Abstract
Jak2 is a 130 kDa tyrosine kinase that is important in a number of cellular signaling pathways. Its function is intrinsically regulated by the phosphorylation of a handful of its 49 tyrosines. Here, we report that tyrosine 972 (Y972) is a novel site of Jak2 phosphorylation, and hence auto-regulation. Specifically, we found that Y972 is phosphorylated and confirmed that this residue resides on the surface of the protein. Using expression plasmids that expressed either wild type Jak2 or a full length Jak2 cDNA containing a single Y972F substitution mutation, we investigated the consequences of losing Y972 phosphorylation on Jak2 function. We determined that the loss of Y972 phosphorylation significantly reduced both Jak2 total tyrosine phosphorylation and phosphorylation of Y1007/Y1008. Additionally, Y972 phosphorylation was shown to be important for maximal kinase function. Interestingly, in response to classical cytokine activation, the Jak2-Y972F mutant exhibited a moderately impaired level of activation when compared to wild type protein. However, when Jak2 was activated via a GPCR ligand, the ability of the Y972F mutant to activate was completely lost, therefore suggesting a differential role of Y972 in Jak2 activation. Finally, we found that phosphorylation of Y972 enhances Jak2 kinase function via a mechanism that appears to stabilize the active conformation of the protein. Collectively, our results suggest that Y972 is a novel site of Jak2 phosphorylation and plays an important differential role in ligand-dependent Jak2 activation via a mechanism that involves stabilization of the Jak2 active conformation.
Keywords: Janus kinase, phosphorylation, tyrosine
Janus Kinase 2 (Jak2) is one of four family members of the Janus family of tyrosine kinases. These proteins mediate signals from the cell surface to the nucleus through tyrosine phosphorylation signaling cascades. The primary cellular role of Jak2 is to phosphorylate members of the Signal Transducers and Activators of Transcription (STAT) family of latent cytoplasmic transcription factors. Once phosphorylated, STAT proteins then dimerize and translocate into the nucleus. In the nucleus, STAT protein complexes bind DNA promoter elements and alter cellular gene transcription patterns.
The relevance of Jak2 to a wide array of disease states highlights the fact that Jak2 function must be exquisitely regulated (1). Jak2 functional regulation is achieved through the cooperation of several different extrinsic and intrinsic elements. Extrinsically, Jak2 function is regulated by a number of proteins that combine to produce a specific Jak/STAT signaling response. These proteins include cell surface receptors, adaptors/activators, and negative regulators (2–4). On an intrinsic level, Jak2 is regulated by the phosphorylation and dephosphorylation of several of its 49 tyrosine residues. For example, our lab has shown that phosphorylation at Y201 is necessary for the interaction between Jak2 and SHP-2, an important adaptor protein that plays a role in angiotensin II-dependent Jak2 activation (5). Tyrosines 221 and 570 have been shown to modulate Jak2 kinase function (6, 7) while Y1007 resides in the Jak2 activation loop and its phosphorylation is required for maximal Jak2 activation (8). Given the importance of these phosphotyrosines to Jak2 function, the identification of new tyrosine phosphorylation sites may advance our understanding of Jak2 function.
Jak2 is able to phosphorylate its substrates and facilitate gene transcription in both a basal catalytic state and a hyper-kinetic, ligand-activated state (9, 10). Ligands that are known to activate Jak2 include those that bind cytokine and G-protein coupled receptors (GPCRs). Of these two receptor signaling paradigms, the cytokine model is more fully understood. In this model, Jak2 is constitutively bound to the cytoplasmic portions of the receptor subunits (11). While it was originally thought that ligand binding at the cell surface led to receptor subunit dimerization, recent evidence suggests that ligands bind pre-dimerized receptor subunits (12–15). Ligand/receptor association is thought to initiate conformational changes in the receptor subunits that ultimately place the constitutively bound Jak2 molecules in close enough proximity to one another to achieve phosphorylation. Once activated, Jak2 phosphorylates the cytoplasmic tail of the receptor to produce recruitment sites for STAT proteins. The recruited STAT proteins are then phosphorylated by Jak2, allowing for subsequent nuclear translocation and alterations in gene transcription patterns.
The GPCR model differs from the cytokine model at a few notable points. First, it is thought that GPCRs activate a cytoplasmic, rather than a receptor-bound pool of Jak2 proteins (16). Additionally, there is currently no evidence to suggest that GPCRs facilitate the same level of physical contact between Jak2 molecules that cytokine receptors are thought to enable. Rather, it is thought that, upon receptor activation, the Gαq receptor subunit activates Jak2 (17). Once activated, Jak2 translocates to the cytoplasmic tail of the GPCR (18). Phosphorylated tyrosines within Jak2 are thought to serve as STAT recruitment sites (19). Once recruited, STAT proteins are phosphorylated by Jak2 and the subsequent events are the same as those described above.
The Src Homology 2 B (SH2B) protein family makes up another important component of Jak2 regulation. This protein family consists of three members; SH2B, APS, and Lnk (20). All members have an SH2 domain, a dimerization domain, and a pleckstrin homology domain (20). With respect to SH2B, there are multiple isoforms of this protein. The SH2B-β isoform is known to bind Jak2 at phosphorylated tyrosine 813 (21). Upon binding, SH2B-β significantly enhances Jak2 kinase activity and thus acts as an extrinsic regulator of Jak2. Additionally, it is thought that SH2B-β 1) facilitates Jak2 dimerization and 2) stabilizes the kinase domain of Jak2 in the active conformation (22). Collectively, these two molecular processes are thought to promote Jak2 phosphorylation (22).
In this study, we identified Y972 as a novel site of Jak2 phosphorylation. Phosphorylation at this residue is critical for the maintenance of both total tyrosine phosphorylation and phosphorylation of Y1007/Y1008. Furthermore, Y972 phosphorylation differentially affects several Jak2-dependent signal transduction mechanisms, such as growth hormone and angiotensin II-dependent Jak2 activation via a mechanism that appears to involve stabilization of the Jak2 active state.
