Abstract
Oxysterols are oxidized species of cholesterol that are derived from exogenous (e.g. dietary) and endogenous (in vivo) sources. Oxysterols play critical roles in normal physiologic functions as well as in pathophysiologic processes in a variety of organ systems. This review provides an overview of oxysterol biology from the vantage point of the biliary system. Several oxysterols have been identified in human bile in the context of biliary tract infection and inflammation. This finding has led to investigations regarding the potential pathophysiologic significance of biliary oxysterols in diseases affecting the biliary system, with an emphasis on cholangiocarcinoma. Emerging evidence implicates specific oxysterols in the development and progression of this malignancy. This review will summarize the literature on oxysterols in the biliary system and discuss how the accumulated evidence contributes to a hypothesis describing the molecular basis of cholangiocarcinogenesis.
Keywords: bile, bile acids, biliary tract, cholangiocarcinoma, cholangiocytes, cholesterol, gallbladder, gallstones, Opisthorchis viverrini, oxysterols
Cholesterol is capable of being oxidized through enzymatic and nonenzymatic pathways. The resulting oxidation products are broadly defined as oxysterols [1]. The term ‘oxysterol’ can be defined as ‘any sterol molecule that is oxidized’, and thus includes not only cholesterol oxidation products, but also phytosterol and shellfish sterol oxidation products [2]. Phytosterols, similar to cholesterol in structure with the major difference residing in the side chain [3], are found in plants and are thus present in the diet. Furthermore, bile acids can also be included in the term ‘oxysterol,’ as these compounds are cholesterol oxidation products. The same definition can apply to cholesterol oxidation products that function as steroid hormones. Despite this justification for a broad definition, the term ‘oxysterol’ as used in the literature generally carries the more narrow definition of cholesterol oxidation products excluding bile acids and steroid hormones. ‘Oxysterol’ as used in this review will adhere to this narrower definition.
The purpose of this review is to provide an overview of the physiologic and pathophysiologic roles of oxysterols from the vantage point of the biliary system. The biliary system, comprising the intra- and extra-hepatic bile ducts and the gallbladder, is the route by which cholesterol (and its major oxidation product, bile acids) exits the liver and thereby leaves the body (although a significant portion of cholesterol and bile acids are reabsorbed in the small intestine). The concentrations of cholesterol and bile acids are high in the biliary system, particularly in the gallbladder where bile is concentrated. A nascent body of work implicates oxysterols in several pathophysiologic processes that affect the biliary system, including the formation of pigment and mixed cholesterol gallstones, and the development and progression of cancer of the bile ducts, or cholangiocarcinoma. Thus, the biliary system provides an additional point of reference by which the vast and unwieldy body of literature regarding oxysterol biology can be examined.
Origin & physiological significance of oxysterols
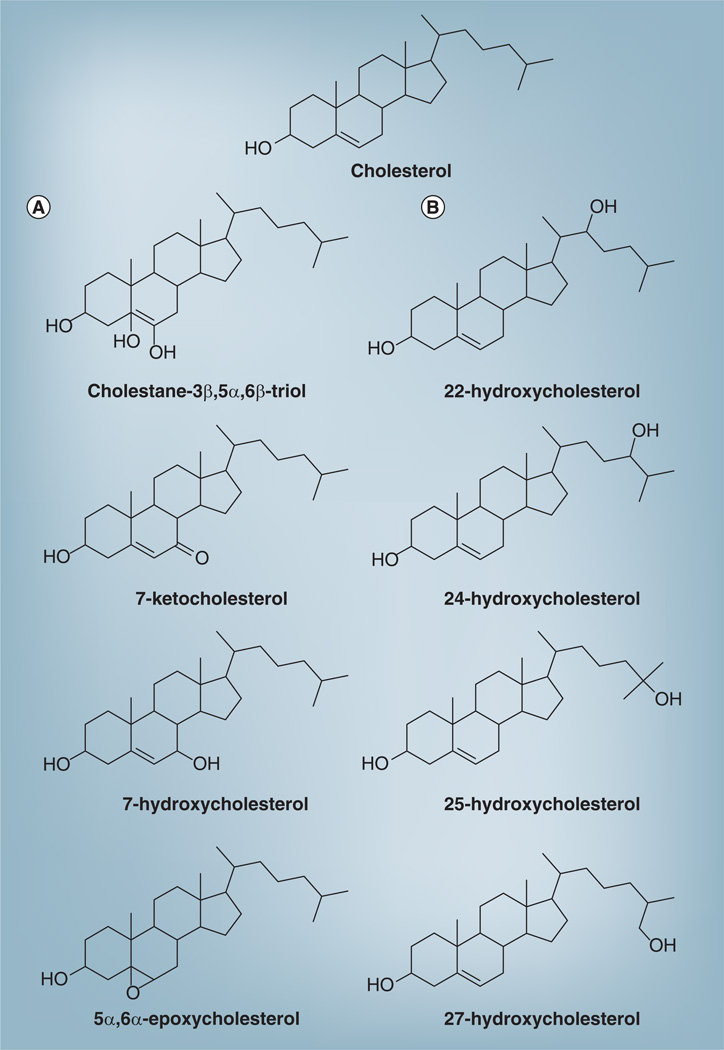
Cholesterol is composed of a cluster of four hydrocarbon rings (designated A, B, C and D), a hydrocarbon side chain and a hydroxyl group at position three. Oxidation can occur on the ring structure and on the side chain. The double bond present on the B ring is a target of free radical attacks and therefore the hydrogen atoms at positions four, five, six and seven are the most vulnerable to spontaneous oxidation. Auto-oxidation on the B ring can lead to the formation of 7α- and 7β-hydroxycholesterol; 7-ketocholesterol; 5α, 6α-epoxycholesterol;5β, 6β-epoxycholesterol and cholestan-3β, 5α, 6β-triol (Figure 1) [1].
