Abstract
OBJECTIVES:
Low vitamin D status may be associated with Crohn's disease. A pilot study was performed in patients with mild-to-moderate Crohn's disease to determine the dose of vitamin D needed to raise serum vitamin D levels above 40 ng/ml.
METHODS:
Patients were evaluated for severity of symptoms using the Crohn's disease activity index (CDAI) and patients with mild-to-moderate (150–400 CDAI scores) Crohn's disease were entered into the study (n=18). Vitamin D3 oral therapy was initiated at 1,000 IU/d and after 2 weeks, the dose was escalated incrementally until patients' serum concentrations reached 40 ng/ml 25(OH)D3 or they were taking 5,000 IU/d. Patients continued on the vitamin D supplements for 24 weeks. CDAI, quality of life measures, bone mineral density, dietary analyses, cytokines, parathyroid hormone, calcium, and several other laboratory measurements were evaluated at baseline and after 24 weeks supplementation.
RESULTS:
Fourteen of eighteen patients required the maximal vitamin D supplement of 5,000 IU/d. Vitamin D oral supplementation significantly increased serum 25(OH)D3 levels from 16±10 ng/ml to 45±19 ng/ml (P<0.0001) and reduced the unadjusted mean CDAI scores by 112±81 points from 230±74 to 118±66 (P<0.0001). Quality-of-life scores also improved following vitamin D supplementation (P=0.0004). No significant changes in cytokine or other laboratory measures were observed.
CONCLUSIONS:
Twenty-four weeks supplementation with up to 5,000 IU/d vitamin D3 effectively raised serum 25(OH)D3 and reduced CDAI scores in a small cohort of Crohn's patients suggesting that restoration of normal vitamin D serum levels may be useful in the management of patients with mild–moderate Crohn's disease.
INTRODUCTION
The incidence of immune-mediated diseases such as inflammatory bowel disease (IBD) has increased in developed countries over the last 50 years. The cause of the increased incidence of IBD is thought to be because of poorly defined environmental factors including changes in the microbial exposure (sometimes referred to as the hygiene hypothesis).1, 2 The hygiene hypothesis proposes that because of vaccination and decreased exposure to helminth infections the immune system has not been properly primed to prevent immune-mediated diseases. For IBD it is clear that the composition of the gastrointestinal microflora is associated with the development of these diseases. IBD is thought to be a disease triggered by the bacteria or dietary antigens in the gut of susceptible individuals.3 There are at least two distinct forms of IBD, ulcerative colitis and Crohn's disease.
A major source of vitamin D for humans results from its manufacture via a photolysis reaction in the skin; vitamin D available from sunlight exposure is significantly less in northern climates, and especially low during the winter.4, 5 In addition, dietary intake of vitamin D is problematic as there are few foods that are naturally rich in vitamin D. The form of vitamin D that is ingested in food and/or released from the skin upon UV light exposure is inactive. Activation involves the metabolism of vitamin D in the liver to form 25(OH)D3, which is the major circulating form of vitamin D. There is mounting evidence for a link between vitamin D availability either from sunshine or diet and the prevalence of autoimmune diseases.6 The use of supplemental vitamin D (500–600 IU) is associated with a 40% reduction in the risk of developing other immune-mediated diseases like multiple sclerosis.7 In addition, vitamin D deficiency is common in patients with Crohn's disease.8, 9, 10 Vitamin D insufficiency in Crohn's disease is due in part to malabsorption of many nutrients as a result of the disease. With our present lifestyles that feature decreased activity outside and diets naturally low in vitamin D, the amount of vitamin D people are exposed to has become more variable.
Vitamin D is essential for bone mineralization and calcium homeostasis; however, other important roles of this vitamin have been described. In 1983,11 the vitamin D receptor was identified in human leukocytes and since that time its ligand has been shown to be an important immunomodulatory compound. In particular, vitamin D is critical for the control of T helper 1/T helper 17-mediated diseases, including experimental IBD. Vitamin D deficiency accelerates the development of experimental IBD,12 and vitamin D receptor knockout models of experimental IBD show a fulminating and often lethal form of the disease.13, 14 Conversely, treatment of mice with active vitamin D3 (1,25(OH)2D3 or calcitriol) inhibits the development of experimental IBD.12
Two recent studies have evaluated the therapeutic effects of vitamin D3 and 1(OH)D3 or 1-α-calcidiol treatment in human IBD. 1-α-Calcidiol is a synthetic form of vitamin D that is hydroxylated at the 1-α position but not the 25 position. A modest but insignificant decrease in markers of inflammation (C-reactive protein, CRP) and Crohn's disease (Crohn's disease activity index, CDAI) was shown following treatment with 1(OH)D3 for 6 weeks.15 There was no effect of 1,000 IU/d vitamin D3 over 6 weeks on either CDAI or CRP levels.15 A randomized double-blind placebo-controlled trial was done in Crohn's patients whose disease was in clinical remission (CDAI score <150).16 The trial showed that 1,200 IU/d vitamin D3 for 3 months improved vitamin D status significantly compared with placebo and the vitamin D treatment group had lower disease relapse rates compared with placebo, but the difference did not reach significance. These two clinical trials suggest that there may be benefits to vitamin D supplementation in patients with Crohn's disease.