METHODS
Mass Spectrometry
Wild-type Jak2 protein was overexpressed and purified to homogeneity as previously described (23). Purified protein was then resolved by SDS-PAGE, Coomassie stained, excised from the gel with a razor blade and digested with trypsin. The trypsinized peptide fragments were analyzed via ms/ms mass spectrometry. Fragments containing putative phosphorylation sites were recognized by a shift in mass of 80 daltons or multiples thereof.
In Silico Molecular Modeling of Jak2
The Swiss Model program was used to generate an atomic homology model of the murine Jak2 kinase domain based on the human Jak2 crystal structure (PDB code: 2B7A). The Definition of Secondary Structure of Proteins (DSSP) program was used to calculate the solvent accessible surface area of Y972 as described (24).
Cell Culture
BSC-40 cells were grown in high glucose (4.5 g/L) DMEM (Cellgro) supplemented with 10% newborn calf serum (Hyclone). COS-7 cells were grown in high glucose DMEM supplemented with 10% fetal bovine serum. Cells treated with angiotensin II or growth hormone were growth-arrested with serum-free DMEM for 18 hrs prior to ligand treatment.
Site Directed Mutagenesis
The pRC-CMV-Jak2-Y972F and pBOS-Jak2-Y972F plasmids were made using the QuikChange Mutagenesis protocol (Stratagene). The sense mutagenic primer sequence was 5′-CTTGGTACAAAAAGGTTTATCCACAGGGACCTG-3′ (Genosys). The anti-sense primer sequence was 5′-CAGGTCCCTGTGGATAAACCTTTTTGTACCAAG-3′. Mutations were confirmed by DNA sequence analysis.
Transient Cell Transfections
For each transfection, plasmid DNA and Lipofectin (Invitrogen) were incubated in separate 0.5 ml aliquots of serum-free DMEM at room temperature for 0.5 hrs. Plasmid DNA and Lipofectin were then combined and incubated at room temperature for 10 min. During this incubation, the cells to be transfected were washed twice with PBS. An additional 2 ml of serum-free DMEM was added to each DNA/Lipofectin solution and the 3 ml transfection mixture was then pipetted onto each plate of cells and incubated at 37°C. After 5 hrs, transfection mixtures were aspirated off of the cells and replaced with 5 ml of serum-containing DMEM. The cells were then allowed to recover overnight. Empty vector plasmid was added at the appropriate amounts to ensure that transfections contained equal amounts of DNA.
Immunoprecipitation
Cells were washed twice with ice-cold PBS containing 1mM sodium orthovanadate and then lysed with 900 μl of ice-cold RIPA buffer containing protease inhibitors. Cellular lysates were placed in microfuge tubes, briefly sonicated at 3.3 Hz, and placed on ice for 1 hr. Samples were centrifuged at 16000 × g for 5 min and the supernatants were transferred to new tubes. Whole cell protein lysate samples were prepared by adding 50 μl of lysate to 15 μl of 4X SDS sample buffer. The remainder of each lysate (~850 μl) was used for immunoprecipitation. For this, 2 μg of the appropriate antibody and 20 μl of Protein A/G beads (Santa Cruz Biotechnology) were added to each lysate. Immunoprecipitations were incubated at 4°C with constant shaking for 4–18 hours. Samples were centrifuged at 7000 rpm for 2 min and the pellets were washed 3 times with 1 ml of IP wash buffer (25 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Triton X-100). The beads were then resuspended in 65 μl of 1X SDS sample buffer. Immunoprecipitated proteins and whole cell lysate samples were separated via SDS-PAGE and then transferred onto nitrocellulose membranes (Bio-Rad).
Western Blotting
All Western blots were executed at room temperature. Nitrocellulose membranes were blocked in 30 ml of either 5% BSA/TBST or 5% milk/TBST for 1 hr. The membranes were then incubated in 25 ml of primary antibody solution for 1–2 hrs and washed in TBST for 1 hr. Membranes were incubated in secondary antibody solution for 30 min and then washed in TBST for 30 min. Proteins were visualized via enhanced chemilluminescence reagents. The antibodies used were all commercially available; anti-Jak2 polyclonal antibodies (Santa Cruz, Cell Signaling Technology and Millipore), anti-HA monoclonal and polyclonal antibodies (Santa Cruz), DYKDDDDK Tag (anti-FLAG) antibody (Cell Signaling Technology), anti-FLAG M1 agarose affinity gel (Sigma-Aldrich), anti-myc tag monoclonal antibody (Cell Signaling Technology), anti-Jak2 pY1007/pY1008 polyclonal antibody (BioSource), and anti-phosphotyrosine mouse monoclonal antibodies (BD Biosciences and Millipore).
RESULTS
Tyrosine 972 is a site of Jak2 phosphorylation and this amino acid resides on the surface of the protein
In order to identify novel sites of Jak2 phosphorylation, wild type murine Jak2 protein was expressed via a vaccinia virus-mediated overexpression system and purified to homogeneity in a manner previously described (23). The purified Jak2 protein was then subjected to ms/ms mass spectrometry. Analysis of the spectra indicated that the peptide encoding Y972 (RYIHRD) was phosphorylated at this position (Fig. 1A). Additionally, we confirmed the phospshorylation of two previously reported sites, Y221 and Y1007 (data not shown).
Figure 1. Tyrosine 972 is a site of Jak2 phosphorylation and this amino acid resides on the surface of the protein.
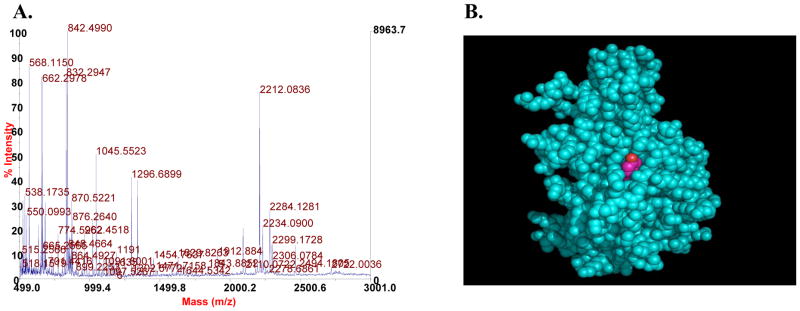
A) Mass spectrometry analysis was performed on purified Jak2 protein. It was determined that Y972 is a site of Jak2 phosphorylation. B) Space filling model of the murine Jak2 kinase domain. Y972, shown in magenta, was found to be on the surface of the structure with a solvent-accessible surface area of 26 Å2.
