syndrome: How well do the emperor’s clothes fit? Diabetes Care. 2004;27(4 ):1011–2. doi: 10.2337/diacare.27.4.1011. [DOI] [PubMed] [Google Scholar]
- 30.Cowey S, Hardy RW. The metabolic syndrome. A high risk state for cancer.? Am J Pathol. 2006;169(5 ):1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity metabolic syndrome insulin resistance and cancer. Proc Nutr Soc. 2012;71(1 ):181–9. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 32.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications A new perspective on an old paradigm. Dia-betes. 1999;48(1 ):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24(2 ):68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee M. Lilly Lecture 1993 Glycation and diabetic complications. Diabetes. 1994;43(6 ):836–41. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 35.Sieri S, Muti P, Claudia A, Berrino F, Pala V, Grioni S, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J Cancer. 2012;130(4 ):921–9. doi: 10.1002/ijc.26071. [DOI] [PubMed] [Google Scholar]
- 36.Reaven GM. Role of insulin resistance in human disease (syndrome X) An expanded definition. Annu Rev. 1993;44:121–31. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 37.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited Are serum triglycerides and plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3(8 ):687–95. [PubMed] [Google Scholar]
- 38.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis A review and critique. Cancer Res. 1992;52(7 Suppl):2040–8s. [PubMed] [Google Scholar]
- 39.Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, et al. Fasting glucose is a risk factor for breast cancer A prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11(11 ):1361–8. [PubMed] [Google Scholar]
- 40.Landsberg L. Insulin mediated sympathetic stimulation Role in the pathogenesis of obesity related hypertension (or how insulin affects blood pressure and why) Hypertension. 2001;19(3 ):523–8. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 41.GoffDC Jr, Zaccaro DJ, Haffner SM, Saad MF. Insulin sensitivity and risk the risk of incident hypertension Insights from the insulin resistance atherosclerosis study. Diabetes Care. 2003;26(3 ):805–9. doi: 10.2337/diacare.26.3.805. [DOI] [PubMed] [Google Scholar]
- 42.Takatori S, Zamami Y, Mio M, Kurosaki Y, Kawasaki H. Chronic hyperinsulinemia enhances adrenergic vasocon-striction and decreases calcitonin gene-related peptide-containing nerve-mediated vasodilation in pithed rats. Hypertens Res. 2006;29(5 ):361–8. doi: 10.1291/hypres.29.361. [DOI] [PubMed] [Google Scholar]
- 43.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293(4 ):H2009–23. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 44.Soler M, Chatenoud L, Negri E, Parazzini F, Franceschi S, LaVecchia C. Hypertension and hormone-related neo-plasms in women. Hypertension. 1999;34(2 ):320–5. doi: 10.1161/01.hyp.34.2.320. [DOI] [PubMed] [Google Scholar]
- 45.Rosato V, Bosetti C, Talamini R, Levi F, Negri E, LaVecchia C. Metabolic syndrome and the risk of breast cancer. Recenti Prog Med. 2011;102(12 ):476–8. doi: 10.1701/998.10859. [DOI] [PubMed] [Google Scholar]
- 46.Healy LA, Ryan AM, Carroll P, Ennis D, Crowley V, Boyle T, et al. Metabolic syndrome central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (R Coll Radiol) 2010;22(4 ):281–8. doi: 10.1016/j.clon.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Kabat GC, Kim M, Chlebowski RT, Khandekar J, Ko MG, McTiernan A, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(7 ):2046–53. doi: 10.1158/1055-9965.EPI-09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altomare E, Vendemiale G, Chicco D, Procacci V, Cirelli F. Increased lipid peroxidation in type 2 poorly controlled diabetic patients. Diabetes Metab. 1992;18(4 ):264–71. [PubMed] [Google Scholar]
- 49.Tsuji S, Kawai N, Tsujii M, Kawano S, Hori M. Review article Inflammation-related promotion of gastrointestinal car-cinogenesis--a perigenetic pathway. Aliment Pharmacol Ther. 2003;18(Suppl 1 ):82–9. doi: 10.1046/j.1365-2036.18.s1.22.x. [DOI] [PubMed] [Google Scholar]