The purpose of this current pilot study was to determine the oral dose of vitamin D3 required to raise 25(OH)D3 levels above 40 ng/ml in patients with mild-to-moderate Crohn's disease. The hypothesis tested was that at baseline Crohn's disease patients would have low-vitamin D status and that vitamin D3 supplementation should improve the serum levels and might affect bone mineral density (BMD), overall health, quality of life, and clinical disease activity.
MATERIALS AND METHODS
Study design
The study design was that of an open-labeled prospective clinical trial and was conducted at two locations both at the Pennsylvania State University, the College of Medicine (Hershey, PA), and the main campus (University Park, PA). Patients with confirmed Crohn's disease between the ages of 18–70 and of either gender were recruited into the study. Inclusion criteria required patients to have CDAI scores between 150 and 400 and serum levels of 25(OH)D3 below 40 ng/ml. Patients were allowed to continue maintenance IBD medications and multivitamins. Patients with ulcerative colitis or other inflammatory bowel conditions were ineligible. Exclusion criteria also included patients with an ostomy or receiving corticosteroid therapy. A total of 31 Crohn's patients were screened for the trial and of those 18 were deemed eligible and completed the 24-week study. All subjects gave their informed written consent before the start of the study. All procedures were approved by the Institutional Review Board of the Pennsylvania State University. The trial has been registered at https://register.clinicaltrials.gov and the trial # is NCT00742781. The primary outcome was to determine the oral dose of vitamin D needed to raise the serum level of 25(OH)D3 to 40 ng/ml in Crohn's patients.
Vitamin D intervention
The vitamin D3 supplement (cholecalciferol) was purchased from Nutraceutical Sciences Institute (Lexington, KY) and the capsules contained 1,000 IU of vitamin D3. Eligible participants were started on 1,000 IU/d of vitamin D3 administered orally once daily for 2 weeks, after which the vitamin D status was evaluated and patients with serum levels of 25(OH)D3 below 40 ng/ml had the dose raised to 2,000 IU/d. The serum 25(OH)D3 levels were measured every 2 weeks and the dose escalated by 1,000 IU/d increments until serum 25(OH)D3 levels above 40 ng/ml were achieved or they were taking 5,000 IU/d vitamin D3. All patients were evaluated at baseline, 2 weeks, 12 weeks, and 24 weeks. Treatment compliance was assessed via log sheets, returned capsule counts, and serum 25(OH)D3 values.
Serum measurements
Serum 25(OH)D3 was measured using 25(OH)-Vitamin D direct ELISA kit (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer's instructions. Serum transaminases, serum calcium, parathyroid hormone (PTH), alkaline phosphatase, and albumin were done to evaluate safety and toxicity from vitamin D. Erythrocyte sedimentation rate (ESR) was monitored as an indicator of inflammation. Serum vascular endothelial growth factor, tumor necrosis factor (TNF-α), interleukin (IL)-10, and IL-17 were measured using a multiplex human kit (Millipore, St Charles, MO) according to the manufacturer's instructions.
Disease activity and overall health assessment
A diary was provided to all patients to record daily bowel function, abdominal pain, sense of general well-being, and anti-diarrhea medication for 7 days before office visits to calculate the CDAI score.17 In addition, patients were asked about any adverse effects or changes in medications. The standard CDAI score criteria were utilized with a score of 220 or greater indicative of active disease, a score of 150 or below indicative of remission, a 70-point or greater decline indicative of a favorable response, and a 100-point or greater increase in score indicative of a flare-up.17 The number of subjects having a 100-point decline was also assessed.18
The IBD questionnaire (IBDQ) was used to measure disease-specific quality of life.19 Baseline and week 24 follow-up physical activity levels were assessed via the short version of the International Physical Activity Questionnaire (IPAQ) (http://www.ipaq.ki.se).
Dietary assessment
Dietary intakes were collected and analyzed at the Penn State Diet Assessment Center (University Park, PA) via a series of unannounced three telephone 24-h recalls (two weekday and one weekend day). The background dietary intake of vitamin D and other related nutrients (e.g., calcium) were assessed in this way. Data were collected at baseline and 24 weeks. Nutrient calculations including both dietary and supplement intake were assessed using the Minnesota Nutrition Data System for Research version 4.06.
Dual energy X-ray absorptiometry scan
The BMD of proximal femur measured by dual energy X-ray absorptiometry was performed at the beginning and end of the study by a trained technician. The T-score and Z-score were calculated according to age and gender.