Figure 1. Cholesterol and several common oxysterols.
(A) Oxysterols derived from oxidation in one of the sterol rings; (B) oxysterols derived from side-chain oxidation.
Adapted from [1] with permission.
Many different oxysterols have been found in human biological samples. These include not only the species listed in the previous paragraph, but also those oxysterols generated by side-chain oxidation, such as 24-hydroxycholesterol, 25-hydroxycholesterol and 26-hydroxycholesterol (Figure 1) [2]. These and other oxysterols have been implicated in a host of normal biological functions, as well as in pathological processes involving a variety of organ systems. Oxysterols have been implicated in the initiation and progression of several chronic diseases, including atherosclerosis, neurodegenerative diseases (e.g. Alzheimer’s and Parkinson’s disease, and multiple sclerosis), osteoporosis and age-related macular degeneration [4,5]. The mechanisms whereby oxysterols trigger apoptosis, activate inflammatory pathways and modulate lipid homeostasis are areas of active investigation [6].
The literature on the normal physiological roles of oxysterols is vast [7,8], while the literature on the pathophysiologic effects of oxysterols is growing rapidly, and encompasses a wide variety of in vitro and in vivo studies involving cell lines, animal studies and human observational studies. Numerous reviews have summarized and put this literature into perspective. For example, Smith provided a summary of the state of knowledge of cholesterol auto-oxidation in 1981 [8]. Schroepfer provided a breathtakingly complete summary of the state of oxysterol research in 1999 in a review article that included over 1200 references [7]. More recent reviews have provided perspectives on oxysterols from a variety of angles, and add to our understanding of the importance of these sterols in normal physiology and their role in a variety of diseases [2,6,9–12]. Despite this wealth of information, much remains to be discovered about the mechanisms of oxysterol-induced pathophysiology in various organ systems.
Sources of oxysterols
Exogenous sources of oxysterols are dietary. Foods containing cholesterol are susceptible to oxidation before ingestion, especially those foods that have been exposed to heat in the presence of oxygen or stored for long periods and subjected to exposure to sunlight and oxygen. The literature with respect to the specific types and amounts of oxysterols found in common foods has recently been reviewed [2].
Endogenous sources also exist and numerous pathways of oxysterol generation have been elucidated. Sterols can be oxidized by enzymatic reactions involving P450 enzymes or nonenzymatic reactions that involve reactive oxygen species (ROS) or reactive nitrogen species. The location and number of oxygenated functional groups on the sterol backbone are quite variable, so that keto-, hydroxyperoxy- and epoxy- forms can be generated. As a general rule, nonenzymatic pathways of oxysterol generation mainly affect the sterol ring, while enzymatic pathways proceed via reactions in the side chain. Exceptions to this rule exist: for example, both pathways can generate 25-hydroxycholesterol and 7α-hydroxycholesterol.
Enzymatically derived oxysterols are important intermediates in steroid and bile acid synthesis [9]. Enzymatic pathways of oxysterol generation involve P450 enzymes such as 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the classical bile acid synthesis pathway that leads to the production of 7α-hydroxycholesterol; and sterol 27-hydroxylase (CYP27A1), the key enzyme in the alternative bile acid synthesis pathway, leading to the production of 27-hydroxycholesterol [11]. CYP7A1 is expressed only in the liver and has limited substrate specificity. CYP27A1 is broadly expressed in tissues and generates several oxysterols. In the liver, its major role is to initiate the side chain oxidation of 7α-hydroxylated intermediates during their metabolism to bile acids. In other tissues, CYP27A1 generates 25(R), 26-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid, which normally circulate in plasma [13], and which can be further metabolized via oxysterol 7α-hydroxylase (CYP7B1) that is also widely expressed [14]. 24-, 25- and 27-hydroxycholesterol are generated by enzymatic side-chain hydroxylation of cholesterol. Cholesterol 25-hydroxylase is the enzyme responsible for generating 25-hydroxycholesterol and is expressed at very low levels. Its product regulates SREBP, a key transcriptional factor that regulates cholesterol synthesis [15]. Another P450 enzyme, CYP46A1, expressed only in the brain, catalyzes the formation of 24(S)-hydroxycholesterol [16,17].