- 50.Munkholm P. Review article The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2 ):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 51.Grote VA, Becker S, Kaaks R. Diabetes mellitus type 2 - an independent risk factor for cancer. Exp Clin Endocrinol Diabetes. 2010;118(1 ):4–8. doi: 10.1055/s-0029-1243193. [DOI] [PubMed] [Google Scholar]
- 52.LaVecchia C, Giordano SH, Hortobagyi GN, Chabner B. Overweight obesity diabetes and risk of breast cancer. Inter-locking pieces of the puzzle. Oncologist. 2011;16(6 ):726–9. doi: 10.1634/theoncologist.2011-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arif JM, Al-Saif AM, Al-Karrawi MA, Al-Sagair OA. Causative relationship between diabetes mellitus and breast cancer in various regions of Saudi Arabia An overview. Asian Pac J Cancer Prev. 2011;12(3 ):589–92. [PubMed] [Google Scholar]
- 54.Baselga J, DiCosimo S, Garcia-Echeverria C, Hackl W, Maira SM, Michelangelo R, Elizalde VS. Combination of (A) a phosphoinositide 3-kinase inhibitor and (B) an antidiabetic compound for use in the treatment of proliferative diseases. US20120059005. 2012
- 55.Sartor BM, Dickey RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci. 2005;330(6 ):336–42. doi: 10.1097/00000441-200512000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Nestler JE. Obesity insulin sex steroids and ovulation. Int J Obes Relat Metab Disord. 2000;24(Suppl 2 ):71–3. doi: 10.1038/sj.ijo.0801282. [DOI] [PubMed] [Google Scholar]
- 57.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen syn-thesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123(2 ):733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 58.Nestler JE, Jakubowicz DJ, deVargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83(6 ):2001–5. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 59.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76(3 ):517–24. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 60.Kim SW, Jun SS, Jo YG, Koo JS, Kim YW. N,N-Dimethyl imidodicarbonimidic diamide acetate method for producing the same and pharmaceutical composition comprising the same. US20120010287. 2012
- 61.Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women apparent relative impairment of 17- 20-lyase activity. J Clin Endocrinol Metab. 1996;81(3 ):881–6. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 62.Cara JF, Fan J, Azzarello J, Rosenfield RL. Insulin-like growth factor-I enhances luteinizing hormone binding to rat ovarian theca-interstitial cells. J Clin Invest. 1990;86(2 ):560–5. doi: 10.1172/JCI114745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaaks R. Nutrition hormones and breast cancer Is insulin the missing link. Cancer Causes Control. 1996;7(6 ):605–25. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 64.Secreto G, Zumoff B. Abnormal production of androgens in women with breast cancer. Anticancer Res. 1994;14(5B ):2113–7. [PubMed] [Google Scholar]
- 65.Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, et al. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112(2 ):312–8. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 66.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86(3-5 ):225–30. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 67.Suba Z. The role of estrogen in health and disease. In: Suba Z, editor. Estrogen versus Cancer. New York: Nova Science Pub-lishers Inc; 2009. pp. 59–84. [Google Scholar]
- 68.Stamataki KE, Spina J, Rangou DB, Chlouverakis CS, Piaditis GP. Ovarian function in women with non-insulin de-pendent diabetes mellitus. Clin Endocrinol (Oxf) 1996;45(5 ):615–21. doi: 10.1046/j.1365-2265.1996.00795.x. [DOI] [PubMed] [Google Scholar]