Statistical analysis
Graphs and some basic statistics were generated using GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA). Baseline and 24-week values were evaluated to determine whether they were normally distributed and compared using either a t-test and/or the Wilcoxon rank sum comparisons. Individual changes in lab tests, CDAI scores, and IBDQ values for a given patient were calculated to assess differences due to the supplementation therapy. Regression analyses were done using PROC GLM in SAS (version 9.3, SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the patients
Based on an initial telephone conversation a total of 31 subjects were evaluated for entry into the study. Of the 31 subjects, 10 subjects were ineligible mainly due to having CDAI scores out of the 150–400 score range. None of the screen failures were due to serum 25(OH)D3 levels above the 40 ng/ml threshold. Three additional patients withdrew from the study leaving a total of 18 patients who completed the study. The main reason stated for withdrawal from the study was compliance with scheduling the multiple visits required to determine appropriate vitamin D dosage (early bi-weekly visits). Table 1 summarizes the baseline characteristics of the 18 patients who completed the study. The study had 61% females and 39% males with a broad age range of 18–66 years and a mean age of 38 years (Table 1). The mean body mass index was 24±3 kg/m2 with a broad range of body mass index values from a low of 18 to a high of 30 (Table 1). Patients were on several different conventional IBD treatments, including anti-TNF-α biologic agents (n=5), purine analogs (n=4), aminosalicylates (n=9), and opioid receptor antagonists (n=4). Several patients were on more than one treatment (n=5) and two patients were not on any Crohn's maintenance drugs (Table 1).
Table 1. Baseline characteristics of participants (n=18).
| Characteristic | Baseline |
|---|---|
| Age (years) | 38±17 |
| Male (%) | 7 (39%) |
| Female (%) | 11 (61%) |
| Body composition (BMI, kg/m2) | 24±3.5 |
| Multivitamin | 12 (67%) |
| Medicationsa | |
| Aminosalicylates | 9 (50%) |
| Purine analogs | 4 (22%) |
| TNF-α blockade | 5 (28%) |
| Opioid receptor antagonists | 4 (22%) |
| None | 2 (11%) |
Abbreviations: BMI, body mass index; TNF, tumor necrosis factor.
In all, 2 patients were on three different medications, 3 were on two different medications, and 11 were only on one drug.
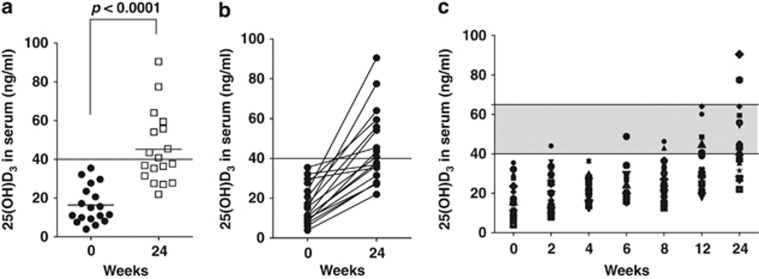
Participants were allowed to continue on multi-vitamins that contained vitamin D. At baseline, 67% of the Crohn's patients were taking nutritional supplements that contained between 120–800 IU/d of vitamin D (Table 1). Baseline vitamin D status of the Crohn's population was low even among those consuming multivitamin supplements (including vitamin D). Serum samples were obtained from 25 Crohn's patients at baseline. In all, 13 of the 25 Crohn's patients (52%) had frank vitamin D deficiency at screening with serum 25(OH)D3 levels below 12 ng/ml. The mean 25(OH)D3 value of the patients entering the study was 16±10 ng/ml (Figure 1a).
Figure 1.
Effect of vitamin D3 supplementation on 25(OH)D3 levels in serum. (a) 25 (OH)D3 levels started below the targeted 40 ng/ml levels at baseline. Twenty-four weeks of vitamin D supplementation significantly raised serum 25(OH)D3 levels and the mean value was above 40 ng/ml. (b) Starting and ending values of 25(OH)D3 are connected with a line. (c) Serum 25(OH)D3 levels continue to rise over the 24-week supplementation scheme. The shadowed area represents the target range of serum vitamin D levels for the supplementation of 40–65 ng/ml. Two individuals went over the targeted range for 25(OH)D3 at the 24-week time point; n=18.
Vitamin D supplementation raises serum 25(OH)D3 levels
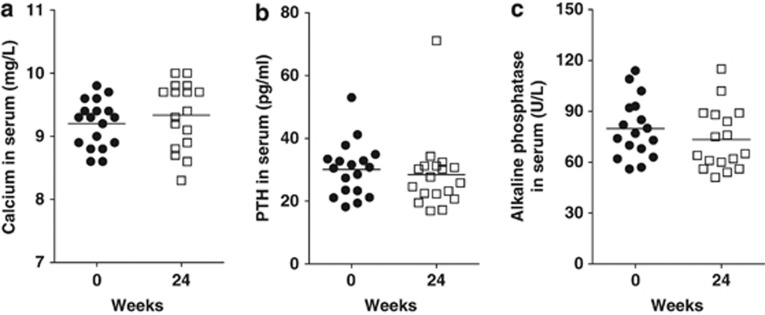