Certain oxysterols are produced by non-enzymatic oxidation (or auto-oxidation) through ROS and reactive nitrogen species [18]. Examples of ROS include hydroxyl radical (OH), hydrogen peroxide (H2O2), singlet oxygen (1O2) and ozone (O3). Representative oxysterols in this group are 7-ketocholesterol and 7α/β-hydroxycholesterol. The attack on cholesterol by ROS targets an allylic hydrogen atom at position seven of the sterol ring. The radical generated can react with oxygen to form a cholesterol peroxyl radical, which further reacts by abstracting hydrogen and generating the relatively stable cholesterol 7α/β hydroxyperoxides [16]. Further nonenzymatic oxidation generates 7α/β-hydroxycholesterols and 7-ketocholesterol, which are the major nonenzymatically generated oxysterols found in most tissues. Nonenzymatic cholesterol oxidation occurs within cell membranes and in circulating lipoproteins [18]. Nonenzymatically derived oxysterols have been detected in various sites including human plasma [19], retinal tissue [20], hepatic bile and gallstones [21,22]. Indeed, oxysterols generated through this mechanism have been proposed to be a marker of oxidative stress in vivo [23].
Oxysterol uptake & transport in vivo
Cholesterol, phytosterols and oxysterols ingested in food or excreted by the liver into bile can be absorbed in the human small intestine through molecularly defined absorption pathways in enterocytes. Cholesterol absorption is modulated by ABCG5/ABCG8, which effluxes a proportion of absorbed cholesterol back into the small intestinal lumen; and by NPC1L1 (the target of the cholesterol absorption inhibitor ezetimibe), which drives cholesterol uptake in enterocytes. A significant proportion of absorbed phytosterols is secreted back into the intestinal lumen by ABCG5/ABCG8 [24]. Conversely, in patients with mutations in ABCG5/ABCG8, significantly higher proportions of phytosterols are absorbed into plasma, leading to phytosterolemia. The extent to which these molecular mechanisms also participate in oxysterol absorption by enterocytes is not well defined, given the diversity of oxysterol species and their relatively low levels in the diet. Most oxysterols measured in plasma and tissues are esterified [25], suggesting that they are substrates for acyl-CoA:cholesterol acyltransferase in cells and lecithin-cholesterol acyltransferase in the circulation. In a process analogous to that for cholesterol, acyl-CoA:cholesterol acyltransferase in enterocytes esterifies oxysterols, which are incorporated into chylomicrons and later to VLDL, LDL and HDL. 7α/β-hydroxycholesterols and 7-ketocholesterol are the oxysterols most often identified in lipoproteins; their concentrations are greater than those found in test diets (i.e. experimental diets fed to subjects), suggesting they are also synthesized endogenously [26].
Physiologic functions of oxysterols
Excluding the well-defined physiologic roles of bile acids and steroid hormones, specific oxysterols have been implicated in physiologic processes. Oxysterols can exert effects through biophysical interactions with cellular membranes and through specific interactions with proteins that follow the paradigm of receptor-ligand binding. For the former type of interaction, apoptotic and cytotoxic effects on cells can result [6]. For the latter, specific signal transduction pathways can be activated which have important roles in a variety of critical cellular functions, including cholesterol homeostasis, morphogenesis and cell-mediated immunity. The following sections provide a brief summary of these functions. Details regarding these physiologic functions are provided in the references cited.
Oxysterols as ligands for the nuclear receptor liver X receptor
Liver X receptor (LXR) functions as a cholesterol sensor. LXR is essential for the maintenance of cholesterol homeostasis, thereby protecting cells from the toxic effects of cellular cholesterol overload. LXR regulates the expression of proteins involved in cholesterol efflux and transport. The most potent of these ligands are the oxysterols 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol and 24(S), 25-epoxycholesterol [9].
Oxysterols as mediators of cholesterol homeostasis
Specific oxysterols play important roles in the control of expression of genes involved in cholesterol synthesis. The cellular pathways that regulate cholesterol synthesis have been delineated and involve a key role for the transcription factor SREBP. SREBP is produced in the endoplasmic reticulum (ER) and controls the expression of cholesterol synthesis genes. SREBP is regulated through binding to SCAP and INSIG proteins. SCAP, acting as a cholesterol sensor, blocks SREBP when cellular cholesterol levels are high. The SCAP–SREBP complex is retained in the ER by binding to INSIG proteins, thereby inhibiting gene transcription [27]. Conversely, if cholesterol levels are low, SCAP releases SREBP and transcription of genes involved in cholesterol synthesis is stimulated. Certain oxysterols have been found to regulate this process. Specifically, 24(S), 25-epoxycholesterol can bind to INSIG proteins, which in turn causes INSIG–SCAP binding that prevents SREPB exit from the ER and thereby inhibits the synthesis of cholesterol [28]. Another mechanism involves 27-hydroxycholesterol, which degrades HMG–CoA reductase, and thereby inhibits cholesterol production [29].
Oxysterols as modulators of hedgehog signaling & morphogenesis
Cholesterol is covalently bonded to the carboxy terminus of Sonic hedgehog [30]. The latter is a 45 kDa protein that undergoes processing to a 20 kDa signaling molecule that becomes lipophilic because of the ester linked cholesterol molecule at the C terminus and a covalently linked palmitic acid at the N terminus [31]. Cholesterol is thought to be important for anchoring Sonic hedgehog to the membrane of responsive cells. Certain oxysterols impact this signaling pathway [32]. 20(S)- and 22(S)-hydroxycholesterol-enhanced induction of Sonic hedgehog target genes [33]. 25-hydroxycholesterol was required for maximal hedgehog signaling [34,35]. The effect of the most potent oxysterol implicated in hedgehog signaling, 20(S)-hydroxycholesterol, was found to be exquisitely stereoselective and functioned through Smoothened [36].
