Abstract
Background
Exercise blood pressure (BP) is an important marker of left ventricular hypertrophy, incident hypertension and future cardiovascular events. Although impaired vascular function is hypothesized to influence the BP response during exercise, limited data exist on the association of vascular function with exercise BP in the community.
Methods and Results
Framingham Offspring cohort participants (n=2115, 53% women, mean age 59 years) underwent a submaximal exercise test (first 2 stages of the Bruce protocol), applanation tonometry and brachial artery flow-mediated dilation (FMD) testing. We related exercise systolic and diastolic BP at second stage of the Bruce protocol to standard cardiovascular risk factors and to vascular function measures. In multivariable linear regression models, exercise systolic BP was positively related to age, standing BP, standing heart rate, smoking, body mass index, and the total cholesterol-to-high-density cholesterol (HDL) ratio (p≤0.01 for all). Similar associations were observed for exercise diastolic BP. Carotid-femoral pulse wave velocity (p=0.02), central pulse pressure (p<0.0001), mean arterial pressure (p=0.04) and baseline brachial flow (p=0.002) were positively associated with exercise systolic BP, whereas FMD was negatively associated (P<0.001). For exercise diastolic BP, forward pressure wave amplitude was negatively related (p<0.0001) whereas mean arterial pressure was positively related (p<0.0001).
Conclusions
Increased arterial stiffness and impaired endothelial function are significant correlates of a higher exercise systolic BP response. Our findings suggest that impaired vascular function may contribute to exaggerated BP responses during daily living, resulting in repetitive increments in load on the heart and vessels and increased cardiovascular disease risk.
Keywords: blood pressure, endothelial function, exercise, vascular function, vascular stiffness
Exercise blood pressure (BP) is an important marker of cardiovascular disease (CVD) risk that is associated with incident hypertension,1–5 myocardial infarction6,7, stroke8 and cardiovascular mortality9,10 in individuals without overt coronary artery disease. In contrast to peak exercise BP, which is highly effort-dependent and affected by fitness level, exercise BP measured during sub-maximal exercise represents a physiological response to low-to-moderate physical activity that is closely correlated with ambulatory BP,11 mean daily BP and end-organ damage.12 Understanding the clinical and vascular correlates of exercise BP response to moderate exercise may provide mechanistic insights into the development of hypertension, left ventricular hypertrophy (LVH) and CVD. However, predictors of exercise BP have not been well characterized in ambulatory individuals in the community.
Individuals with a hypertensive exercise BP response are frequently characterized by limited exercise tolerance, LVH and impaired LV diastolic function13, which have been attributed to abnormalities in vascular function despite limited data to support this claim. In addition, the relative contributions of increased vascular stiffness and impaired endothelial function to exaggerated BP response to exercise have not been evaluated in a large community-based sample. We hypothesized that abnormal arterial stiffness, microvascular function and endothelial function would be associated with an increased systolic BP response to exercise. Accordingly, using data from the community-based Framingham Offspring Study, we evaluated the cross-sectional relations of exercise BP response to treadmill exercise with CVD risk factors and measures of pulsatile arterial load (central pulse pressure and its main components, forward pressure wave amplitude and augmented pressure), steady-flow arterial load (mean arterial pressure [MAP]), aortic stiffness (carotid-femoral pulse wave velocity) and endothelial function (flow-mediated dilatation [FMD] and forearm microvascular reactivity).
Methods
Study Sample
The Framingham Offspring Study enrolled the children of the original Framingham Study participants and the spouses of these children starting in 1971.14,15 Participant are examined approximately every four to six years. For the present analyses, participants attending the seventh examination cycle (1998–2001) were eligible. Of 2441 participants who underwent an exercise test at this examination, we excluded 326 individuals who did not complete both stages of the protocol or had missing one or more exercise BP variables. After all exclusions, 2,115 participants remained eligible for the present investigation. The study protocols were approved by the Institutional Review Board of Boston University Medical Center and the Massachusetts General Hospital and all participants provided written informed consent.
Assessment of Vascular Risk Factors
At the seventh examination cycle, risk factor information was collected from routine medical history, physical examination, and laboratory assessment. Serum total cholesterol and high-density lipoprotein cholesterol (HDL-C) were determined on fasting blood samples using standardized biochemical methods. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. BP was determined on the left arm of seated participants using a mercury column sphygmomanometer, a cuff of appropriate size and a standardized protocol; the mean of 2 such physician-obtained readings was taken as the examination BP. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dl (≥7.0 mmol/L) or use of insulin or other hypoglycemic medications. A physician elicited self-reported cigarette smoking (based on regular cigarette smoking within the last year) and medication use (including anti-hypertensive and lipid-lowering therapy) using a standardized questionnaire.