- 69.MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update An overview The essential roles of estrogens in pubertal growth epiphyseal fusion and bone turnover: Lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res. 1998;49(Suppl 1 ):2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- 70.Alemany M. Do the interactions between glucocorticoids and sex hormones regulate the development of the metabolic syndrome? Front Endocrinol (Lausanne) 2012:3–27. doi: 10.3389/fendo.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity insulin resistance and cancer risk. Diabetol Metab Syndr. 2011:3–12. doi: 10.1186/1758-5996-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobi D, Stanya KJ, Lee CH. Adipose tissue signaling by nuclear receptors in metabolic complications of obesity. Adi-pocyte. 2012;1(1 ):4–12. doi: 10.4161/adip.19036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6 ):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 74.Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev. 1999;10(1 ):5–14. doi: 10.1016/s1359-6101(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 75.Arcidiacono B, Iiritano S, Nocera A, Possidente K, Nevolo MT, Ventura V. Insulin resistance and cancer risk An overview of the pathogenetic mechanisms. Exp Diabetes Res 2012. 2012:789174. doi: 10.1155/2012/789174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fantuzzi G. Adipose tissue adipokines and inflammation. J Allergy Clin Immunol. 2005;115(5 ):911–9. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 77.Cirillo D, Rachiglio AM, laMontagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: An overview. J Cell Biochem. 2008;105(4 ):956–64. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 78.Schäffler A, Schölmerich J, Buechler C. Mechanisms of disease Adipokines and breast cancer - endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;(4 ):345–54. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 79.Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis - focus on mammary tumorigenesis. Biochimie. 2012;94(10 ):2164–71. doi: 10.1016/j.biochi.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Celis JE, Moreira JM, Cabezón T, Gromov P, Friis E, Rank F, et al. Identification of extracellular and intracellular signaling components of the mammary adipose tissue and its interstitial fluid in high risk breast cancer patients: Toward dissecting the molecular circuitry of epithelial-adipocyte stromal cell interactions. Mol Cell Proteomics. 2005;4(4 ):492–522. doi: 10.1074/mcp.M500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 81.Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: Mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11(9 ):1133–46. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- 82.Pallottini V, Bulzomi P, Galluzzo P, Martini C, Marino M. Estrogen regulation of adipose tissue functions. Involvement of estrogen receptor isoforms. Infect Disord Drug Targets. 2008;8(1 ):52–60. doi: 10.2174/187152608784139631. [DOI] [PubMed] [Google Scholar]
- 83.Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4 ):803–12. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: A controlled longitudinal study. Ann Intern Med. 1995;123(9 ):673–5. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- 85.Choi SB, Jang JS, Park S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology. 2005;146(11 ):4786–94. doi: 10.1210/en.2004-1653. [DOI] [PubMed] [Google Scholar]
- 86.Tagawa N, Yuda R, Kubota S, Wakabayashi M, Yamaguchi Y, Kiyonaga D, et al. 17Beta-estradiol inhibits 11beta-hydroxysteroid dehydrogenase type 1 activity in rodent adipocytes. J Endocrinol. 2009;202(1 ):131–9. doi: 10.1677/JOE-09-0021. [DOI] [PubMed] [Google Scholar]
- 87.Baghaei F, Rosmond R, Westberg L, Hellstrand M, Eriksson E, Holm G, et al. The CYP19 gene and associations with androgens and abdominal obesity in premenopausal women. Obes Res. 2003;11(4 ):578–85. doi: 10.1038/oby.2003.81. [DOI] [PubMed] [Google Scholar]
- 88.Diamanti-Kandarakis E, Baillargeon JP, Iuorno MJ, Jakubowicz DJ, Nestler JE. A modern medical quandary: Polycystic ovary syndrome insulin resistance and oral contraceptive pills. J Clin Endocrinol Metab. 2003;88(5 ):1927–32. doi: 10.1210/jc.2002-021528. [DOI] [PubMed] [Google Scholar]