If Y972 was a potential site of phosphorylation, we hypothesized that it should sit on the surface of the Jak2 molecule. To test this, an atomic homology model of the murine Jak2 kinase domain was generated based on the crystal structure of the human Jak2 kinase domain (25). We found that Y972 was very much on the surface of the protein and was therefore accessible for phosphorylation and dephosphorylation events (Fig. 1B). To put a quantitative measure on this level of surface accessibility, we used the Definition of Secondary Structure of Proteins (DSSP) program and found that the solvent accessible surface area of Y972 is 26 angstroms squared, and the criteria for designating a residue as being solvent accessible is ~15 angstroms squared. Keep in mind however, that this value was calculated from a crystal structure that encodes only the kinase domain and in the context of the entire protein, this value may be higher or lower.
We next examined how well Y972 is conserved within Jak2 across different species as well as between different Jak family members in humans. Analysis of the primary amino acid sequence indicated that Y972 is conserved within Jak2 in species ranging from rodents to primates (Table 1). When looking at this specific residue across different Jak family members in humans, we found that Y972 is conserved in Jak1, Jak2 and Tyk2, but not Jak3 (Table 1).
Table 1. Y972 is highly conserved within Jak2.
Comparison of the primary amino acid sequence of Y972 (underlined) in Jak2 amongst different species as well as within different human Jak kinase family members.
| Protein | Species | Sequence |
|---|---|---|
| Jak2 | Mouse | LGTKRYIHRDL |
| Jak2 | Rat | LGTKRYIHRDL |
| Jak2 | Monkey | LGTKRYIHRDL |
| Jak2 | Human | LGTKRYIHRDL |
| Jak2 | Human | LGTKRYIHRDL |
| Jak1 | Human | LGSRQYVHRDL |
| Jak3 | Human | LGSRRCVHRDL |
| Tyk2 | Human | LHAQHYIHRDL |
Taken together, these data suggest that Y972 is a site of Jak2 phosphorylation.
Y972 affects Jak2 total tyrosine phosphorylation and phosphorylation of Y1007
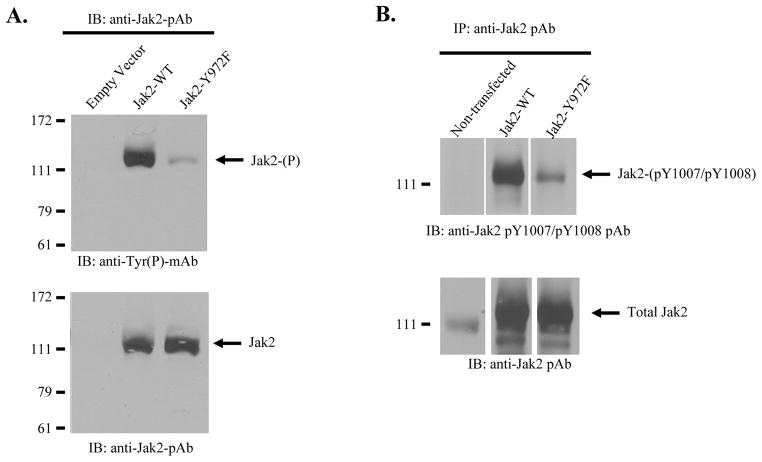
Previous studies have shown that phosphotyrosines can play important roles in regulating Jak2 phosphorylation levels (6–8). To determine the effect of Y972 on total Jak2 tyrosine phosphorylation, BSC-40 cells were transfected with 10 μg of plasmid encoding either empty vector control, Jak2 wild-type, or Jak2-Y972F. The expressed Jak2 protein was then allowed to phosphorylate at every possible tyrosine via high level over expression. The levels of total Jak2 tyrosine phosphorylation were measured by immunoprecipitating the samples with an anti-Jak2 antibody and then Western blotting them with an anti-phosphotyrosine antibody (Fig. 2A, top). We found that conversion of Tyr 972 to Phe greatly reduced the levels of Jak2 tyrosine phosphorylation when compared to wild type protein. The levels of total Jak2 protein were subsequently confirmed by blotting the same membrane with anti-Jak2 antibody (Fig. 2A, bottom).
Figure 2. The effect of Y972 phosphorylation on Jak2 total and Y1007 phosphorylation.
A) BSC-40 cells were transfected with 10 μg of the indicated plasmid DNA. These constructs were overexpressed and allowed to phosphorylate via a vaccinia virus-delivered T7 RNA polymerase. Western blot analysis shows that the loss of phosphorylation at Y972 drastically reduces Jak2 total tyrosine phosphorylation (top). The membrane was stripped and re-blotted with an anti-Jak2 antibody to verify equal protein loading (bottom). B) COS-7 cells were transfected with the indicated plasmids and Y1007/Y1008 phosphorylation was measured via Western blot using an anti-pY1007/Y1008 phospho-specific antibody (top). The membrane was stripped and re-blotted with an anti-Jak2 antibody to verify equal protein loading (bottom). Although not contiguous, the bands are from the same membrane. Shown is one of five (A) or three (B) representative results.
To directly assess the affect of Y972 on Y1007 phosphorylation, COS-7 cells were transfected as described in Fig. 2A, but this time however, the Jak2 immunoprecipitates were Western blotted with anti-Jak2-pY1007/pY1008 antibody. COS-7 cells were used in place of the BSC-40 cells in this assay because phosphorylation of Y1007 does not require incredibly high expression levels. We found that the loss of phosphorylation at Y972 dramatically reduced the phosphorylation of the critically important Y1007 residue (Fig. 2B, top). Again, we confirmed the levels of total protein by Western blotting the samples with anti-Jak2 antibody (Fig. 2B, bottom).
Collectively, the data indicate that when compared to wild type Jak2 protein, the Y972F mutant exhibits marked reduction, but not complete elimination, of its tyrosine phosphorylation capabilities. Furthermore, the loss of Y972 phosphorylation affects both Jak2 total tyrosine phosphorylation and phosphorylation at the critically important Y1007 residue.