Sub-maximal Exercise Test
A sub-maximal exercise test (first 2 stages of the standard Bruce protocol) was performed on a treadmill using standard procedures including continuous electrocardiographic monitoring, as described previously.16 Systolic and diastolic BP were recorded at the following time-points during the exercise test: (1) in a standing position on the stationary treadmill before exercise; during exercise at (2) the midpoint of the first stage and (3) at the midpoint of the second exercise stage. In addition, BP was also recorded in the supine position at the end of each minute during the 4 minute recovery stage.
Arterial Tonometry Measurements
Using a commercially available applanation tonometer (SPT-301, Millar Instruments, Houston, TX), tonometry was performed as previously described.17 Briefly, with the participants in the supine position arterial waveforms were obtained from the carotid, brachial, radial and femoral arteries with simultaneous acquisition of the electrocardiogram using custom hardware and software (Cardiovascular Engineering, Inc., Norwood, MA) and BP was measured using an oscillometric device. Waveforms were signal averaged using the ECG R-wave as the fiducial point. Oscillometric systolic and diastolic pressures were used to calibrate the peak and trough of the signal-averaged brachial waveform. MAP was assessed by integrating the calibrated brachial pressure waveform. Other waveforms were then calibrated using brachial MAP and diastolic pressure as previously described.16 Carotid-femoral pulse wave velocity was calculated from the transit distances (from body surface measurements) and transit times (based on the timing of carotid and femoral waveforms), as previously reported.17 The forward pressure wave amplitude and augmented pressure amplitude were derived from the calibrated carotid waveform, as previously described.18 The calibrated carotid pressure was used as a surrogate for the central pressure.19
Brachial Artery Measurements
Brachial artery measurements were performed using a commercial ultrasound system (Toshiba SSH-140A). Baseline brachial artery diameter and flow velocity were assessed and then a cuff on the right forearm was inflated to 50 mm Hg above the participant’s systolic BP for 5 minutes. Hyperemic flow was assessed during the first 20 seconds after deflation of the cuff. We assessed Doppler flow at baseline and during reactive hyperemia by using a 3.75 MHz probe and correcting for insonation angle. Mean baseline flow velocity and hyperemic flow velocity were obtained from signal-averaged Doppler flow spectra using a semi-automated technique as reported previously20. FMD was expressed as percent change in diameter from baseline. Reproducibility results for brachial artery measurements have been reported21. “The intraobserver and interobserver correlation coefficients for baseline and deflation diameters were 0.99. The absolute error between measurements ranged from 0 to 0.12 mm (FMDmm) and 0.02% to 2.99% (FMD%). For both FMDmm and FMD%, the correlation coefficients ranged between 0.78 and 0.92.”21
Statistical Analysis
The dependent variables for analyses were systolic and diastolic BP recorded during the second stage of the exercise test. Cigarette smoking, diabetes and use of medications (i.e. lipid-lowering and anti-hypertensive) were treated as binary (yes/no) variables; all other variables were treated as continuous. For exercise systolic and diastolic BP at the second stage of exercise, linear regression models were fitted using a backward elimination process (using a criterion p<0.10 to stay in the model) to select a parsimonious set of risk factor covariates that were associated with exercise BP. Sex and age were forced in, whereas the following covariates were candidates for removal: resting systolic BP, diastolic BP and resting heart rate, diabetes, body mass index, smoking history, total cholesterol to HDL ratio, waist circumference and exercise technician. We also evaluated statistical interactions of sex or hypertension (including hypertension treatment) with resting BP. Having constituted a model incorporating standard risk factors for each exercise BP variable, we then entered the vascular stiffness variables (including carotid-femoral pulse wave velocity, central pulse pressure, forward pressure wave amplitude, augmented pressure, augmentation index, MAP) and the brachial artery variables (including baseline and hyperemic artery dimensions and flow velocities). The vascular stiffness variables and the brachial artery variables were added to the model as 2 separate groups. If one variable remained significant (p<0.10) when the group was added, we then tested each variable in the group for retention in the model using a backward elimination strategy (P<0.10).
To evaluate the joint relations of aortic stiffness and brachial flow variables, we fitted a final model in which the baseline risk factors for each exercise outcome were forced in and all vascular function variables were considered simultaneously using a backward variable selection strategy with a p<0.10 for retention. Due to the exploratory nature of these analyses we did not adjust for multiple testing. For variables for which we obtained P < 0.001, results are unlikely to be false positive findings. However, any variable for which we obtained 0.001 < P <= 0.05 should be considered as “possibly of interest”, pending further replication by others. All analyses were performed in SAS 9.1.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Sample characteristics
The study sample consisted of 2115 participants, of which 53% were women. Mean age was 59±9 years (limits 34–81 years). Other clinical characteristics of the study sample are summarized in Table 1.