- 89.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5 ):538–54. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 90.Reckelhoff JF. Sex steroids cardiovascular disease and hypertension unanswered questions and some speculations. Hypertension. 2005;45(2 ):170–4. doi: 10.1161/01.HYP.0000151825.36598.36. [DOI] [PubMed] [Google Scholar]
- 91.Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas. 1990;12(4 ):321–31. doi: 10.1016/0378-5122(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 92.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17 ):1196–204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 93.Hamden K, Carreau S, Ellouz F, Masmoudi H, El FA. Protective effect of 17beta-estradiol on oxidative stress and liver dysfunction in aged male rats. J Physiol Biochem. 2007;63(3 ):195–201. doi: 10.1007/BF03165782. [DOI] [PubMed] [Google Scholar]
- 94.Harrison-Bernard LM, Schulman IH, Raij L. Postovariectomy hypertension is linked to increased renal AT1 receptor and salt sensitivity. Hypertension. 2003;42(6 ):1157–63. doi: 10.1161/01.HYP.0000102180.13341.50. [DOI] [PubMed] [Google Scholar]
- 95.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33(1 Pt 2 ):323–8. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- 96.Widder J, Pelzer T, vonPoser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, et al. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension. 2003;42(5 ):991–6. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- 97.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens. 2011;24(7 ):740–9. doi: 10.1038/ajh.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dantas AP, Tostes RC, Fortes ZB, Costa SG, Nigro D, Carvalho MH. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension. 2002;39(2 Pt 2 ):405–11. doi: 10.1161/hy0202.102993. [DOI] [PubMed] [Google Scholar]
- 99.Viña J, Sastre J, Pallardó FV, Gambini J, Borrás C. Role of mitochondrial oxidative stress to explain the different longevity between genders: Protective effect of estrogens. Free Radic Res. 2006;40(12 ):1359–65. doi: 10.1080/10715760600952851. [DOI] [PubMed] [Google Scholar]
- 100.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors New players in diabetes mellitus. Trends Mol Med. 2006;12(9 ):425–31. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional ß-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6 ):342–51. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 102.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia. 2006;49(3 ):588–97. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 103.Nagira K, Sasaoka T, Wada T, Fukui K, Ikubo M, Hori S, et al. Altered subcellular distribution of estrogen receptor alpha is implicated in estradiol-induced dual regulation of insulin signaling in 3T3-L1 adipocytes. Endocrinology. 2006;147(2 ):1020–8. doi: 10.1210/en.2005-0825. [DOI] [PubMed] [Google Scholar]
- 104.Welch RD, Gorski J. Regulation of glucose transporters by estradiol in the immature rat uterus. Endocrinology. 1999;140(8 ):3602–8. doi: 10.1210/endo.140.8.6923. [DOI] [PubMed] [Google Scholar]
- 105.Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: Decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993;264(2 Pt 1 ):197–202. doi: 10.1152/ajpendo.1993.264.2.E197. [DOI] [PubMed] [Google Scholar]
- 106.Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25(5 ):693–721. doi: 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- 107.Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183(2 ):1393–402. doi: 10.4049/jimmunol.0803157. [DOI] [PubMed] [Google Scholar]