The loss of Y972 phosphorylation affects Jak2 kinase activity, but does not confer a dominant negative phenotype
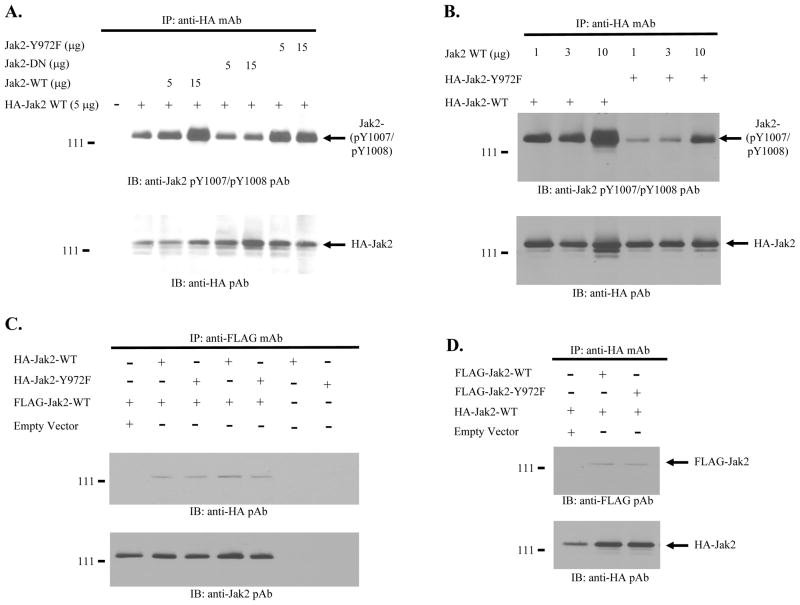
One possible explanation for the dramatic losses in phosphorylation seen above is that the loss of Y972 phosphorylation confers an inhibitory phenotype to Jak2. Structural and conformational changes within the Jak2 kinase domain can generate such a phenotype (26). To evaluate the inhibitory potential of Jak2 in the absence of Y972 phosphorylation, BSC-40 cells were transfected with 5 μg of a plasmid encoding an HA-tagged Jak2 wild-type protein. Additionally, these cells were co-transfected with increasing amounts (5 or 15 μg) of plasmids encoding either Jak2 wild type protein, a known Jak2 dominant negative protein (W1020G/E1024A) (26), or the Jak2-Y972F mutant. The expressed Jak2 proteins were then allowed to phosphorylate via high level over expression. The cells were lysed and HA-tagged Jak2 was immunoprecipitated from the lysates via anti-HA antibody. The levels of Y1007/Y1008 specific phosphorylation of the HA-tagged Jak2 wild type protein were then determined by Western blot analysis (Fig. 3A. top). We found that the titration of additional Jak2 wild type protein resulted in increased phosphorylation at Y1007/Y1008 of the HA-tagged wild type protein, and this was dose-dependent. Addition of the known dominant negative protein however, failed to increase the Y1007 phosphorylation of the HA-tagged protein. Finally, with the addition of the Jak2-Y972F mutant, while it did not inhibit phosphorylation at Y1007 of the HA-tagged Jak2 wild-type protein, it also did not increase it. The membrane was eventually stripped and re-probed with an anti-HA polyclonal antibody to verify equal protein loading (Fig. 3A, bottom).
Figure 3. The loss of Y972 phosphorylation affects Jak2 kinase activity, but does not affect Jak2 dimerization.
A) The indicated plasmids were overexpressed in BSC-40 cells using the vaccinia virus overexpression system. The cells were lysed and HA-tagged Jak2 wild-type protein was immunoprecipitated from the lysates. The Y1007/Y1008 phosphorylation levels of HA-tagged Jak2 wild-type protein were measured via Western blot (top). The membrane was stripped and re-probed with an anti-HA antibody to verify equal protein loading (bottom). B) The indicated plasmids were transfected into BSC-40 cells. Lysates were prepared and HA-tagged Jak2 wild-type and Jak2-Y972F proteins were immunoprecipitated from the lysates. The Y1007/Y1008 phosphorylation levels of the HA-tagged Jak2 proteins were determined via Western blot (top). The membrane was stripped and re-probed with an anti-HA antibody to verify equal protein loading (bottom). C) The indicated plasmids were expressed in COS-7 cells (10 μg of FLAG-Jak2 WT and 10 μg of either HA-Jak2 Y972F or HA-Jak2 WT). All cells were lysed and FLAG-tagged Jak2 wild type protein was immunoprecipitated from the lysates. The levels of co-precipitated HA-tagged Jak2 protein were determined by Western blot (top). The membrane was then stripped and reprobed with an anti-Jak2 polyclonal antibody to verify equal protein loading. D) The indicated plasmids were expressed in COS-7 cells (10 μg of HA-Jak2 WT and10 μg of either FLAG-Jak2 WT or FLAG Jak2 Y972F). All cells were lysed and HA-tagged Jak2 wild-type protein was immunoprecipitated from the lysates. The levels of co-precipitated FLAG-tagged Jak2 protein were determined via Western blot (top). The membrane was stripped and protein levels were verified by Western blot (bottom). Shown is one of three representative results.
Another biochemical property that may explain the inability of the Y972F mutant to support Jak2 tyrosine phosphorylation is the ability of Jak2 itself to act as a substrate of phosphorylation. Thus, in a similar experiment to the one described above, we evaluated the ability of either Jak2 wild type or Jak2-Y972F to act as a substrate when incubated with increasing amounts of Jak2 wild type protein. Specifically, BSC-40 cells were transfected with 10 μg of either an HA-tagged Jak2 wild type plasmid or an HA-tagged Jak2-Y972F plasmid. Additionally, the cells were co-transfected with increasing amounts (1, 3, or 10 μg) of Jak2 wild type plasmid. The levels of Y1007/Y1008 phosphorylation on the HA-tagged Jak2 isoforms were then determined via Western blot analysis (Fig. 3B, top). We found that although the Jak2-Y972F mutant was able to act as a substrate for phosphorylation at Y1007, the level of phosphorylation was ~10-fold less than that of Jak2 wild type protein. Equal protein loading was subsequently determined via Western blot analysis with anti-HA antibody (Fig. 3B, bottom).