Table 1.
Baseline Participant Characteristics and Measures of Vascular Function
| Participants (N=2115) | |
|---|---|
| Baseline Characteristics | |
| Age, yrs (limits) | 59±9 (34–81) |
| Male, n (%) | 991 (47) |
| Standing SBP, mm Hg | 118±18 |
| Standing DBP, mm Hg | 75±11 |
| Standing HR, bpm | 77±12 |
| BMI, kg/m2 | 27.5±4.6 |
| Total cholesterol/HDL ratio | 4.0±1.3 |
| Diabetes, n (%) | 146 (7) |
| Smoking, n (%) | 263 (12) |
| Lipid treatment, n (%) | 304 (14) |
| Hypertension treatment, n (%) | 514 (24) |
| Arterial Tonometry Variables | |
| Carotid-femoral pulse wave velocity, meters/second | 9.24±2.7 |
| Mean arterial pressure, mm Hg | 90.6±11.0 |
| Central pulse pressure, mm Hg | 47.6±13.9 |
| Forward pressure wave amplitude, mm Hg | 38.7± 11.0 |
| Augmentation pressure, mm Hg | 7.67±6.6 |
| Augmentation index, % | 14.3±12.5 |
| Brachial Artery Variables | |
| Resting flow, cm/s | 8.4±4.9 |
| Hyperemic flow, cm/s | 54.3±21.0 |
| Baseline brachial artery, mm | 4.22±0.9 |
| Flow-mediated dilatation, % | 3.16±2.9 |
Data are presented as mean ± standard deviations, except where otherwise specified.
Profile of BP responses over time during exercise test
Unadjusted profiles for systolic and diastolic BP during various stages of the exercise test are presented in Supplemental Figure, Panels A–F. Systolic BP was higher at all exercise time-points in men (compared to women), in participants ≥60 years of age (compared to <60 years of age) and in individuals with a higher BMI category.
Relations between baseline risk factors and BP responses during exercise test
Mean blood pressure during the second stage of exercise was 166±25/75±15 mm Hg and mean heart rate was 127±19 beats per minute. Standard risk factor associations with exercise responses are summarized in Table 2. In separate multivariable models, exercise systolic BP was positively related to age, standing BP, standing heart rate, cigarette smoking, BMI and the total/HDL cholesterol ratio. For exercise diastolic BP, age, standing systolic BP, standing diastolic BP, smoking and BMI were positively associated.
Table 2.
Relations of standard Risk Factors to Second Stage Exercise BP Responses in Multivariable Models
| Exercise Systolic Blood Pressure | Exercise Diastolic Blood Pressure | |||
|---|---|---|---|---|
| β̂ (±SE) | P | β̂ (±SE) | P | |
| Male sex | 0.6±0.9 | 0.48 | 0.4±0.5 | 0.38 |
| Age | 5.3 ± 0.4 | <0.0001 | 0.6±0.3 | 0.014 |
| Standing SBP | 12.9±0.4 | <0.0001 | 1.6±0.3 | <0.0001 |
| Standing DBP | - | - | 5.1±0.3 | <0.0001 |
| Standing HR | 3.9±0.4 | <0.0001 | - | - |
| Smoking | 3.2±1.2 | 0.011 | 1.8±0.7 | 0.007 |
| Diabetes | 2.9±1.6 | 0.071 | - | - |
| BMI | 3.3±0.4 | <0.0001 | 1.3±0.2 | <0.0001 |
| Total Cholesterol/HDL | 1.7±0.4 | 0.0001 | - | - |
| Anti-HTN treatment | - | - | 1.1±0.5 | 0.050 |
| model R2 | 0.48 | 0.53 | ||
Data are estimated regression coefficient (β per SD increment continuous variables, or presence vs absence for binary ones) ± standard error (SE). Sex and age were forced into all models. Additional covariates with p<0.10 retained by backward selection are reported. All continuous variables are presented per 1 standard deviation (See Table 1 for SD of variables).
Relations between vascular stiffness and exercise BP responses
Relations of tonometry and brachial measures with exercise BP are summarized in Table 3. After adjustment for cardiovascular risk factors, we found positive associations of exercise systolic BP with carotid-femoral pulse wave velocity, MAP and central pulse pressure. Forward pressure wave amplitude was negatively associated whereas MAP were positively associated with exercise diastolic BP (Table 3). Overall, tonometry measures explained a small proportion of the variance in exercise systolic BP (partial R2=1.7%) and diastolic BP (partial R2=3.6%). For llustrative purposes, Figure 1 panels A and B show the unadjusted BP responses for individuals with carotid-femoral pulse wave velocity above versus below the median.