- 108.Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93(5 ):399–405. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- 109.Llaneza P, González C, Fernandez-Iñarrea J, Alonso A, Diaz F, Arnott I, et al. Soy isoflavones diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine. 2011;18(4 ):245–50. doi: 10.1016/j.phymed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 110.Adlercreutz HJ. Phytoestrogens and breast cancer. Steroid Biochem Mol Biol. 2002;83(1-5 ):113–8. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 111.Veldhuis JD, Frystyk J, Iranmanesh A, Ørskov H. Testosterone and estradiol regulate free insulin-like growth factor I (IGF-I) IGF binding protein 1 (IGFBP-1) and dimeric IGF-I/IGFBP-1 concentrations. J Clin Endocrinol Metab. 2005;90(5 ):2941–7. doi: 10.1210/jc.2004-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58(3 ):627–32. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- 113.Xu J, Xiang Q, Lin G, Fu X, Zhou K, Jiang P, et al. Estrogen improved metabolic syndrome through down-regulation of VEGF and HIF-1α to inhibit hypoxia of periaortic and intra-abdominal fat in ovariectomized female rats. Mol Biol Rep. 2012;39(8 ):8177–85. doi: 10.1007/s11033-012-1665-1. [DOI] [PubMed] [Google Scholar]
- 114.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II. The role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13(10 ):422–7. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: Exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(Suppl 1 ):15–24. doi: 10.1677/erc.1.01273. [DOI] [PubMed] [Google Scholar]
- 116.Stoica A, Saceda M, Doraiswamy VL, Coleman C, Martin MB. Regulation of estrogen receptor-alpha gene expression by epidermal growth factor. J Endocrinol. 2000;165(2 ):371–8. doi: 10.1677/joe.0.1650371. [DOI] [PubMed] [Google Scholar]
- 117.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2(3 ):321–7. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 118.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21 ):2395–402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 119.Harris HR, Willett WC, Terry KL, Michels KB. Body fat distribution and risk of premenopausal breast cancer in the Nurses' Health Study II. J Natl Cancer Inst. 2011;103(3 ):273–8. doi: 10.1093/jnci/djq500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, et al. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999;28(6 ):1026–31. doi: 10.1093/ije/28.6.1026. [DOI] [PubMed] [Google Scholar]
- 121.Page JH, Rexrode KM, Hu F, Albert CM, Chae CU, Manson JE. Waist-height ratio as a predictor of coronary heart disease among women. Epidemiology. 2009;20(3 ):361–6. doi: 10.1097/EDE.0b013e31819f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fagherazzi G, Chabbert-Buffet N, Fabre A, Guillas G, Boutron-Ruault MC, Mesrine S, et al. Hip circumference is associated with the risk of premenopausal ER-/PR- breast cancer. Int J Obes (Lond) 2012;36(3 ):431–9. doi: 10.1038/ijo.2011.66. [DOI] [PubMed] [Google Scholar]
- 123.Caprio S, Hyman LD, Limb C, McCarthy S, Lange R, Sherwin RS, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269(1 Pt 1 ):118–26. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 124.Gurtcheff SE, Klein NA. Diminished ovarian reserve and infertility. Clin Obstet Gynecol. 2011;54(4 ):666–74. doi: 10.1097/GRF.0b013e3182353c65. [DOI] [PubMed] [Google Scholar]
- 125.Tobisch B, Blatniczky L, Barkai L. Correlation between insulin resistance and puberty in children with increased cardiometabolic risk. Orv Hetil. 2011;152(27 ):1068–74. doi: 10.1556/OH.2011.29159. [DOI] [PubMed] [Google Scholar]
- 126.Theintz G. From obesity to type-2 diabetes in children and adolescents. Rev Med Suisse. 2005;1(7 ):477–80. [PubMed] [Google Scholar]
- 127.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85(11 ):2400–9. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 128.Bardia A, Vachon CM, Olson JE, Vierkant RA, Wang AH, Hartmann LC, et al. Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2 ):374–8. doi: 10.1158/1055-9965.EPI-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stoll BA. Teenage obesity in relation to breast cancer risk. Int J Obes Relat Metab Disord. 1998;22(11 ):1035–40. doi: 10.1038/sj.ijo.0800769. [DOI] [PubMed] [Google Scholar]
- 130.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: Prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4(5 ):567–71. [PubMed] [Google Scholar]