Vitamin D3 supplementation for 24 weeks effectively and significantly raised serum 25(OH)D3 levels to 45±19 ng/ml from 16±10 ng/ml (Figure 1a). Two weeks of 1,000 IU/d of vitamin D3 was not adequate for raising 25(OH)D3 levels in any of the 18 individuals in the study. In 14 of the 18 patients (78%), the maximal dose of 5,000 IU/d was needed for the 24-week study. Two of the patients who required less than 5,000 IU/d of vitamin D3 had higher levels of 25(OH)D3 at baseline (over 30 ng/ml). At the discretion of their physician, two patients had a dose reduction of vitamin D3 from 5,000 to 4,000 IU/d due to unrelated health problems and those two patients remained on the lower dose of 4,000 IU/d for the remainder of the study. Even on the maximum dose of 5,000 IU/d dose for 24 weeks half of the participants failed to achieve serum 25(OH)D3 levels above 40 ng/ml. Figure 1b shows the beginning and ending value of 25(OH)D3 for each individual patient. Those subjects who started with higher vitamin D levels at baseline had reduced improvement in serum 25(OH)D3 and those who started with low vitamin D levels showed the greatest improvement (Figure 1b). The maximum increase in serum 25(OH)D3 was 67 ng/ml, and smallest increase was 5 ng/ml. (Figure 1b). There was a gradual improvement in serum 25(OH)D3 levels over the 24 weeks supplementation (Figure 1c). By week 24, two patients had serum 25(OH)D3 levels above our targeted range of 65 ng/ml (Figure 1c). Compliance was good based on the pill counts and was 96% (range 72–100%) for the study overall. The improvement in serum vitamin D status was not associated with a change in serum PTH (from 30±9 to 28±12 pg/ml), calcium (from 9.2±0.4 to 9.3±0.5 mg/dl), or alkaline phosphatase (from 80±17 to 73±18 U/l) following 24 weeks of vitamin D3 supplementation (Figure 2). There was a small but insignificant increase in the BMD of both the right and left hip Z-score after 24 weeks of vitamin D3 intake (data not shown). PTH and calcium values were all within the normal range both at the start and at the end of the study, suggesting that the vitamin D3 dose was not in the toxic range for any patient. There was no significant correlation between 25(OH)D3 and either PTH or calcium at either time points (Table 2).
Figure 2.
Effect of vitamin D3 supplementation on calcium, parathyroid hormone (PTH), and alkaline phosphatase levels in serum. There were no changes in the calcium, PTH, or alkaline phosphatase levels between baseline and 24 weeks post vitamin D3 supplementation.
Table 2. Selected dietary characteristics of participants (n=18)a.
| Baseline mean (s.d.) | Follow-up mean (s.d.) | Change mean (s.d.)b | P-valuec | |
|---|---|---|---|---|
| Energy (kcal) | 1,739 (603) | 1,542 (444) | 189 (431) | 0.10 |
| Carbohydrates (% kcal) | 51 (9.8) | 51 (5.4) | 0 (10.3) | 0.83 |
| Protein (% kcal) | 17 (4.6) | 18 (4.3) | −1 (4.6) | 0.56 |
| Fat (% kcal) | 32 (7.2) | 31 (4.0) | 1 (8.3) | 0.63 |
| Dietary calcium (mg) | 670 (388) | 648 (279) | −1 (290) | 0.99 |
| (mg/1,000 kcal) | 379 (155) | 442 (203) | −73 (160) | 0.09 |
| Calcium supplements (mg) | 228 (576) | 506 (970) | −250 (418) | 0.03 |
| Total calcium (mg) | 897 (606) | 1153 (879) | −251 (490) | 0.06 |
| (mg/1,000 kcal) | 538 (408) | 791 (648) | −245.2 (320) | 0.008 |
| Dietary vitamin D (μg) | 3.7 (2.4) | 5.3 (6.6) | −1.7 (6.8) | 0.33 |
| (μg/1,000 kcal) | 2.2 (1.5) | 3.9 (5.1) | −1.7 (4.8) | 0.17 |
| Vitamin D supplement (μg)d | 4.9 (5.9) | 4.8 (5.5) | 0.6 (3.5) | 0.48 |
| Total vitamin D (μg) | 8.5 (6.4) | 10.1 (9.5) | −1.0 (5.6) | 0.46 |
| (μg/1,000 kcal) | 5.3 (4.5) | 7.2 (7.0) | 1.6 (3.7) | 0.11 |
| Total vitamin A (μg RAE) | 1322 (1005) | 1365 (914) | 62 (348) | 0.49 |
| (μg RAE/1,000 kcal) | 799 (691) | 954 (707) | −88.78 (338.1) | 0.31 |
| Total vitamin C (mg) | 113 (106) | 272 (746) | −173 (754) | 0.37 |
| (mg/1,000 kcal) | 64 (53) | 139 (327) | −80 (334) | 0.35 |
| Total vitamin E (mg ATE) | 19 (16) | 22 (17) | −2.0 (10.8) | 0.48 |
| (mg ATE/1,000 kcal) | 11 (10) | 15 (12) | −3 (9) | 0.19 |
| Total iron (μg) | 37 (41) | 38 (61) | 2 (30) | 0.78 |
| (μg/1,000 kcal) | 24 (31) | 27 (46) | −1.1 (19) | 0.82 |
| Total selenium (μg) | 114 (54) | 103 (46) | 13 (49) | 0.31 |
| (μg/1,000 kcal) | 67 (26) | 68 (27) | 0.15 (27) | 0.98 |
| Total zinc (mg) | 9.3 (4.5) | 9.9 (5.5) | −0.66 (4.9) | 0.59 |
| (mg/1,000 kcal) | 5.2 (1.3) | 6.5 (3.5) | −1.2 (3.0) | 0.12 |
Abbreviations: ATA, alpha-tocopherol equivalents; RAE, retinal activity equivalents.