Finally, another possible explanation for the lack of Jak2-Y972F phosphorylation observed is that the Y972F mutant may have a reduced ability to form Jak2 dimers. In order to evaluate the impact of Y972 phosphorylation on Jak2 dimerization, cells were transfected with 10 μg of a plasmid encoding FLAG-tagged Jak2 wild type protein. The cells were co-transfected with 10 μg of a plasmid encoding either HA-tagged Jak2 wild type protein or HA-tagged Jak2-Y972F protein. Two days later, the cells were lysed and FLAG-tagged Jak2 wild type protein was immunoprecipitated from the lysates. The levels of HA-tagged Jak2 protein that co-precipitated with the FLAG-tagged Jak2 protein were determined by Western blot with an anti-HA antibody. We found that the loss of Y972 phosphorylation did not affect the ability of HA-tagged Jak2 protein (either mutant or wild type) to co-precipitate with the wild-type FLAG-tagged protein (Fig. 3C). The reciprocal experiment, in which the ability of FLAG-tagged Jak2 protein (either mutant or wild type) to co-precipitate with HA-tagged Jak2 wild type protein, was also performed. The ability of FLAG-tagged Jak2 wild type protein to co-precipitate with HA-tagged Jak2 protein was unaffected by the loss of Y972 phosphorylation (Fig. 3D). The membranes were subsequently stripped and re-probed to verify equal protein loading for both experiments (Figs. 3C and 3D, bottom).
Taken together, the data in Fig. 3 indicate that the Y972F mutant displays a defect in its ability to tyrosine phosphorylate wild type protein. Additionally, the ability of the mutant to act as a substrate for wild type protein is also reduced. However, the ability of Jak2 to form dimers is not affected by the loss of Y972 phosphorylation.
Phosphorylation of Y972 enhances growth hormone-dependent Jak2 activation
The growth hormone (GH) signaling pathway is an excellent example of the cytokine model of Jak2-dependent activation. Thus, we sought to determine the impact of Y972 phosphorylation on Jak2 activation, in response to GH. COS-7 cells were transiently transfected with plasmids encoding the growth hormone receptor (GHR) and either empty vector control, Jak2 wild type, or Jak2-Y972F. The cells were subsequently growth arrested, treated with GH for the indicated times, and whole cell lysates were prepared. We first measured the relative phosphorylation levels of Y1007/Y1008 as a function of Y972 phosphorylation (Fig. 4A, top). We found that the loss of phosphorylation at Y972 significantly reduced the growth hormone dependent increase in Y1007 phosphorylation in response to GH, when compared to cells expressing wild type protein. The membrane was subsequently stripped and equal protein expression was verified via Western blot with an anti-Jak2 antibody (Fig. 4A, bottom).
Figure 4. Phosphorylation of Y972 enhances growth hormone-dependent Jak2 activation.
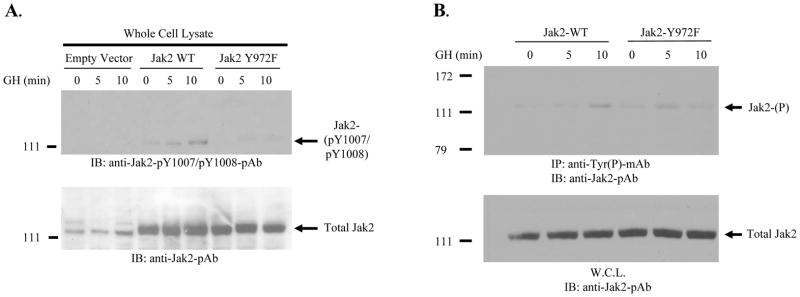
COS-7 cells were transiently transfected with 10 μg of a growth hormone receptor (GHR) plasmid and 2.5 μg of either empty vector control plasmid, Jak2 wild-type plasmid, or Jak2-Y972F plasmid. After serum starvation, the cells were treated with 250 ng/ml GH for the indicated times. A) Phosphorylation at the critically important Y1007 residue was measured via Western blot of whole cell protein lysates (top). The levels of expressed Jak2 protein were confirmed via anti-Jak2 Western blot analysis of the same membrane (bottom). B) Lysates were first immunoprecipitated with anti-phosphotyrosine antibody and then Western blotted with anti-Jak2 antibody to measure GH-mediated Jak2 total phosphorylation (top). Whole cell lysate samples were Western blotted with an anti-Jak2 antibody to verify equal expression of both wild-type and mutant plasmids (bottom). Shown is one of two (A) or three (B) representative results.
In order to determine the affect of Y972 on all the remaining tyrosine residues, cells were transfected exactly as described in Fig. 4A, but this time the protein lysates were first immunoprecipitated with anti-phosphotyrosine antibody and then Western blotted with anti-Jak2 antibody (Fig. 4B, top). We found that loss of phosphorylation at Y972 had virtually no effect on growth hormone-mediated Jak2 total tyrosine phosphorylation levels, when compared to wild type protein. The expression of both wild type and Y972F mutant proteins were confirmed by blotting aliquots from these samples with anti-Jak2 antibody (Fig. 4B, bottom).
In summary, the data in Fig. 4 demonstrate that in response to GH, Y972 has a marked affect on Y1007 phosphorylation and little affect on the phosphorylation of the remaining Jak2 tyrosine residues.
Y972 is critically important for Jak2 activation in response to the GPCR ligand, angiotensin II
As discussed above, GPCRs can also activate Jak2, but they do so through a different mechanism than that of cytokine receptors. Therefore, we next wanted to determine whether Y972 is important for Jak2 activation in the context of the GPCR signaling paradigm. For these studies, we used the angiotensin II type 1 receptor (AT1R) and its high affinity ligand, angiotensin II. Specifically, COS-7 cells were transfected with plasmids encoding the AT1R and either empty vector control, Jak2 wild type, or Jak2-Y972F. The cells were eventually treated with angiotensin II for the indicated times and then lysed. Jak2 protein was immunoprecipitated from the lysates and total Jak2 tyrosine phosphorylation levels were measured via Western blot analysis with anti-phosphotyrosine antibody (Fig. 5A, top). Unlike with GH, we found that the loss of phosphorylation at Y972 completely abolished the ability of angiotensin II to promote any degree of Jak2 tyrosine phosphorylation. As was done previously, total precipitation levels were confirmed by blotting the same membrane with anti-Jak2 antibody (Fig. 5A, bottom).