Table 3.
Relations of Tonometry and Brachial Artery Measures with second Stage Exercise BP Responses
| ExerciseSystolic BP | Exercise Diastolic BP | |||
|---|---|---|---|---|
| β̂ (±SE) | P | β̂ (±SE) | P | |
| Tonometry | ||||
| Carotid-femoral pulse wave velocity | 1.7±0.7 | <0.01 | - | - |
| Central pulse pressure | 2.7±0.6 | <0.0001 | - | - |
| Forward pressure wave amplitude | - | - | −1.6±0.3 | <0.0001 |
| Augmented pressure | - | - | - | - |
| Augmentation index | - | - | - | - |
| Mean arterial pressure | 1.6±0.7 | 0.02 | 2.0±0.4 | <0.0001 |
| Brachial artery dimensions | ||||
| Baseline | - | - | - | - |
| Flow-mediated dilation | −2.0±0.5 | <0.0001 | - | - |
| Brachial artery blood flow velocity | ||||
| Baseline, | 1.8±0.6 | 0.001 | - | - |
| Hyperemic | −1.7±0.6 | 0.004 | - | - |
Linear regression models for second stage systolic BP and second stage diastolic BP were adjusted for risk factors listed in Table 2. Each set of vascular function variables (in bold) was evaluated in a separate model that did not include other vascular function variables. Results are presented as estimated regression coefficients (β) per 1 SD increment in the independent variables ± standard error (SE). (See Table 1 for SD of variables)
Figure 1.
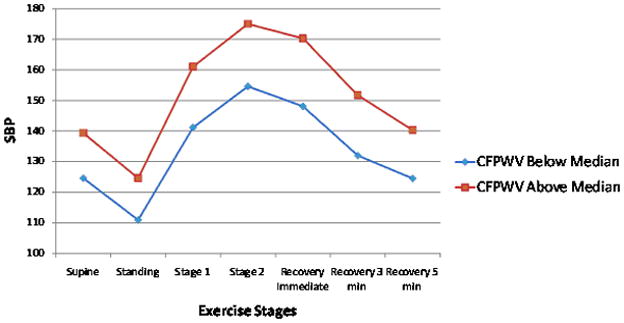
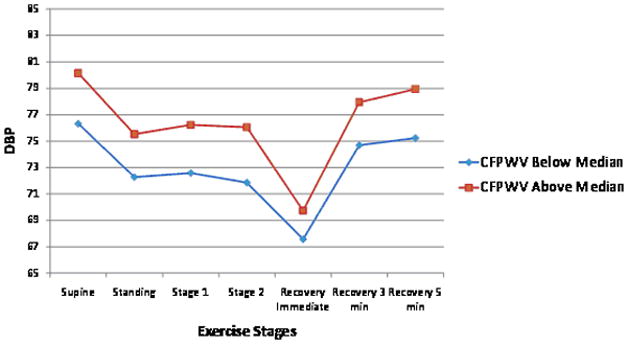
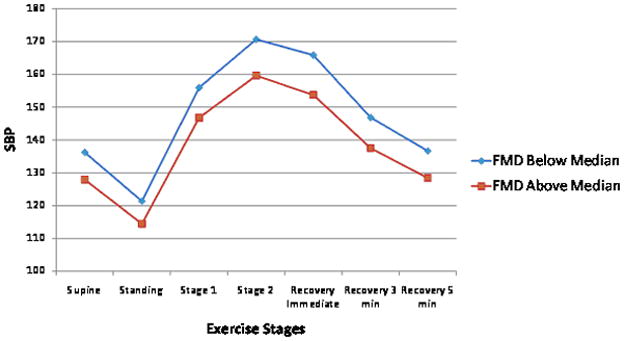
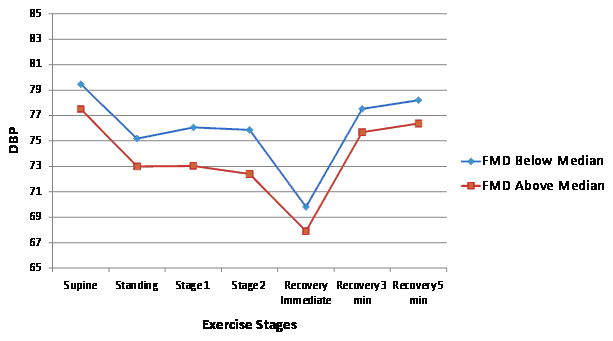
Unadjusted exercise systolic and diastolic blood pressure (BP) responses during submaximal exercise test according to higher (above median) versus lower (below median) carotid-femoral pulse wave velocity (CFPWV, Panel A and B) and flow mediated dilation percent (FMD, Panel C and D). Systolic BP standard error of the mean (SEM) varied from 0.5 to 0.9 depending on the time-point for CFPWV above and below the median. Diastolic BP SEM varied from 0.5 to 0.9 depending on the time-point for CFPWV above and below the median. Systolic BP SEM varied from 0.5 to 1.01 depending on the time-point for FMD above and below the median. Diastolic BP SEM varied from 0.3 to 0.6 depending on the time-point for FMD above and below the median.