Oxysterols as immune cell mediators
Oxysterol-activated signaling pathways have been implicated in immune cell migration. The oxysterol 7α,25-dihydroxycholesterol is a potent and selective agonist for the G-protein-coupled receptor EBI2, which is required for humoral immune responses [37,38].
Physiologic roles of oxysterol-binding proteins
Oxysterol-binding proteins (OSBPs)/OSBP-related proteins comprise a family of 27 genes encoded in the human genome that have been implicated in sterol signaling and/or sterol transport [39,40]. Members of this family are highly conserved from yeast to humans and share cholesterol and/or oxysterol-binding motifs that bind oxysterols and/or cholesterol and transfer these sterols between the ER and Golgi compartments. Sterol binding by OSBPs is linked to the activation of signaling pathways and interactions with the cytoskeleton [3,9]. Consequently, downstream effects of sterol binding include effects on cholesterol secretion pathways, lipid homeostasis, vesicular transport, nonvesicular sterol transfer, and cell growth and differentiation [41].
Pathogenic effects of oxysterols
Several recent reviews describe the mechanisms involved in the pathophysiologic effects of oxysterols, with implications for diseases as diverse as atherosclerosis, osteoporosis, multiple sclerosis, Alzheimer’s disease, cancers in various organ systems and Parkinson’s disease [6,9,42]. This literature is heterogeneous with respect to the types of methodologies used, the doses and types of oxysterols studied and the organ systems investigated. A critical appraisal of the literature on the pathophysiologic role of oxysterols must include an appreciation of the minute concentrations of oxysterols found in vivo, the likely transient existence of free oxysterol species in vivo, and the relative paucity of knowledge regarding the precise cellular concentrations and bioavailability of various oxysterols in different organ systems. Furthermore, intracellular oxysterol levels are likely to be more important than extracellular levels (e.g., in plasma or bile), but the intracellular concentrations of oxysterols found in vivo are difficult to ascertain and are likely modulated by binding to OSBPs/OSBP-related proteins [43]. These methodologic shortcomings are illustrated by the discrepancy with respect to oxysterols used for in vitro studies and oxysterols that have been identified in vivo. The in vitro literature, using various cell lines, is replete with studies using 25-hydroxycholesterol or 7-ketocholesterol. These two oxysterols, however, were not found to be physiologically relevant in a human study in which artifactual generation of oxysterols via auto-oxidation was avoided [44]. 25-hydroxycholesterol was not detected in plasma and the concentration of 7-ketocholesterol was one order of magnitude less than that of 25(R), 26-hydroxycholesterol, the most abundant oxysterol measured [44]. This observation highlights the need for the use of naturally occurring oxysterols rather than available surrogates that may not be truly representative of in vivo effects. In addition, this observation indicates the need to identify and quantitate oxysterol species more accurately in vivo [10], particularly in intracellular compartments.
Conversion of certain oxysterols through sulfation reactions can affect oxysterol function in a beneficial manner. For example, the sulfotransferase SULT2B1b sulfonates 7-ketocholesterol and abrogates its cytotoxic effects [45]. In hepatocytes, the oxysterol 25-hydroxycholesterol and its sulfated derivative 25-hydroxycholesterol-3-sulfate regulate lipid metabolism and inflammation in a diametrically opposing fashion through LXR–SREBP-1c and IκB–NFκB signaling pathways [46]. This sulfation reaction, which also occurs via SULT2B1b, occurs in human aortic endothelial cells and serves to maintain intracellular lipid homeostasis [47]. The same sulfated oxysterol attenuates the inflammatory response via PPARγ signaling in human macrophages [48]. In hepatocytes, 25-hydroxycholesterol-3-sulfate significantly decreased serum and lipid levels in mouse models of nonalcoholic fatty liver disease, suggesting an important role in regulation of lipid biosynthesis in the liver [49]. Thus, this emerging body of evidence supports the notion that sulfation is a mechanism that can counteract the harmful intracellular effects of specific oxysterols.
Pathophysiologic effects of oxysterols in the biliary tract
Gallstones form through the concerted action of multiple factors such as biliary cholesterol hypersecretion, gallbladder hypomotility, gallbladder mucus hypersecretion, chronic inflammation in the gallbladder, genetic factors such as Lith genes and mutations in the cholesterol efflux transporter ABCG8, and lifestyle and dietary factors [50,51]. Recent evidence suggests that oxysterols also play a role in the formation of pigmented and mixed cholesterol gallstones.
Oxysterols in human bile & gallstones
Fresh human bile and gallstones were initially reported to contain two oxysterols, cholesta-4, 6-diene-3-one and cholest-4-ene-3-one [21]. When more than a hundred human gallstones were analyzed by gas chromatography/mass spectrometry, concentrations of these oxysterols were highest in pigment gallstones, where bacterial DNA was most abundant [52].