Values for the daily recommended intakes (DRIs) for people aged 19–30 years are as follows. For all nutrients except iron the lower value is for females, higher value is for males, and if there is only one value it is the same DRI for males and females: 1,000 mg calcium, 15 μg vitamin D, 700–900 μg vitamin A, 75–90 mg vitamin C, 15 mg vitamin E, 8–18 μg iron, 55 μg selenium, and 8–11 mg zinc.
Change calculated as baseline—follow up.
P-value is for significant change between time points.
There were nine users of multivitamin supplements that contained vitamin D at baseline; all but one of these remained multivitamin users at follow-up. Background vitamin D supplement use ranged between 3 and 20 μg/d or 120 and 800 IU/d.Bold values denote significant difference.
Crohn's disease severity
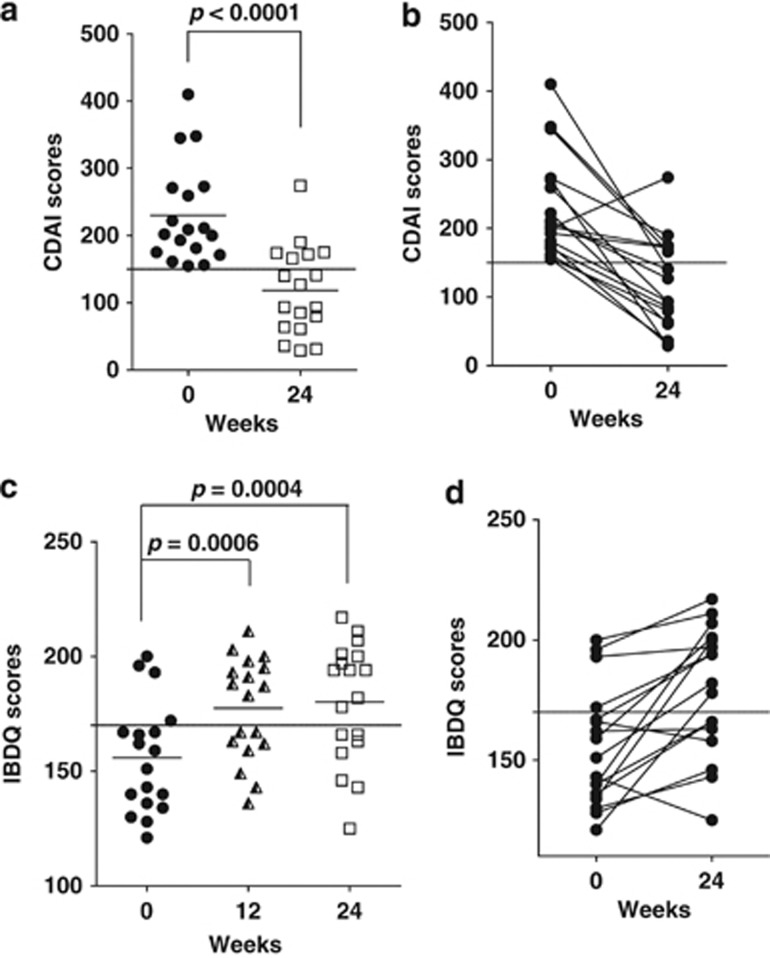
The mean CDAI score for patients at the beginning of the study was 230±74 indicating active disease (Figure 3a). After 24 weeks of vitamin D, the CDAI scores were below 150 for 67% of the participants (Figure 3). The mean CDAI scores after 24 weeks vitamin D3 supplementation also suggested that the patients were in remission. The mean CDAI score at follow-up was 118±66 (Figure 3a) with 12 out of 18 patients (67%) in remission with CDAI<150. Furthermore, 17 patients (95%) achieved a response, with an average decrease in CDAI score of 112±81 points after vitamin D supplement use for 24 weeks (Figure 3). The maximum decrease was 269 points (from 410–141) with 78% of the patients experiencing a decrease in CDAI score of >70 points. Of the remaining participants, three experienced a decrease of less than 70 points (decrease of 21–69 points) and one experienced an increase of 74 points. Two of the non-responders were patients who started with high serum 25(OH)D3 and ended up on less than 5,000 IU/d vitamin D3. There was at least one vitamin D non-responder on each IBD treatment (TNF-α blockages etc.).
Figure 3.
Effect of vitamin D on CDAI and IBDQ scores. (a) CDAI scores were decreased significantly after vitamin D3 supplementation for 24 weeks. (b) Beginning and ending CDAI for each patient are connected by a line. All but one patient had a lower CDAI score at the end of the study than at the beginning. Horizontal dashed lines indicate the CDAI score of 150 that indicates remission. (c) IBDQ scores increased significantly after vitamin D3 supplementation for either 12 weeks or 24 weeks. (d) The beginning and ending IBDQ scores are connected by a line. Most (15 of 18) of the participants showed improvement in the IBDQ scores following vitamin D3 supplementation. Horizontal dashed line is at 170, which is the threshold indicating poor quality of life.