Relations between endothelial function, brachial artery flow and exercise BP response
FMD was inversely related to exercise systolic BP after adjustment for standard risk factors (Table 3), explaining <1% of its variance. We found no significant associations between FMD and exercise diastolic BP. Both baseline and hyperemic brachial artery flow were positively associated with exercise stage 2 systolic BP but explained only ~1% of its variance. No other statistically significant relations were identified for brachial flow measures and exercise diastolic BP (Table 3). For illustrative purposes, Figure 1 panels C and D show the unadjusted BP responses for individuals with FMD above versus below the median.
Joint relations of all vascular function variables and exercise BP responses
For each exercise outcome measure, we constructed a final model that included the baseline risk factors and all vascular function variables to identify a final set of predictor variables (Table 4). For exercise systolic BP, carotid-femoral pulse wave velocity, central pulse pressure, MAP and mean baseline brachial flow velocity were positively associated, whereas FMD was negatively associated. These vascular function variables as a group explained 8.0% of the variance of exercise systolic BP. For diastolic BP, MAP was positively associated, whereas forward wave amplitude was negatively associated. The vascular function variables together explained 3.7% of the variance in exercise diastolic BP.
Table 4.
Vascular Function Variables Retained in Final Models for Exercise Systolic and Diastolic Blood Pressure
| β̂ (±SE) | P | |
|---|---|---|
| Exercise Systolic Blood Pressure | ||
| Carotid-femoral pulse wave velocity | 1.5±0.7 | 0.02 |
| Central pulse pressure | 2.8±0.6 | <0.0001 |
| Mean arterial pressure | 1.5±0.7 | 0.04 |
| Baseline brachial flow | 1.6±0.5 | 0.002 |
| Flow-mediated dilation | −1.8± 0.5 | 0.001 |
| Exercise Diastolic Blood Pressure | ||
| Forward pressure wave amplitude | −1.6±0.3 | <0.0001 |
| Mean arterial pressure | 2.0±0.4 | <0.0001 |
Discussion
Principal Findings
In our community-based sample of over 2100 individuals, we identified important relations between exercise BP responses and cardiovascular risk factors, including: age, BP, cigarette smoking, BMI, and cholesterol. In addition, our results demonstrate that measures of arterial stiffness are associated with second stage systolic BP and diastolic BP during a sub-maximal exercise test. These relations persisted after adjustment for the baseline cardiovascular risk factors and resting BP. In addition, we also found that lower levels of endothelial function, as measured by FMD, were associated with higher exercise systolic BP responses. Overall, our results implicate several vascular function measures in the BP response to exercise and may partially explain the increased cardiovascular risk associated with a hypertensive response to exercise.
In the context of the current literature
Exercise BP is an important marker of mean ambulatory BP22 and LVH12,23,24 and a predictor of future hypertension2,5,9,25 and cardiovascular events.16,26–28 Despite the importance of exercise BP as a marker of cardiovascular risk, limited data exist regarding the predictors of exercise BP in large cohorts of ambulatory individuals. Prior studies evaluating cardiovascular risk factors and exercise BP have been limited by small sample sizes29,30 and evaluation of predictors solely in women.31 Despite these limitations, our findings are consistent with previous published reports of important associations with age, smoking, BMI, resting BP and total cholesterol with exercise systolic BP.29–32 In addition to the importance of exercise systolic BP, we have previously shown that exercise diastolic BP is a predictor of cardiovascular events.16 We identified several cardiovascular risk factors that were also important predictors of exercise diastolic BP response.