Figure 5. Tyrosine 972 is critical for angiotensin II-dependent Jak2 phosphorylation.
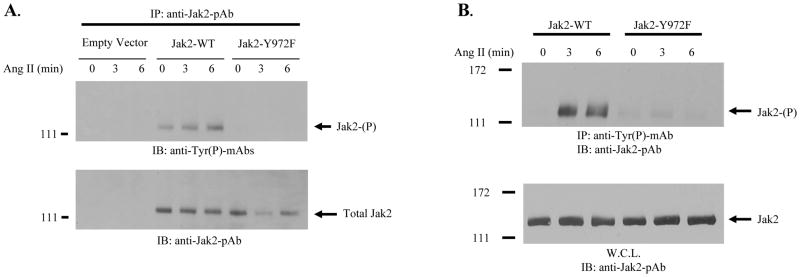
A) COS-7 cells were transiently transfected with 10 μg of AT1 receptor plasmid and 2.5 μg of either empty vector control plasmid, Jak2 wild-type plasmid, or Jak2-Y972F plasmid. After transfection and subsequent serum starvation, the cells were treated with 100 nM angiotensin II for the indicated times. The cells were lysed and Jak2 protein was immunoprecipitated from the lysates. Jak2 total tyrosine phosphorylation levels were determined via Western blot analysis with anti-phosphotyrosine antibody (top). The membrane was stripped and re-probed with anti-Jak2 antibody to verify equal protein loading (bottom). B) Cells were transfected as described in Fig. 5A. After serum starvation and angiotensin II treatment, lysates were immunoprecipitated with anti-phosphotyrosine antibody and then Western blotted with anti-Jak2 antibody (top). Whole cell protein lysates from the same samples were Western blotted with anti-Jak2 antibody to validate expressed Jak2 protein levels (bottom). Shown is one of three representative results for each.
To confirm this marked affect of Y972 on angiotensin II-mediated Jak2 activation via an alternate means, cells were once again transfected as described in Fig. 5A and then treated with angiotensin II. This time however, the protein lysates were immunoprecipitated with anti-phosphotyrosine antibody and then Western blotted with anti-Jak2 antibody (Fig. 5B, top). We found that while angiotensin II was able to markedly activate Jak2 wild type protein, it was completely unable to promote any level of tyrosine phosphorylation on the Jak2-Y972F mutant. We confirmed the levels of expressed Jak2 by Western blotting aliquots from these same samples with anti-Jak2 antibody (Fig. 5B, bottom). Taken together, the results suggest that Y972 plays a prominent role in Jak2 activation when the Jak2 molecules are activated by GPCR ligands such as angiotensin II.
SH2B-β restores Y1007/Y1008 phosphorylation in the Jak2-Y972F mutant
The activation of Jak2 is known to require two critical steps. First, at least two separate Jak2 molecules must dimerize. Once bound, the activation loops of both molecules must be stabilized in the active conformation for the kinases to be hyper-kinetic. SH2B-β is an important component of Jak2 activation. Initial work by Carter-Su demonstrated that SH2B-β potently activates Jak2 (2). Subsequent work by this group suggests that the mechanism for this activation is that SH2B-β promotes Jak2 dimer formation (21) and then stabilizes the active state of Jak2 (22). The data in Fig. 3C and 3D indicate that the Y972F mutant is able to dimerize with wild type protein quite normally. Therefore, we hypothesized that the inability of the Y972F mutant to phosphorylate was due to the decreased stability of the Jak2 active conformation. Furthermore, we hypothesized that this deficiency could be overcome via SH2B-β expression.
To test this hypothesis, COS-7 cells were transiently transfected with low levels (3.0 μg per dish) of either Jak2 wild-type or Jak2 Y972F plasmid. Unlike the high level of expression that was done in Fig. 2, this low level of Jak2 expression results in very little Jak2 activation/phosphorylation. Additionally, the cells were co-transfected with increasing amounts (0, 0.5 and 1.0 μg) of a Myc-tagged SH2B-β construct. Thirty-six hrs later, the cells were lysed and Jak2 protein was immunoprecipitated from the lysates. The stability of the Jak2 active conformation was directly accessed via Western blotting the samples with an antibody that is directed against the activation loop; namely, the anti-Jak2 pY1007/pY1008 antibody (Fig. 6, top). We found that in the absence of SH2B-β, there was no active Jak2 protein for both Jak2 wild type and Jak2-Y972F samples (lanes 1 and 4). However, addition of 0.5 μg of SH2B-β plasmid resulted in a marked increase in Y1007/Y1008 phosphorylation for both Jak2 wild type and Jak2-Y972F (lanes 2 and 5). Finally, addition of 1.0 μg of SH2B-β protein resulted in no additional Jak2 activation (lanes 3 and 6), therefore suggesting that the 0.5 μg amount was fully activating Jak2. To confirm that Jak2 was precipitated at similar levels, the membrane was Western blotted with anti-Jak2 antibody (Fig. 6, middle). Finally, to visualize the levels of expressed Myc-tagged SH2B-β, whole cell lysate aliquots from these same samples were Western blotted with anti-Myc antibody (Fig. 6, bottom).
Figure 6. SH2B-β restores Y1007/Y1008 phosphorylation in the Jak2-Y972F mutant.
COS-7 cells were transfected with a fixed amount (3 μg per dish) of either Jak2 wild-type or Jak2-Y972F plasmid and increasing amounts (0, 0.5 and 1.0 μg) of a Myc-tagged SH2B-β plasmid. Two days later, protein lysates were immunoprecipitated with anti-Jak2 antibody and Y1007 phosphorylation was measured via Western blot analysis (top). The membrane was stripped and equal Jak2 precipitation was verified via anti-Jak2 Western blot analysis (middle). The levels of expressed Myc-tagged SH2B-β protein were visualized via anti-Myc Western blot analysis of the corresponding whole cell lysate samples (bottom). Shown is one of three representative results.