Based on univariate Pearson Correlations, there was no relationship between CDAI and 25(OH)D3 status either before or after 24 weeks of vitamin D3 supplementation (see Supplementary Table 1 online). The change in CDAI scores was inversely related to CDAI score at baseline indicating that patients with high CDAI scores at baseline showed the greatest vitamin D treatment effects (see Supplementary Table 1 online). CDAI scores showed a positive association with serum PTH before supplementation, but the relationship disappeared after supplementation (see Supplementary Table 1 online).
Baseline IBDQ scores of 156±24 were reported indicating poor quality of life (Figure 3c). After supplementation for 12 weeks, 56% of patients had more favorable IBDQ scores with mean values of 178±22 (P=0.0006; Figure 3c). At week 24, the IBDQ scores were maintained and were also significantly higher than at baseline 180±26 (P=0.0004) but not different from the 12-week scores (Figure 3c). The one patient who showed an increase in CDAI score also showed a decreased IBDQ score from 143 to 125 after 24 weeks and as noted previously, a serum 25(OH)D3 level that did not reach the 40 ng/ml target (32 ng/ml after 24 weeks) (Figures 1 and 3). There was a significant inverse correlation between CDAI scores and IBDQ score both before and after supplementation (see Supplementary Table 1 online). Serum 25(OH)D3 level and the change in serum 25(OH)D3 level after supplementation revealed a positive correlation with IBDQ score but not before (see Supplementary Table 1 online).
Multiple linear regression analyses showed that at follow-up mean CDAI score was decreased by 112 points (96 for women, 95% confidence interval (CI)=55–138; 136 for men, 95%=CI 83–189) after accounting for gender, change in serum vitamin D, and baseline CDAI score (data not shown). Change in serum vitamin D over the follow-up time was marginally associated with change in CDAI score (P=0.09). In addition, at follow-up the mean IBDQ scores increased by 24 points (24 for women, 95% CI=10–37; and 25 for men, 95% CI=7–42) after accounting for gender, change in serum vitamin D, and baseline IBDQ. Change in serum vitamin D over the follow-up time was significantly associated with change in IBDQ score (P=0.0302). The addition of other covariates, including body mass index, age, and baseline serum vitamin D, did not affect the results in either model.
Dietary status
The diets of all participants were evaluated using a series of 3- to 24-h recall surveys at baseline and follow-up 24 weeks later. The nutrient intakes are reported as total and the overall intakes per 1,000 kcal as individuals who consume more energy would be more likely to consume more nutrients. There were no significant changes in the dietary composition of most nutrients between baseline and 24 weeks (Table 2). As part of the evaluation, supplement use was recorded at baseline and 24 weeks for all participants (Table 2). There were nine patients already taking vitamin D supplements at baseline that ranged from 120–800 IU/d or 3–20 μg/d vitamin D (Table 2). At the end of the trial, all, except one, of the original nine patients were still taking the vitamin D supplements. At baseline, the mean total vitamin D intakes were below the daily recommended intake for all, but two participants who were supplementing their vitamin D intakes. Dietary vitamin D, supplemental vitamin D (as background), and total vitamin D did not change significantly between baseline and 24 weeks (Table 2). There was an increase in the use of calcium supplements at 24 weeks compared with baseline calcium supplement use (Table 2). This resulted in an increase in total calcium (P<0.06) and a significant difference in total calcium reported for 1,000 kcal energy intake between 24 weeks and baseline (Table 2). There were no differences in the intakes of vitamin A, vitamin C, vitamin E, iron, selenium, or zinc between baseline and 24-week follow-up (Table 2).
Secondary outcomes
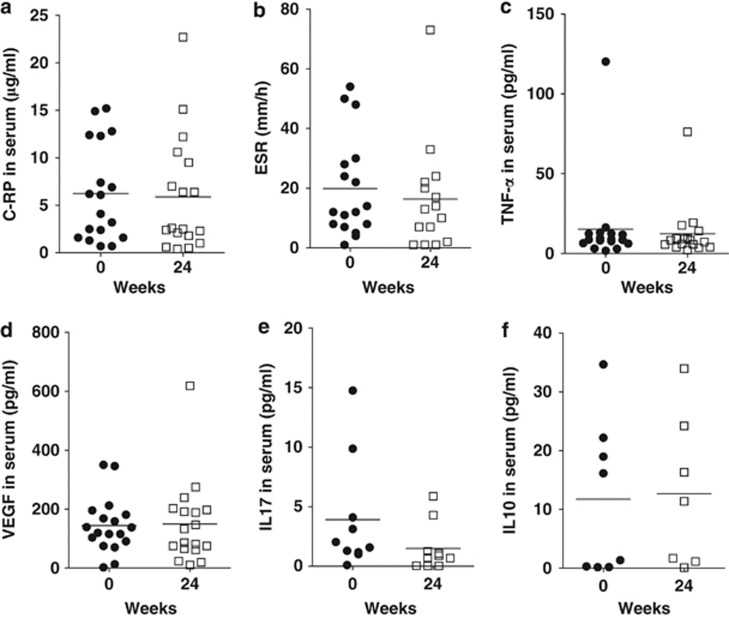
Serum from the Crohn's patients before and after vitamin D3 supplementation was assessed to determine whether there were changes in markers of inflammation associated with the vitamin D3 supplementation. CRP, ESR, TNF-α, IL-17, IL-10, and vascular endothelial growth factor were all found not to be affected by the vitamin D supplementation (Figure 4).