These observations suggested that biliary oxysterols are associated with the presence of bacteria and may be generated as a consequence of the inflammatory response to bacterial infection of the biliary tract. This hypothesis was supported by a follow-up study performed on hepatic bile obtained from eight patients with biliary tract diseases, in which multiple different species of oxysterols were identified and quantitated [22]. Bile samples were differentiated into uninfected and infected bile, and analyses were also performed on gallstones and gallbladder bile. Oxysterols isolated from hepatic bile included 7α-hydroxycholesterol, 7β-hydroxycholesterol, cholestan-3β, 5α, 6β-triol, 25-hydroxycholesterol, 26-hydroxycholesterol, 7-ketocholesterol and 7α-hydroxy-4-cholestene-3-one. Levels of 7α-hydroxycholesterol and 7β-hydroxycholesterol were increased in infected bile. Serum C-reactive protein levels, a marker of systemic inflammation, correlated positively with biliary levels of 7α-hydroxycholesterol, 7β-hydroxycholesterol, cholestan-3β, 5α, 6β-triol, 7α-hydroxy-4-cholesten-3-one and 7-ketocholesterol. Several oxysterols were found in gallstones, including 3-keto-cholest-4-ene, 3-keto-cholesta-4, 6-diene and 7-ketocholesterol [22].
In a subsequent in vitro study, incubation of human leukocytes with model bile in the presence of bacterial lipopolysaccharide resulted in increased concentrations of oxysterols [53]. Model or human bile incubated with lipopolysaccharide produced a variety of oxysterols, including 3-ketocholest-4-ene, 3-keto-cholesta-4, 6-diene, 7β-hydroxycholesterol, 7-ketocholesterol and 7-ketocholesta-3, 5-diene [53]. Taken together, the results from this series of studies suggest that during bacterial infection of the biliary tract, biliary cholesterol can be converted to a variety of oxysterols.
Functional consequences of exposure to biliary oxysterols for biliary epithelial cells
The finding of multiple different species of oxysterols in bile and the correlation between oxysterol types and concentrations with bacterial infection of the biliary tract led to questions regarding their pathophysiologic significance. In vitro studies were performed to begin to understand the interactions between biliary oxysterols and the biliary epithelium. A fundamental question was whether low concentrations of biliary oxysterols altered biliary epithelial cell integrity or function. Therefore, the effects of biliary oxysterols were investigated in a cell culture model of the biliary epithelium using dog gallbladder epithelial cells [54]. Cells were exposed to culture media containing the oxysterols cholest-4-en-3-one cholesta-4, 6-dien-3-one and cholesta-3, 5-dien-7-one. The culture media was formulated so as to model the predominant components in bile and therefore contained cholesterol, the bile acid taurocholate and the phospholipid, phosphatidylcholine. Oxysterol exposure resulted in apoptosis of cultured biliary epithelial cells [55]. Apoptosis involved cytochrome C release, implicating a mitochondrial pathway [55]. The relatively more hydrophobic bile acid taurodeoxycholic acid and the hydrophilic bile acid tauroursodeoxycholic acid modulated oxysterol-induced apoptosis. Taurodeoxycholic acid enhanced, and tauroursodeoxycholic acid diminished, oxysterol-induced apoptosis [56], suggesting that the composition of bile acids in bile could modify oxysterol-induced apoptosis in these cells.
Modulation by bile acids of oxysterol-induced apoptosis of biliary epithelial cells also suggests that a more broad functional relationship may exist between the biliary tract bile acid profile and biliary oxysterols. We can speculate that bile acids, acting via the bile acid receptor TGR5 and via activation of the nuclear hormone receptor FXR (both of which are expressed in biliary epithelial cells) could protect against oxysterol-induced effects. In this scenario, the biliary milieu, as represented by the relative compositions of bile acids, could provide a means to defend against threats to the integrity and function of the biliary epithelium [57]. Activation of TGR5 could lead to downstream signaling events that are protective through anti-inflammatory effects, such as blockade of NFκB as demonstrated in hepatocytes, that serve to maintain immune system homeostasis [58,59]. Integrity of the biliary epithelial layer in response to oxysterol exposure could be modulated by the relative abundance of bile acids in bile, as reported with intestinal epithelia [60].
A differential effect of oxysterols was also noted with respect to mucin secretion, an important cellular function of gallbladder epithelial cells that has been implicated in cholesterol gallstone pathogenesis [50]. Cholestan-3β, 5α, 6β-triol suppressed taurodeoxycholic acid-induced mucin secretion, an effect that was not observed with 7-ketocholesterol [61]. These results suggested that biliary oxysterols were not functionally equivalent, and induced differential effects on biliary epithelial cell integrity and function.
Biliary tract oxysterols & cholangiocarcinoma: casual association or causal connection?