Collectively, the data demonstrate that the Jak2-Y972F mutant exhibits a low level of kinase power as measured by decreased activation loop phosphorylation. However, this deficiency can be completely overcome when the Jak2-Y972F mutant is expressed in the presence of SH2B-β, a protein that is known to stabilize the active conformation of Jak2.
DISCUSSION
Jak2 is a ubiquitous tyrosine kinase that is activated via a number of different mechanisms including cytokine and GPCR ligand-dependent activation. In the end, it integrates a number of intrinsic and extrinsic signals in order to maintain an appropriate level of kinase activity; too little or too much kinase activity can be deleterious. For example, mice that expressed a Jak2 protein that was completely devoid of kinase activity died during embryonic development because they were unable to make red blood cells (27). Conversely, hyper-kinetic Jak2 kinase activity is known to be the cause of numerous hematological and myeloproliferative disorders seen in humans (28–30). Thus, an appropriate level of Jak2 kinase activity is imperative for normal cellular function.
One of the mechanisms by which Jak2 regulates its kinase activity is via the phosphorylation and dephosphorylation of a handful of its 49 tyrosine residues. In a previous report, Matsuda and colleagues synthesized twenty unique peptides encoding 28 different Jak2 tyrosine residues (31). Each peptide was incubated in vitro with ATP and recombinant wild type Jak2 protein in order to determine potential sites of Jak2 phosphorylation. They found that a number of the peptides could be phosphorylated in this cell free system including one that encoded Y972. The limitation of this work however, was that one wondered whether Y972 could phosphorylate in the context of a full length Jak2 protein (as opposed to on a small peptide) and whether this phosphorylation could occur within an intact cell (as opposed to in a test tube). Our work here suggests that the answer to both of these questions is yes. In a vein similar to this, a recent report by Funakoshi-Tago and colleagues confirmed that yet another tyrosine residue, Y119, is not only a site of Jak2 phosphorylation on a synthetic peptide, but also within the full length protein and inside cultured cells (32).
Tyrosine 972 was investigated within the context of both cytokine and GPCR-mediated Jak2 activation. One of the key differences between these two models is that, while cytokine receptors are thought to activate Jak2 molecules by bringing them into close physical contact with one another, no evidence exists to suggest that GPCRs facilitate such intimate contact. Since cytokine receptors facilitate interaction between Jak2 molecules, they may be better able to take advantage of suboptimal Jak2 activation states than GPCRs (9). This difference may explain why moderate increases in Jak2 total phosphorylation were seen in response to GH (Fig. 4B), but completely lacking in response to angiotensin II (Fig. 5B). As such, these data may also support the hypothesis that Y972 phosphorylation offers stability to the maximally active conformation of Jak2.
Further support for this model arises when comparing the effects of losing Y972 phosphorylation in GH-dependent Y1007/Y1008 phosphorylation versus SH2B-β mediated Y1007/Y1008 phosphorylation. Both the GHR and SH2B-β facilitate Jak2/Jak2 interactions. However, unlike the GHR, SH2B-β is also known to stabilize the Jak2 active conformation (22). The putative stability deficit of this conformation produced by the loss of Y972 phosphorylation may be too great to be fully overcome by the GHR alone. This would explain why GHR activation largely restored Jak2 global phosphorylation, but not Y1007 phosphorylation, in the Y972F mutant. Maximal activation, as indicated by Y1007 phosphorylation within the Jak2 activation loop, was achieved only in the presence of SH2B-β (Fig. 6), a molecule that promotes both Jak2/Jak2 dimer formation (21) and increased stability of the Jak2 active conformation (22). This suggests that SH2B-β is able to provide an extra degree of stability to the Y972F mutant, allowing it to become maximally activated.
In conclusion, the loss of phosphorylation at Y972 has significant consequences for Jak2 biological function. We assert that this loss confers a degree of instability to the Jak2 active conformation, as evidenced by the Y972-dependent loss in Y1007/Y1008 phosphorylation. This instability hinders Jak2 kinase function, as is demonstrated by the fact that the Y972F mutant could not fully phosphorylate HA-tagged wild-type Jak2. It also manifests itself in the severe reduction of total tyrosine phosphorylation in overexpressed Jak2. With respect to signal transduction, the putative Y972-dependent stability deficit is a hurdle to activating agents that have not been shown to facilitate Jak2 interaction, such as the AT1 receptor. However, the loss of Y972 phosphorylation does not irreversibly hinder Jak2 function. A potent activator, such as SH2B-β was able to restore maximal activation to the Y972F mutant. Thus, we assert that Y972 phosphorylation is necessary for maximal Jak2 function.
Acknowledgments
This work was supported by a Biomedical Research Support Program for Medical Schools Award to the University of Florida College of Medicine by the Howard Hughes Medical Institute, a University of Florida Opportunity Fund Award, an American Heart Association Florida/Puerto Rico Affiliate Grant in Aid (#0555359B), and National Institutes of Health Award R01-HL67277.
We are indebted to Melissa A. Johns for exceptional technical assistance. We wish to thank Dr. D.M. Wojchowski for the Jak2 expression plasmid, Dr. O. Silvennoinen for the HA-tagged Jak2 expression plasmid and Dr. C. Carter-Su for the FLAG-tagged Jak2 and Myc-tagged SH2B-β plasmids.