Figure 4.
Effects of vitamin D3 supplementation on systemic inflammation markers and cytokines in serum. Serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), vascular endothelial growth factor (VEGF), cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-10, and IL-17 levels at baseline and 24 weeks following vitamin D3 supplementation. No significant differences were observed for any measurement comparing the baseline with the 24-week vitamin D3 supplemented values.
IPAQ questionnaires showed that of the 14 patients evaluated there was the same level of physical activity before and after vitamin D3 supplementation (data not shown). Three of the participants increased their activity, three of the participants decreased their activity, and the remaining eight had no change in physical activity following vitamin D3 supplementation (data not shown). The IPAQ scores were therefore unaffected by the vitamin D3 supplementation.
Adverse effects
All adverse events reported during the trial are shown in Table 3. There were no significant adverse effects that could be directly attributed to vitamin D3 supplementation. Complaints among the patients that may have been related to the supplement included headache, dizziness, fatigue, abdominal pain, constipation, and rash. Fifteen subjects reported one or more adverse events. The most common reported adverse event was headache, which occurred in five (28%) subjects. Four (22%) subjects reported mild abdominal pain. Four (22%) subjects reported rash that resolved within 1 day. Four (22%) subjects reported fatigue. All of these reported adverse events were mild and no infectious or serious adverse events occurred during the treatment.
Table 3. Adverse events.
| Adverse events | n (%) |
|---|---|
| No adverse events | 3 (17%) |
| Headache | 5 (28%) |
| Hyperanxiety | 1 (6%) |
| Dizziness | 1 (6%) |
| Fatigue | 4 (22%) |
| Eye irritation | 3 (17%) |
| Sinusitis | 3 (17%) |
| Nausea | 2 (11%) |
| Abdominal pain | 4 (22%) |
| Back pain | 1 (6%) |
| Joint pain | 1 (6%) |
| Muscle pain | 3 (17%) |
| Constipation | 1 (6%) |
| Rash | 4 (22%) |
DISCUSSION
In a small clinical trial in central PA, vitamin D3 supplementation for 24 weeks effectively improved vitamin D status, decreased CDAI scores, and improved IBDQ scores in 18 patients. By raising serum vitamin D levels, Crohn's patients with mild-to-moderate symptoms exhibited a decrease in CDAI scores such that the majority of patients achieved remission. The effect of vitamin D3 on the CDAI scores was remarkable with all but one patient having a decrease in CDAI score and 78% of the patients had a drop of 70 points or more, which is considered a treatment response in the clinic.20, 21 The vitamin D3 effect was also reflected in the improved IBDQ scores, which is a second validated assessment tool used to monitor therapeutic efficacy in clinical trials of IBD.22, 23 The IBDQ questionnaire consists of 32 questions scored in four domains: bowel symptoms, emotional health, systemic systems, and social function. As established by other investigators, the IBDQ scores were significantly correlated to the CDAI scores at both baseline and 24 weeks indicating that vitamin D3 effectively improved both quality of life and CDAI scores. Even with the small number of participants and the lack of a placebo the study points to a strong effect of vitamin D3 supplementation on Crohn's disease symptoms.
The primary goal of the study was to determine what dose of vitamin D3 would be required to raise serum 25(OH)D3 levels into a target range of over 40 ng/ml. Consistent with what has been published by others, we found that Crohn's patients have low serum 25(OH)D3 levels.8, 910 Even though the majority of the patients were on vitamin D supplements at baseline, the level of supplementation was insufficient to sustain serum 25(OH)D3 above 40 ng/ml. In fact, half of the Crohn's patients screened for the study had frank vitamin deficiency with serum levels of 25(OH)D3 suggesting malabsorption. The maximal amount of vitamin D3 (5,000 IU/d) was needed to raise serum 25(OH)D3 above 40 ng/ml. The serum levels of 25(OH)D3 were slow to respond to the vitamin D3 supplement and even between 12 weeks and 24 weeks serum 25(OH)D3 levels continued to go up even though the dose of vitamin D3 was not changed. It seems likely that the increased level of 25(OH)D3 between the 12-week and 24-week time points reflects improvement in CDAI scores, a healthier gut and better absorption of the vitamin D supplement. There were no signs of vitamin D toxicity based on the fact that both calcium and PTH were unaffected by the vitamin D3 supplementation. In addition, there were no reported adverse events that required a decrease or discontinuation of the vitamin D dose. Although the higher serum levels of 25(OH)D3 were not toxic, there are no reported benefits associated with high serum levels of 25(OH)D3. In future studies, it would be prudent to check the serum 25(OH)D3 levels at least every 3 months to monitor the 25(OH)D3 levels and to adjust the vitamin D3 supplementation downward if needed. This study suggests that in patients with mild-to-moderate Crohn's disease a safe and tolerable dose of vitamin D that effectively raises serum 25(OH)D3 levels is 5,000 IU/d.