The finding that certain biliary oxysterols induced apoptosis in cultured biliary epithelial cells led to a clinically relevant question: could biliary oxysterols be implicated in the development and progression of cancer of the biliary system? Cholangiocarcinoma can affect the intra- or extra-hepatic biliary system. While the incidence of extrahepatic cholangiocarcinoma is decreasing in western countries, that of intrahepatic cholangiocarcinoma is rising [62]. Risk factors for cholangiocarcinoma include primary sclerosing cholangitis, chronic hepatitis B and C virus infection, exposure to the radiologic contrast agent Thorotrast, and chronic infection with the liver fluke Opisthorchis viverrini [63]. Chronic biliary tract infection with O. viverrini, acquired through the ingestion of certain types of freshwater fish, leads to the development of cholangiocarcinoma. This association is so well-established that this organism has been classified as a class I carcinogen by WHO. The highest incidence of chronic infection with O. viverrini is in Northeastern Thailand; not coincidentally, the world’s highest incidence of cholangiocarcinoma is also in this region [64]. A focused effort to understand the pathophysiologic basis of the origin and progression of cholangiocarcinoma in the setting of O. viverrini infection has resulted in significant advances in our understanding of the cellular and molecular basis of cholangiocarcinogenesis [65,66]. This effort has been aided by the development of a hamster model of O. viverrini-induced cholangiocarcinoma, along with insights gained from the study of cholangiocarcinoma in humans chronically infected with this parasite [67–70]. Recent findings provide intriguing insights into a potentially important role for biliary oxysterols in the development and progression of cholangiocarcinoma in both the hamster model and in humans.
OSBPs & Opisthorchis viverrini-induced cholangiocarcinoma
The hamster model of cholangiocarcinoma involves ingestion of O. viverrini metacerceriae in concert with exposure to the carcinogen N-nitrosodimethylamine [67]. This potent combination reliably leads to the development of cholangiocarcinoma over a 24-week period. In this model, OSBPL-8 was overexpressed [68]. Furthermore, in human O. viverrini-induced cholangiocarcinoma, certain OSBPs were increased. Specifically, significantly increased levels of OSBPL-7 mRNA were observed in human tumor compared with nontumor liver tissues. Higher levels of OSBP2 and OSBPL-7 mRNA were found in blood samples from cholangiocarcinoma patients compared with healthy controls. These findings suggest that OSBP2 and OSBPL-7 might serve as serum markers of cholangiocarcinoma metastasis [71]. More broadly, these findings lead to questions regarding the role of the ligands of these cytosolic OSBPs, that is, specific oxysterols, in the development and progression of cholangiocarcinoma. Thus, the significance of these findings might be in providing insights into the molecular basis of cholangiocarcinoma development.
Evidence for a molecular basis for oxysterol-mediated cholangiocarcinogenesis
To this end, further investigation using the hamster model showed that while five oxysterols were present in bile, two of these (cholestan-3β, 5α, 6β-triol and 3 keto-cholest-4-ene) were present at significantly higher levels in the livers of hamsters with O. viverrini-induced cholangiocarcinoma compared with control livers. A human cholangiocyte cell line called MMNK-1, immortalized by transduction with SV40T and hTERT, was then used to investigate the cellular effects of these two oxysterols. Both oxysterols induced apoptosis via a mitochondrial pathway. These oxysterols also induced the formation of the DNA adducts 1,N6-etheno-2’-deoxyadenosine, 3, N4-etheno-2’-deoxycytidine and 8-oxo-7, 8-dihydro-2’deoxyguanosine in MMNK-1 cells [72].
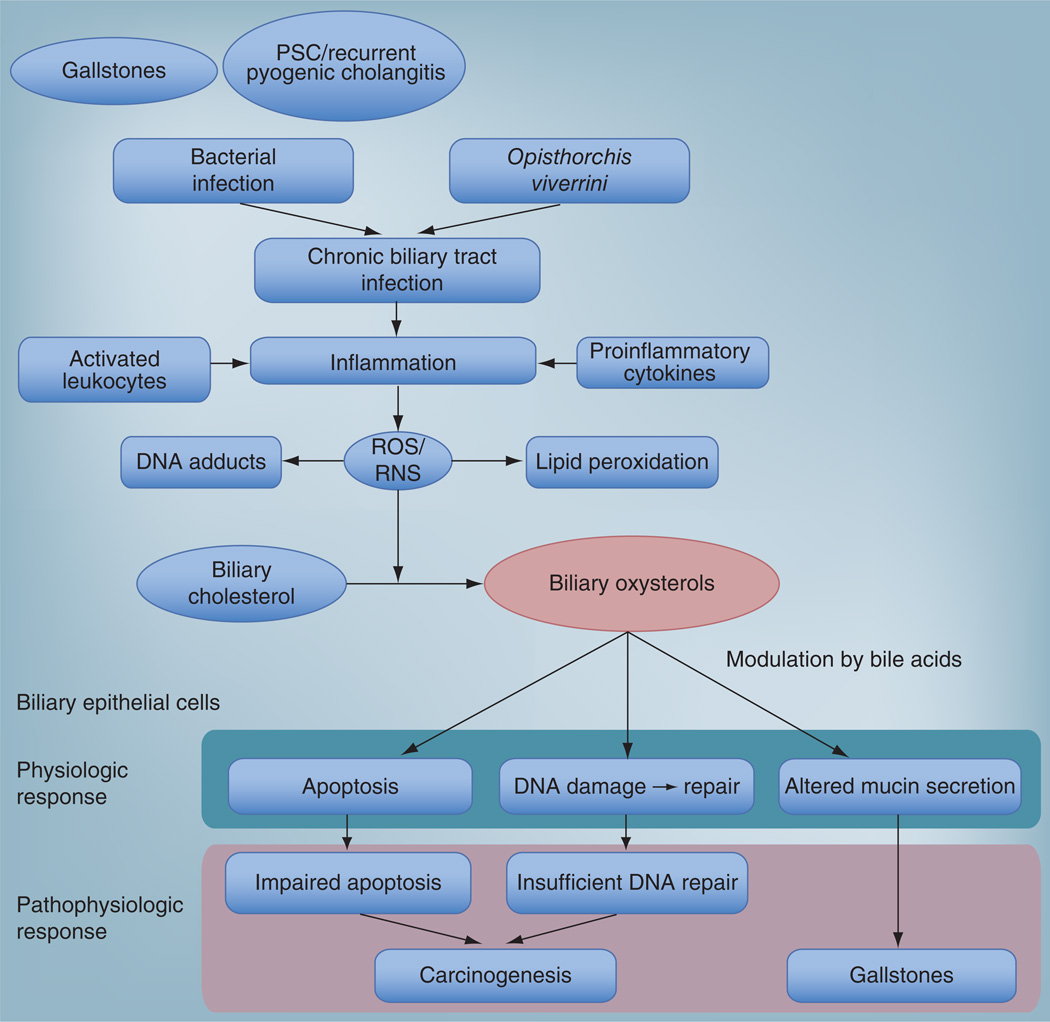