While abnormal vascular function has been suggested as a possible mechanism for an exaggerated systolic BP response to exercise, few studies have systematically evaluated the relations between measures of vascular function (stiffness or endothelial function) with exercise BP responses. Tsioufis et al. evaluated 171 individuals and found that carotid-femoral pulse wave velocity was associated with an exaggerated systolic BP response to exercise but this was not a significant predictor in multivariable models.30 Conversely, Stewart et al. reported an association of reduced FMD with exercise and systolic BP, but failed to detect any association between carotid-femoral pulse wave velocity and exercise BP in a small study of 82 mildly hypertensive individuals.29 In a much larger study of over 9000 individuals, central arterial stiffness, as measured by pulse pressure, was associated with exercise systolic BP in univariate analyses; however, multivariable methods were not employed and tonometric data was not available to further evaluate this relation.33 In our study, of over 2000 participants with exercise BP, tonometric data, brachial flow velocity and FMD, we demonstrate that arterial stiffness, microvascular reactivity and endothelial function (as assessed by FMD) are significant correlates of exercise BP thereby implicating abnormal vascular function as a contributing factor in the exaggerated BP response observed with exercise.
Possible mechanisms
During normal exercise, cardiac output increases in response to the demand of working muscle due to a sympathetically-mediated increase in heart rate and stroke volume. The BP response to the increase in cardiac output during exercise is moderated, in part, by a fall in peripheral artery resistance via endothelium-dependent vasodilation and microcirculatory reserve.13,34 Chronic exposure to vascular risk factors, including cholesterol, cigarette smoking and increased BP, have been shown to promote endothelial dysfunction and arterial stiffness by reducing availability of nitric oxide35,36 and by altering the normal balance and spatial organization of arterial structural elements (including collagen and elastin).37,38 Our results suggest that a greater increase in BP during exercise is likely due to the combination of a stiffer aorta, as measured by increased carotid-femoral pulse wave velocity and higher central pressure pulsatility, and by impaired endothelial function (as measured by higher resting brachial flow, lower reactive hyperemia and reduced FMD).
Interestingly, we also found an inverse association between exercise diastolic BP and forward pressure wave velocity, however the underlying mechanism for this association is not entirely clear. It is conceivable that such an association reflects the fall in diastolic BP in response to a transient increase in cardiac load posed by acute exercise. In addition, since the model also included MAP, the association may simply indicate a lower diastolic BP in those with a higher forward wave amplitude at a given MAP.
Clinical Implications
Our findings that impaired vascular function, including increased arterial stiffness and abnormal endothelial function, are associated with increased exercise BP responses may provide an important mechanistic link between a hypertensive response to exercise, LVH and increased risk of cardiovascular events. Our observations suggest that individuals with a hypertensive response to exercise likely have underlying impaired vascular function, which limits their ability to compensate for the increased cardiac output associated with the low-moderate exercise frequently encountered during normal activities and thus resulting in frequent rises in BP. Although such individuals may have normal (or near-normal) resting BP, the frequent transient increases in BP may increase the propensity for developing LVH, and may increase the risk for future cardiovascular disease events. Furthermore, large artery stiffness, as measured by carotid-femoral pulse wave velocity, contributes to central pulse pressure, which represents the pressure directly exerted on the heart and brain.39 Increased stiffness leads to increased systolic afterload40,41 providing the stimulus for LVH and atherosclerosis.
Both arterial stiffness42–46 and impaired endothelial function47,48 are important predictors of future cardiovascular events, which may further explain the observed associations between a hypertensive response to exercise and cardiovascular events. It has been suggested that the increased pulse wave velocity and the earlier timing of the reflected wave occurring during systole, rather than diastole, may reduce coronary perfusion.39 In addition, impaired FMD may be a marker for vascular inflammation and propensity for thrombosis.49 Progressive stiffening of the aorta may also attenuate the normal interface between central and peripheral arteries, equalizing the impedance of central and peripheral arteries, reducing wave reflections and subjecting the peripheral microcirculation to potentially deleterious pulsatile energy that increases the propensity for end-organ damage.17
Despite the knowledge that a hypertensive BP response is a risk factor for future hypertension and cardiovascular events, the use of exercise testing as a screening test for pre-hypertension has not been recommended, given the low predictive value of a hypertensive response for subsequent cardiovascular events.34 Furthermore, despite recent evidence for improved risk reclassification with non-invasive measures,17,48 the use of FMD or tonometry as routine screening tests is not currently recommended nor widely available. However, because exercise testing is a common test that is performed routinely, a hypertensive response to exercise may represent an easily available clinical marker of increased vascular stiffness or impaired endothelial function. Further research exploring the value of exercise BP as a preliminary assessment of vascular function may be warranted.