Calcium intakes were increased in the participants at week 24 compared with baseline (Table 3). A closer inspection of the dietary data provided by the individuals seemed to indicate that some individuals at follow-up consumed a calcium supplement every day of the 3 days of recall but only 2 of the 3 days at baseline. Therefore, it seems unlikely that the relatively modest change in calcium for a few individuals would account for the observed changes in CDAI and IBDQ scores. Other known immuno-modulatory nutrients, including vitamin A, vitamin C, vitamin E, selenium, iron, and zinc, were not different between baseline and 24 week follow-up. It is possible that at week 24 absorption of numerous nutrients (other than vitamin D) had improved concomitant with the decrease in CDAI scores; in future, vitamin D intervention would be interesting to determine the concentrations of other micronutrients biochemically.
The Crohn's patients in this study were on several different maintenance therapies except glucocorticoids. The rationale for the exclusion of the glucocorticoids was the fact that usually glucocorticoid treatment is used to suppress Crohn's disease flares and the goal was to have patients with active but mild-to-moderate Crohn's symptoms. The study was not powered to identify whether vitamin D3 supplementation might be more or less effective based on the other treatments being used. However, as vitamin D reduced CDAI scores in all, but one patient it seems that the exclusion of any of the common maintenance therapies may not be warranted.
Several serum proteins were measured in the patient population. Acute-phase proteins such as CRP have been shown to increase with inflammation and to correspond with active Crohn's disease. ESR measurements are another nonspecific measure of inflammation. There was no effect of vitamin D3 supplementation or improvement in CDAI on the levels of either CRP or ESR in this cohort. In addition, TNF-α and IL-17 were detectable but not affected by the vitamin D3 supplementation. Vascular endothelial growth factor is another serum protein that has been shown to correlate with active Crohn's disease.24 There was no effect of vitamin D3 on any of the serum proteins measured and there was no correlation between these inflammatory biomarkers and the improvement in CDAI scores seen. At this time, it is unclear by what mechanisms vitamin D might be improving the CDAI scores. This would be an important area for future research.
This small pilot study establishes that vitamin D3 supplementation of patients with mild-to-moderate Crohn's disease is effective at improving the quality of life and suppressing clinical symptoms to induce remission. An effective dose of vitamin D3 for improving vitamin D status in Crohn's patients was identified as being 5,000 IU/d. The major limitations of this study are the small sample size and lack of placebo control. The study suggests that future larger studies that include placebos and go on for longer periods of time are warranted.
Study Highlights
Acknowledgments
We thank Chris McGovern for technical assistance, Joseph Henderson Ashmore for statistical analyses, and Nicole Marie Zeky for assistance with the dietary analyses.
Guarantor of the article: Margherita T. Cantorna
Specific author contributions: L.Y., V.W., and S.B. collected and analyzed the data; L.Y. wrote the manuscript; V.W. and S.B. supervised the study; J.P.S., T.J.H., and M.T.C. conceived and directed the project, secured funding, and edited the manuscript.
Financial support: GCRC funding NIH M01 RR010732 and C06-RR016499 and the Clinical and Translational Science Pilot Project Awards, College of Medicine, Hershey PA. This project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and TranslationalGastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- Koloski NA, Bret L, Radford-Smith G. Hygiene hypothesis in inflammatory bowel disease: a critical review of the literature. World J Gastroenterol. 2008;14:165–173. doi: 10.3748/wjg.14.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida YR, Rolfe VE. Host-bacterial interactions in inflammatory bowel disease. Clin Sci. 2004;107:331–341. doi: 10.1042/CS20040136. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. Vitamin D. Nutrition Today. 1993;28:6–11. [Google Scholar]
- Clemens TL, Adams JS, Nolan JM, et al. Measurement of circulating vitamin D in man. Clin Chim Acta. 1982;121:301–308. doi: 10.1016/0009-8981(82)90239-x. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med. 2004;229:1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- Ardizzone S, Cassinotti A, Bevilacqua M, et al. Vitamin D and inflammatory bowel disease. Vitam Horm. 2011;86:367–377. doi: 10.1016/B978-0-12-386960-9.00016-2. [DOI] [PubMed] [Google Scholar]
- Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–1961. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence. Proc Soc Exp Biol Med. 2000;223:230–233. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, et al. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miheller P, Muzes G, Hritz I, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis. 2009;15:1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology. 1994;106:287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- Trinder MW, Lawrance IC. Efficacy of adalimumab for the management of inflammatory bowel disease in the clinical setting. J Gastroenterol Hepatol. 2009;24:1252–1257. doi: 10.1111/j.1440-1746.2009.05786.x. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Schreiber S, et al. Dosage adjustment during long-term adalimumab treatment for Crohn's disease: clinical efficacy and pharmacoeconomics. Inflamm Bowel Dis. 2011;17:141–151. doi: 10.1002/ibd.21328. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103:3132–3141. doi: 10.1111/j.1572-0241.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Sostegni R, Daperno M, Scaglione N, et al. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17 (Suppl 2:11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Ciccocioppo R, Armellini E, et al. Serum bFGF and VEGF correlate respectively with bowel wall thickness and intramural blood flow in Crohn's disease. Inflamm Bowel Dis. 2004;10:573–577. doi: 10.1097/00054725-200409000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.