The findings from these studies, along with the prior published work on oxysterol-binding-protein expression in O. viverrini-induced hamster and human cholangiocarcinoma, provide data that can be used to formulate hypotheses regarding the molecular basis for cholangio-carcinoma development and progression that permits inclusion of an active role for certain oxysterols. As elucidated by Jusakul et al., chronic inflammation in the biliary tract, such as that induced by O viverrini, leads to the production of ROS and reactive nitrogen species by activated leukocytes [72]. Overproduction and insufficient clearance mechanisms allow these reactive species to induce the generation of DNA adducts. A normal cellular stress response allows cells to undergo programmed cell death, thereby eliminating damaged cells. However, in a subset of cells, under the pressure of ongoing inflammation, oxysterol generation and DNA damage, this normal cellular response is perturbed or absent, leading to insufficient DNA repair. Consequently, a subset of cells becomes resistant to DNA repair and/or the apoptotic response, giving rise to a selected clonal population that undergoes expansion leading to malignancy (Figure 2). This model is supported by the finding that low concentrations of cholestan-3β, 5α, 6β-triol and 3 keto-cholest-4-ene induced high levels of DNA-adducts but low cell death, suggesting that certain cells become resistant to apoptosis [72].
Figure 2. A conceptual framework for the role of biliary oxysterols in biliary tract diseases.
Chronic or recurrent biliary tract infection, as represented by recurrent pyogenic cholangitis, bacterial colonization in gallstones and Opisthorchis viverrini infection, induces inflammation. This chronic inflammatory state is associated with activated leukocytes, which produce ROS mediated by NADPH oxidase and via the induction of proinflammatory cytokines such as IL-1 and IL-6. The resultant pro-oxidative milieu, represented by the relative overabundance of ROS/RNS induces the production of various oxysterol species through oxidation of biliary cholesterol. Biliary epithelial cells that are chronically exposed to these oxysterol species undergo apoptosis, DNA damage and repair, and altered mucin secretion. These physiologic effects are modulated by the bile acid milieu, perhaps through FXR- and TGR5-mediated mechanisms. Over time, a pathophysiologic response develops through failure of apoptosis and/or defective DNA repair, allowing a clone of resistant cells to undergo neoplastic change. Altered mucin secretion can also induce changes in the biliary milieu to favor gallstone formation.
ROS: Reactive oxygen species; RNS: Reactive nitrogen species.
This mechanism is likely to be a contributor to multifaceted molecular mechanisms that lead to the development and progression of cholangiocarcinoma. Increased proinflammatory cytokines, such as IL-1, IL-6 and TNF-α [73]; and oxidative stress response enzymes such as COX-2 and free radical molecules [74–76], may disturb the balance of oxidant/antioxidant molecules and DNA repair mechanisms leading to enhanced survival [72]. Additional participants might be mRNAs that modulate the expression of these contributing molecules [77]. Thus, while a strict causality between specific oxsterols and the development and progression of cholangiocarcinoma has not been established, the data implicate oxysterols (and, by association, OSBPs/OSPBLs) as components of the proinflammatory milieu in which biliary tract cancer develops.
Conclusion
The biliary system provides a unique perspective to study oxysterol pathophysiology. Multiple different species of oxysterols have been identified in human bile. An association between chronic infection of the biliary system and gallstones (both pigmented and mixed cholesterol types) has been established. Recent studies using the well-characterized model of O. viverrini-induced cholangiocarcinoma in humans and in an animal model provide insights into the molecular mechanisms involved in oxysterol-associated cholangiocarcinogenesis. This emerging data concerning the role of oxysterols in the biliary system adds to the existing literature on the multifaceted roles of oxysterols in liver physiology and pathophysiology, where oxysterols have been found to play important roles in bile acid synthesis, cholesterol homeostasis, and in diseases such as cerebrotendinous xanthomatosis [9,11,78].