- 131.Pilia S, Casini MR, Foschini ML, Minerba L, Musiu MC, Marras V, et al. The effect of puberty on insulin resistance in obese children. J Endocrinol Invest. 2009;32(5 ):401–5. doi: 10.1007/BF03346475. [DOI] [PubMed] [Google Scholar]
- 132.Vanhala MJ, Vanhala PT, Keinänen-Kiukaanniemi SM, Kumpusalo EA, Takala JK. Relative weight gain and obesity as a child predict metabolic syndrome as an adult. Int J Obes Relat Metab Disord. 1999;23(6 ):656–9. doi: 10.1038/sj.ijo.0800898. [DOI] [PubMed] [Google Scholar]
- 133.Apter D, Vihko R. Endocrine determinants of fertility Serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990;71(4 ):970–4. doi: 10.1210/jcem-71-4-970. [DOI] [PubMed] [Google Scholar]
- 134.Baer HJ, Colditz GA, Willett WC, Dorgan JF. Adiposity and sex hormones in girls. Cancer Epidemiol Biomarkers Prev. 2007;16(9 ):1880–8. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- 135.Velentgas P, Daling JR. Risk factors for breast cancer in younger women. J Natl Cancer Inst Monogr. 1994;(16 ):15–24. [PubMed] [Google Scholar]
- 136.Suba Z. Common soil of smoking-associated and hormone-related cancers: Estrogen deficiency. Oncol Rev. 2010;4(2 ):73–87. [Google Scholar]
- 137.Terry KL, Willett WC, Rich-Edwards JW, Hunter DJ, Michels KB. Menstrual cycle characteristics and incidence of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(6 ):1509–13. doi: 10.1158/1055-9965.EPI-05-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol. 1994;140(12 ):1081–90. doi: 10.1093/oxfordjournals.aje.a117208. [DOI] [PubMed] [Google Scholar]
- 139.Yuan JM, Yu MC, Ross RK, Gao YT, Henderson BE. Risk factors for breast cancer in Chinese women in Shanghai. Cancer Res. 1988;48(7 ):1949–53. [PubMed] [Google Scholar]
- 140.Clavel-Chapelon F. Cumulative number of menstrual cycles and breast cancer risk: Results from the E3N cohort study of French women. Cancer Causes Control. 2002;13(9 ):831–8. doi: 10.1023/a:1020684821837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Titus-Ernstoff L, Longnecker MP, Newcomb PA, Dain B, Greenberg ER, Mittendorf R, et al. Menstrual factors in rela-tion to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7(9 ):783–9. [PubMed] [Google Scholar]
- 142.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, et al. Association of menstrual and reproductive factors with breast cancer risk. Results from the Sanghai Breast Cancer Study. Int J Cancer. 2000;87(2 ):295–300. doi: 10.1002/1097-0215(20000715)87:2<295::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 143.denTonkelaar I, deWaard F. Regularity and length of menstrual cycles in women aged 41-46 in relation to breast cancer risk: Results from the DOM-project. Breast Cancer Res Treat. 1996;38(3 ):253–8. doi: 10.1007/BF01806143. [DOI] [PubMed] [Google Scholar]
- 144.Wu AH, Ziegler RG, Pike MC, Nomura AM, West DW, Kolonel LN, et al. Menstrual and reproductive factors and risk of breast cancer in Asian-Americans. Br J Cancer. 1996;73(5 ):680–6. doi: 10.1038/bjc.1996.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Parazzini F, LaVecchia C, Negri E, Franceschi S, Tozzi L. Life-long menstrual patterns and risk of breast cancer. On-cology. 1993;50(4 ):222–5. doi: 10.1159/000227183. [DOI] [PubMed] [Google Scholar]
- 146.Terry KL, Willett WC, Rich-Edwards JW, Michels KB. A prospective study of infertility due to ovulatory disorders ovulation induction and incidence of breast cancer. Arch Intern Med. 2006;166(22 ):2484–9. doi: 10.1001/archinte.166.22.2484. [DOI] [PubMed] [Google Scholar]
- 147.Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Polycystic ovary syndrome and gynecological cancers Is there a link? Gynecol Endocrinol. 2005;20(4 ):200–8. doi: 10.1080/09513590400021201. [DOI] [PubMed] [Google Scholar]
- 148.deFrança NetoAH, Rogatto S, DoAmorim MMR, Tamanaha S, Aoki T, Aldrighi JM. Oncological repercussions of polycystic ovary syndrome. Gynecol Endocrinol. 2010;26(10 ):708–11. doi: 10.3109/09513590.2010.490607. [DOI] [PubMed] [Google Scholar]