Abbreviations
- Jak2
Janus Kinase 2
- STAT
Signal Transducers and Activators of Transcription
- GH
growth hormone
- GHR
growth hormone receptor
- SH2B
Src Homology 2 B
- GPCRs
G-protein coupled receptors
References
- 1.Sandberg EM, Wallace TA, Godeny MD, Vonderlinden D, Sayeski PP. Jak2 tyrosine kinase: a true jak of all trades? Cell Biochem Biophys. 2004;41:207–232. doi: 10.1385/cbb:41:2:207. [DOI] [PubMed] [Google Scholar]
- 2.Rui L, Carter-Su C. Indentification of SH2-b beta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999;96:7172–7177. doi: 10.1073/pnas.96.13.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273:2344–2354. doi: 10.1074/jbc.273.4.2344. [DOI] [PubMed] [Google Scholar]
- 4.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godeny MD, Sayyah J, Vonderlinden D, Johns M, Ostrov DA, Caldwell-Busby J, Sayeski PP. The N-terminal SH2 domain of the tyrosine phosphatase, SHP-2, is essential for Jak2-dependent signaling via the angiotensin II type AT1 receptor. Cell Signal. 2007;19:600–609. doi: 10.1016/j.cellsig.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Feener EP, Rosario F, Dunn SL, Stancheva Z, Myers MG. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol Cell Biol. 2004;24:4968–4978. doi: 10.1128/MCB.24.11.4968-4978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argetsinger LS, Kouadio JL, Steen H, Stensballe A, Jensen ON, Carter-Su C. Phosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol Cell Biol. 2004;24:4955–4967. doi: 10.1128/MCB.24.11.4955-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatti K, Farrar WL, Duhe RJ. Tyrosine phosphorylation of the Janus kinase 2 activation loop is essential for a high-activity catalytic state but dispensable for a basal catalytic state. Biochemistry. 2004;14:4272–4283. doi: 10.1021/bi036109b. [DOI] [PubMed] [Google Scholar]
- 10.Wallace TA, VonDerlinden D, He K, Frank SJ, Sayeski PP. Microarray analyses identify JAK2 tyrosine kinase as a key mediator of ligand-independent gene expression. Am J Physiol Cell Physiol. 2004;287:C981–991. doi: 10.1152/ajpcell.00085.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bach EA, Tanner JW, Marsters S, Ashkenazi A, Aguet M, Shaw AS, Schreiber RD. Ligand-induced assembly and activation of the gamma interferon receptor in intact cells. Mol Cell Biol. 1996;16:3214–3221. doi: 10.1128/mcb.16.6.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 13.Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12:814–821. doi: 10.1038/nsmb977. [DOI] [PubMed] [Google Scholar]
- 14.Lu X, Gross AW, Lodish HF. Active conformation of the erythropoietin receptor: random and cysteine-scanning mutagenesis of the extracellular juxtamembrane and transmembrane domains. J Biol Chem. 2006;281:7002–7011. doi: 10.1074/jbc.M512638200. [DOI] [PubMed] [Google Scholar]
- 15.Yang N, Wang X, Jiang J, Frank SJ. Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol. 2007;21:1642–1655. doi: 10.1210/me.2006-0458. [DOI] [PubMed] [Google Scholar]
- 16.Sayeski PP, Ali MS, Frank SJ, Bernstein KE. The angiotensin II-dependent nuclear translocation of STAT1 is mediated by the Jak2 protein motif 231YRFRR. J Biol Chem. 2001;276:10556–10563. doi: 10.1074/jbc.M008856200. [DOI] [PubMed] [Google Scholar]
- 17.Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 18.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272:23382–23388. doi: 10.1074/jbc.272.37.23382. [DOI] [PubMed] [Google Scholar]
- 19.Ali MS, Sayeski PP, Bernstein KE. Jak2 acts as both a STAT1 kinase and as a molecular bridge linking STAT1 to the angiotensin II AT1 receptor. J Biol Chem. 2000;275:15586–15593. doi: 10.1074/jbc.M908931199. [DOI] [PubMed] [Google Scholar]
- 20.Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab. 2007;18:38–45. doi: 10.1016/j.tem.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O’Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 phosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurzer JH, Saharinen P, Silvennoinen O, Carter-Su C. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol Cell Biol. 2006;26:6381–6394. doi: 10.1128/MCB.00570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Sayeski PP. Vaccinia virus-mediated high level expression and single step purification of recombinant Jak2 protein. Protein Expr Purif. 2004;35:181–189. doi: 10.1016/j.pep.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 25.Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE, Walter M, Burns CJ, Treutlein H, Wilks AF, Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107:176–183. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- 26.Zhuang H, Patel SV, He TC, Sonsteby SK, Niu Z, Wojchowski DM. Inhibition of erythropoietin-induced mitogenesis by a kinase deficient form of Jak2. J Biol Chem. 1994;269:21411–21414. [PubMed] [Google Scholar]
- 27.Frenzel K, Wallace TA, McDoom I, Xiao HD, Capecchi MR, Bernstein KE, Sayeski PP. A functional Jak2 tyrosine kinase domain is essential for mouse development. Exp Cell Res. 2006;312:2735–2744. doi: 10.1016/j.yexcr.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Tomasson MH, Williams IR, Li S, Kutok J, Cain D, Gillessen S, Dranoff G, Van Etten RA, Gilliland DG. Induction of myeloproliferative disease in mice by tyrosine kinase fusion oncogenes does not require granulocyte-macrophage colony-stimulating factor or interleukin-3. Blood. 2001;97:1435–1441. doi: 10.1182/blood.v97.5.1435. [DOI] [PubMed] [Google Scholar]
- 29.Tefferi A, Gilliland DG. JAK2 in myeloproliferative disorders is not just another kinase. Cell Cycle. 2005;4:1053–1056. [PubMed] [Google Scholar]
- 30.Villeval JL, James C, Pisani DF, Casadevall N, Vainchenker W. New insights into the pathogenesis of JAK2 V617F-positive myeloproliferative disorders and consequences for the management of patients. Semin Thromb Hemost. 2006;32:341–351. doi: 10.1055/s-2006-942755. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda T, Witthuhn BA, Sekine Y, Ihle JN. Determination of the transphosphorylation sites of Jak2 kinase. Biochem Biophys Res Commun. 2004;325:586–594. doi: 10.1016/j.bbrc.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 32.Funakoshi-Tago M, Pelletier S, Matsuda T, Parganas E, Ihle JN. Receptor specific downregulation of cytokine signaling by phosphorylation in the FERM domain of Jak2. EMBO J. 2006;25:4763–4772. doi: 10.1038/sj.emboj.7601365. [DOI] [PMC free article] [PubMed] [Google Scholar]