Strengths and limitations
A major strength of our study is the measurement of exercise BP, tonometry and brachial artery flow measurements on the same morning in each participant, thereby allowing for an unbiased assessment of the cross-sectional relations between these variables. In addition, our large community-based sample of individuals with detailed cardiovascular risk factor data allowed for relations to be explored in ambulatory individuals and permitted multivariable modeling of exercise BP to elucidate the effects of vascular function on exercise BP with adequate statistical power. Our study also had a number of limitations which deserve mention. First, given the observational nature of our study, causality cannot be inferred for any of the reported associations. Second, because exercise BP responses and vascular function measurements occurred years after enrollment into the Offspring cohort and were conditional on survival to the 7th examination cycle of the study, our sample may be biased toward a healthier group of participants. Third, participants underwent a submaximal exercise test and thus BP responses do not represent peak BP responses, which could potentially result in underestimation of the impact of abnormal vascular function on maximal exercise BP responses. However, BP measured during submaximal exercise correlates well with ambulatory BP and may be more representative measure of the physiological rise in BP with exercise. Fourth, our sample was predominantly white of European descent; reported results may not be generalizable to other ethnicities.
Conclusions
In our investigation of a moderate-sized community-based sample, we demonstrate that cardiovascular risk factors (including resting BP, smoking, cholesterol and BMI) are important correlates of BP responses during a sub-maximal exercise test. Most importantly, we have demonstrated that increased arterial stiffness and impaired endothelial function are associated with exercise BP supporting the notion that abnormal vascular function may contribute to exaggerated BP responses to exercise.
Supplementary Material
Clinical Summary.
Exercise blood pressure (BP) is an important marker of cardiovascular disease (CVD) risk that is associated with incident hypertension, myocardial infarction and cardiovascular mortality in individuals without overt coronary artery disease. It has been hypothesized that individuals with a hypertensive response to exercise may have abnormalities in vascular function that limit the compensatory mechanisms necessary to dampen the rise in blood pressure with exercise. Using data from over 2000 individuals from the Framingham Offspring Study, we demonstrate that increases in blood pressure during low intensity exercise (i.e. walking) is associated with both endothelial dysfunction and increased aortic stiffness. Our findings suggest that impaired vascular function may contribute to exaggerated BP responses during activities of daily living, resulting in repetitive increments in load on the heart and vessels and increased cardiovascular disease risk. Our results provide an important mechanistic link between the hypertensive response to exercise, left ventricular hypertrophy and the associated increased risk of cardiovascular events.
Acknowledgments
Sources of Funding: This work was supported by the Ellison Medical Foundation (SC) and the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and the following grants: 6R01-NS 17950, R01HL080124 (RSV). Dr. Thanassoulis was supported by a research fellowship from the Canadian Institute for Health Research and the Fonds de Recherche en Santé du Québec.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 2.Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high-normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high-normal blood pressure. J Am Coll Cardiol. 2000;36:1626–1631. doi: 10.1016/s0735-1097(00)00903-7. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima M, Miura K, Kido T, Saeki K, Tamura N, Matsui S, Morikawa Y, Nishijo M, Nakanishi Y, Nakagawa H. Exercise blood pressure in young adults as a predictor of future blood pressure: a 12-year follow-up of medical school graduates. J Hum Hypertens. 2004;18:815–821. doi: 10.1038/sj.jhh.1001749. [DOI] [PubMed] [Google Scholar]
- 4.Sharabi Y, Ben-Cnaan R, Hanin A, Martonovitch G, Grossman E. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J Hum Hypertens. 2001;15:353–356. doi: 10.1038/sj.jhh.1001157. [DOI] [PubMed] [Google Scholar]
- 5.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 6.Laukkanen JA, Kurl S, Rauramaa R, Lakka TA, Venalainen JM, Salonen JT. Systolic blood pressure response to exercise testing is related to the risk of acute myocardial infarction in middle-aged men. Eur J Cardiovasc Prev Rehabil. 2006;13:421–428. doi: 10.1097/01.hjr.0000198915.83234.59. [DOI] [PubMed] [Google Scholar]
- 7.Laukkanen JA, Kurl S, Salonen R, Lakka TA, Rauramaa R, Salonen JT. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle-aged men. Hypertension. 2004;44:820–825. doi: 10.1161/01.HYP.0000148460.95060.f2. [DOI] [PubMed] [Google Scholar]
- 8.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic Blood Pressure Response to Exercise Stress Test and Risk of Stroke. Stroke. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- 9.Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol. 1999;83:371–375. doi: 10.1016/s0002-9149(98)00871-6. [DOI] [PubMed] [Google Scholar]