- 149.Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51(7 ):581–6. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 150.Papaioannou S, Tzafettas J. Anovulation with or without PCO hyperandrogenaemia and hyperinsulinaemia as promoters of endometrial and breast cancer. Best Pract Res Clin Obstet Gynaecol. 2010;24(1 ):19–27. doi: 10.1016/j.bpobgyn.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 151.Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopau-sal women with endometrial cancer. Obstet Gynecol. 2005;105(3 ):575–80. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 152.Uccella S, Cha SS, Melton LJ, 3rd, Bergstralh EJ, Boardman LA, Keeney GL, et al. Risk factors for developing multiple malignancies in patients with endometrial cancer. Int J Gynecol Cancer. 2011;21(5 ):896–901. doi: 10.1097/IGC.0b013e318219711f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berinder K, Akre O, Granath F, Hulting AL. Cancer risk in hyperprolactinemia patients: A population-based cohort study. Eur J Endocrinol. 2011;165(2 ):209–15. doi: 10.1530/EJE-11-0076. [DOI] [PubMed] [Google Scholar]
- 154.Mujagic Z, Srabovic N, Mujagic H. The role of prolactin in human breast cancer. Biochemia Medica. 2009;19(3 ):236–49. [Google Scholar]
- 155.Wolpert BJ, Amr S, Ezzat S, Saleh D, Gouda I, Loay I, et al. Estrogen exposure and bladder cancer risk in Egyptian women. Maturitas. 2010;67(4 ):353–7. doi: 10.1016/j.maturitas.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity other reproductive factors and risk of pancreatic cancer mortality in a large cohort of U.S. women. Cancer Causes Control. 2005;16(9 ):1035–40. doi: 10.1007/s10552-005-0332-4. [DOI] [PubMed] [Google Scholar]
- 157.Bosetti C, Negri E, Franceschi S, Conti E, Levi F, Tomei F. Risk factors for oral and pharyngeal cancer in women: A study from Italy and Switzerland. Br J Cancer. 2000;82(1 ):204–7. doi: 10.1054/bjoc.1999.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Key TJ. Hormones and cancer in humans. Mutat Res. 1995;333(1-2 ):59–67. doi: 10.1016/0027-5107(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 159.Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocr Relat Cancer. 2007;14(4 ):907–33. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- 160.Lay YC, Hamazaki K, Yoshizawa K, Kawanaka A, Kuwata M, Kanematsu S, et al. Short-term pregnancy hormone treatment of N-methyl-N-nitrosourea-induced mammary carcinogenesis in relation to fatty acid composition of serum phospholipids in female Lewis rats. In vivo. 2010;24(4 ):553–60. [PubMed] [Google Scholar]
- 161.Opdahl S, Alsaker MD, Janszky I, Romundstad PR, Vatten LJ. Joint effect of nulliparity and other breast cancer risk factors. Br J Cancer. 2011;105(5 ):731–6. doi: 10.1038/bjc.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Newcomb PA, Trentham-Dietz A, Hampton JM, Egan KM, Titus-Ernstoff L, WarrenAndersen S, et al. Late age at full term birth is strongly associated with lobular breast cancer. Cancer. 2011;117(9 ):1946–56. doi: 10.1002/cncr.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cetin I, Cozzi V, Antonazzo P. Infertility as a cancer risk factor - a review. Placenta. 2008;29(Suppl B ):169–77. doi: 10.1016/j.placenta.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 164.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Risk of breast cancer in a cohort of infertile women. Gynecol Oncol. 1996;60(1 ):3–7. doi: 10.1006/gyno.1996.0002. [DOI] [PubMed] [Google Scholar]
- 165.Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Malignancies among women who gave birth after in vitro fertilization. Hum Reprod. 2011;26(1 ):253–8. doi: 10.1093/humrep/deq307. [DOI] [PubMed] [Google Scholar]
- 166.Schneider JG, Tompkins C, Blumenthal RS, Mora S. The metabolic syndrome in women. Cardiol Rev. 2006;14(6 ):286–91. doi: 10.1097/01.crd.0000233757.15181.67. [DOI] [PubMed] [Google Scholar]
- 167.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause central body fatness and insulin resistance: Effects of hormone-replacement therapy. Coron Artery Dis. 1998;9(8 ):503–11. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 168.Deligeoroglou E, Michailidis E, Creatsas G. Oral contraceptives and reproductive system cancer. Ann NY Acad Sci. 2003;997:199–208. doi: 10.1196/annals.1290.023. [DOI] [PubMed] [Google Scholar]
- 169.ESHRE Capri Workshop Group. Ovarian and endometrial function during hormonal contraception. Hum Reprod. 2001;16(7 ):1527–35. doi: 10.1093/humrep/16.7.1527. [DOI] [PubMed] [Google Scholar]
- 170.Tejura B. Methods of treating hyperandrogenism and conditions associated therewith by administering a fatty acid ester of an estrogen or an estrogen derivative. US20100286105. 2010
- 171.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Wrighting Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women s Health Initiative randomized controlled trial. JAMA. 2002;288(3 ):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 172.Suba Z. Re-evaluation of the Epidemiological Associations of female Sexual Steroids and Cancer Risk. In: Suba Z, editor. Estrogen versus Cancer. New-York: Nova Science Publishers Inc; 2009. pp. 107–126. [Google Scholar]
- 173.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. A randomized controlled trial. JAMA. 2011;305(13 ):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shapiro S, Farmer RD, Mueck AO, Seaman H, Stevenson JC. Does hormone replacement therapy cause breast cancer? An application of causal principles to three studies Part 3 The Women's Health Initiative: Unopposed estrogen. J Fam Plann Reprod Health Care. 2011;37(4 ):225–30. doi: 10.1136/jfprhc-2011-0091. [DOI] [PubMed] [Google Scholar]
- 175.Ragaz J, Shakeraneh S. Estrogen Prevention of Breast Cancer: A Critical Review. In: Suba Z, editor. Estrogen Prevention for Breast Cancer. New York: Nova Science Publishers Inc; 2012. [Google Scholar]
- 176.Howell A, Evans GD. Hormone replacement therapy and breast cancer. Recent Results Cancer Res. 2011;188:115–24. doi: 10.1007/978-3-642-10858-7_10. [DOI] [PubMed] [Google Scholar]
- 177.Chlebowski RT, Anderson GL. Changing concepts Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104(7 ):517–27. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stevenson JC, Hodis HN, Pickar JH, Lobo RA. HRT and breast cancer risk A realistic perspective. Climacteric. 2011;14(6 ):633–6. doi: 10.3109/13697137.2011.590618. [DOI] [PubMed] [Google Scholar]
- 179.Darbre PD, Charles AK. Environmental oestrogens and breast cancer Evidence for combined involvement of dietary household and cosmetic xenoestrogens. Anticancer Res. 2010;30(3 ):815–27. [PubMed] [Google Scholar]
- 180.Jung EM, An BS, Yang H, Choi KC, Jeung EB. Biomarker genes for detecting estrogenic activity of endocrine disruptors via estrogen receptors. Int J Environ Res Public Health. 2012;9(3 ):698–711. doi: 10.3390/ijerph9030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Suba Z. Light deficiency confers breast cancer risk by endocrine disorders. Recent Pat Anticancer Drug Discov. 2012;7(3 ):337–44. doi: 10.2174/157489212801820048. [DOI] [PubMed] [Google Scholar]
- 182.Suba Z. Antiestrogen or Estrogen as Anticancer Drug. In: Hoffmann AB, editor. Sex Hormones: Development, Regulation and Disorders. New York: Nova Science Publishers Inc; 2011. pp. 75–94. [Google Scholar]
- 183.Leick MB, Jordan VC. Evolution of Estrogen Action in Breast Cancer: From Culprit to Killer. In: Suba Z, editor. Estrogen prevention for breast cancer. New York: Nova Science Publishers Inc; 2012. [Google Scholar]
- 184.Kelly GE. Methods for treating or reducing predisposition to breast cancer, premenopausal syndrome or symptoms associated with menopause by administration of phyto-estrogen. US6562380. 1997
- 185.Kotake Y, Nlijima J, Fukuda Y, Nagai M, Kanada RM, Nakashima T, Yoshida M, Tsuchida T. Novel physiologically active substances. US20080275059. 2008
- 186.Schwartz AG, Lewbart ML. Steroids useful as anti-cancer and anti-obesity agents. US5696106. 1997