- 10.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- 11.Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: comparing office, ambulatory and exercise blood pressures. J Hum Hypertens. 2001;15:627–633. doi: 10.1038/sj.jhh.1001229. [DOI] [PubMed] [Google Scholar]
- 12.Ren JF, Hakki AH, Kotler MN, Iskandrian AS. Exercise systolic blood pressure: a powerful determinant of increased left ventricular mass in patients with hypertension. J Am Coll Cardiol. 1985;5:1224–1231. doi: 10.1016/s0735-1097(85)80029-2. [DOI] [PubMed] [Google Scholar]
- 13.Lim PO, MacFadyen RJ, Clarkson PB, MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intern Med. 1996;124:41–55. doi: 10.7326/0003-4819-124-1_part_1-199601010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Dawber TR, Meadors GF, Moore FEJ. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2008;101:1614–1620. doi: 10.1016/j.amjcard.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local Shear Stress and Brachial Artery Flow-Mediated Dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical Correlates and Heritability of Flow-Mediated Dilation in the Community. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 22.Lim PO, Donnan PT, MacDonald TM. How well do office and exercise blood pressures predict sustained hypertension? A Dundee Step Test Study. J Hum Hypertens. 2000;14:429–433. doi: 10.1038/sj.jhh.1001041. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, Brown J, Zoltick J, Fletcher RD. Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Intern Med. 1990;112:161–166. doi: 10.7326/0003-4819-112-3-161. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Levy D, Anderson KM, Plehn JF. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study. Ann Intern Med. 1992;116:203–210. doi: 10.7326/0003-4819-116-3-203. [DOI] [PubMed] [Google Scholar]
- 25.Manolio TA, Burke GL, Savage PJ, Sidney S, Gardin JM, Oberman A. Exercise blood pressure response and 5-year risk of elevated blood pressure in a cohort of young adults: the CARDIA study. Am J Hypertens. 1994;7:234–241. doi: 10.1093/ajh/7.3.234. [DOI] [PubMed] [Google Scholar]
- 26.Filipovsky J, Ducimetiere P, Safar M. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- 27.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24:56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- 28.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise Blood Pressure Predicts Mortality From Myocardial Infarction. Hypertension. 1996;27:324–329. doi: 10.1161/01.hyp.27.3.324. [DOI] [PubMed] [Google Scholar]
- 29.Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP, Ouyang P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. doi: 10.1016/S0895-7061(03)01003-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsioufis C, Dimitriadis K, Thomopoulos C, Tsiachris D, Selima M, Stefanadi E, Tousoulis D, Kallikazaros I, Stefanadis C. Exercise Blood Pressure Response, Albuminuria, and Arterial Stiffness in Hypertension. The American Journal of Medicine. 2008;121:894–902. doi: 10.1016/j.amjmed.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil. 2002;22:178–183. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Criqui M, Haskell W, Heiss G, Tyroler H, Green P, Rubenstein C. Predictors of systolic blood pressure response to treadmill exercise: the Lipid Research Clinics Program Prevalence Study. Circulation. 1983;68:225–233. doi: 10.1161/01.cir.68.2.225. [DOI] [PubMed] [Google Scholar]
- 33.Jae SY, Fernhall B, Lee M, Heffernan KS, Lee MK, Choi YH, Hong KP, Park WH. Exaggerated blood pressure response to exercise is associated with inflammatory markers. J Cardiopulm Rehabil. 2006;26:145–149. doi: 10.1097/00008483-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Le VV, Mitiku T, Sungar G, Myers J, Froelicher V. The blood pressure response to dynamic exercise testing: a systematic review. Prog Cardiovasc Dis. 2008;51:135–160. doi: 10.1016/j.pcad.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM. 2002;95:423–429. doi: 10.1093/qjmed/95.7.423. [DOI] [PubMed] [Google Scholar]
- 36.Gilligan D, Panza J, Kilcoyne C, Waclawiw M, Casino P, Quyyumi A. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- 37.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of Alterations in Structure and Function of Elastin in the Arterial Media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 38.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. doi: 10.1161/01.HYP.0000164580.39991.3d. [DOI] [PubMed] [Google Scholar]
- 39.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large A. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. Curr Hypertens Rep. 2004;6:436–441. doi: 10.1007/s11906-004-0037-1. [DOI] [PubMed] [Google Scholar]
- 41.Saeki A, Recchia F, Kass DA. systolic flow augmentation in hearts ejecting into a model of stiff aging vasculature. Influence on myocardial perfusion-demand balance. Circ Res. 1995;76:132–141. doi: 10.1161/01.res.76.1.132. [DOI] [PubMed] [Google Scholar]
- 42.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 43.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 44.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 45.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Health ABCS. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 46.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 47.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 48.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson TJ. Prognostic significance of brachial flow-mediated vasodilation. Circulation. 2007;115:2373–2375. doi: 10.1161/CIRCULATIONAHA.107.697045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