Future perspective
The ability to identify and quantitate oxysterol species in bile has led to an appreciation of the potential importance of these compounds in disease pathogenesis in the biliary tract. The foundation for future studies into the pathophysiologic roles of specific oxysterols in the development and progression of two major diseases affecting the biliary system, that is, gallstones and cholangiocarcinoma, is now in place. Several approaches can be taken to expand our knowledge regarding the role of oxysterols in biliary tract diseases:
-
▪
Cell culture studies using normal cholangiocytes/gallbladder epithelial cells or cholangiocarcinoma cells, including the use of the in vitro 3D co-culture model [79], can be used to gain insights into the effects of specific biliary oxysterols on cholangiocyte integrity and function. For example, long-term, low-dose, in vitro exposure of normal biliary epithelial cells to specific biliary oxysterols could provide insights into early cellular mechanisms that are operative during cancer development. The mechanisms whereby specific bile acids modulate oxysterol effects is another avenue of investigation that is likely to prove rewarding.
-
▪
Animal studies, for example, the hamster model of O. viverrini-induced cholangiocarcinoma, can be used to probe the roles of specific OSBPs and oxysterols in cholangiocarcinoma development and progression. Specific areas of investigation include the mechanisms whereby oxysterols interact with known carcinogenic pathways, such as those defined for inflammatory cytokines such as IL-6 and COX-2, DNA mismatch repair, oxidative and nitrative DNA damage and repair, mRNAs, and epigenetic mechanisms involving DNA methylation.
-
▪
Human studies involving subjects chronically infected with O. viverrini and those with pigment or mixed cholesterol gallstones, patients with chronic inflammation of the biliary tract, such as those with primary sclerosing cholangitis and patients in whom cholangiocarcinoma has been diagnosed. Areas of investigation with potential clinical relevance include the search for serum biomarkers that may involve oxysterols or OSBPs. These serum biomarkers could include those that signal early stages of neoplastic progression, metastatic disease or disease relapse following treatment.
Executive summary.
Oxysterol: definitions & semantics
-
▪
While bile acids and steroid hormones, as well as oxidation products of noncholesterol sterols, such as phytosterols and shellfish sterols, could be described as oxysterols, this review follows the conventional definition of oxysterols as cholesterol oxidation products that excludes bile acids and steroid hormones.
Origin of oxysterols
-
▪
Oxysterols are produced through the enzymatic and nonenzymatic oxidation of cholesterol. Exogenous (e.g. dietary) and endogenous (e.g., via nonenzymatic oxidation of cholesterol via P450 enzyme-mediated oxidation) sources of cholesterol exist.
-
▪
Many different types of oxysterols have been found in human tissues, with implications for normal physiology and for disease pathogenesis. Oxysterols have been implicated in a variety of diseases affecting various organ systems.
Physiologic functions of oxysterols
-
▪
Oxysterols have been implicated in a wide variety of normal physiologic functions, including as ligands for the nuclear receptor Liver X receptor, as mediators of cholesterol homeostasis, as mediators and intermediates of bile acid synthesis, and as modulators of hedgehog signaling and morphogenesis.
Pathophysiologic effects of oxysterols in the biliary tract
-
▪
Human bile and gallstones contain a variety of oxysterols and are associated with the presence of bacterial infection and biliary tract inflammation, suggesting that biliary oxysterol generation occurs via leukocyte-mediated production of reactive oxygen species.
-
▪
Biliary oxysterols interact with biliary epithelial cells, inducing apoptosis via a mitochondrial pathway, and modulating biliary epithelial cell functions such as mucin secretion. Bile acids differentially modulate oxysterol-induced apoptosis.
-
▪
Biliary tract oxysterols are associated with cholangiocarcinoma. Two oxysterols, cholestan-3β, 5α, 6β-triol and 3 keto-cholest-4-ene, are present at significantly higher levels in the livers of hamsters with Opisthorchis viverrini-induced cholangiocarcinoma.
Acknowledgements
The author acknowledges the assistance of L Blaker Sanders in the preparation of this manuscript.
This work was supported by NIH grant CA114403.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Jusakul A, Yongvanit P, Loilome W, Namwat N, Kuver R. Mechanisms of oxysterol-induced carcinogenesis. Lipids Health Dis. 2011;10:44. doi: 10.1186/1476-511X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otaegui-Arrazola A, Menendez-Carreno M, Ansorena D, Astiasaran I. Oxysterols: a world to explore. Food Chem. Toxicol. 2010;48:3289–3303. doi: 10.1016/j.fct.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Hovenkamp E, Demonty I, Plat J, Lutjohann D, Mensink RP, Trautwein EA. Biological effects of oxidized phytosterols: a review of the current knowledge. Prog. Lipid Res. 2008;47:37–49. doi: 10.1016/j.plipres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Guardiola F, Codony R, Addis PB, Rafecas M, Boatella J. Biological effects of oxysterols: current status. Food Chem. Toxicol. 1996;34:193–211. doi: 10.1016/0278-6915(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 5.Sottero B, Gamba P, Gargiulo S, Leonarduzzi G, Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr. Med. Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- 6.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Aspects Med. 2009;30:153–170. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Schroepfer GJ., Jr Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80:361–554. doi: 10.1152/physrev.2000.80.1.361. [DOI] [PubMed] [Google Scholar]
- 8.Smith LL. Cholesterol Autoxidation. NY, USA: Plenum Press; 1981. [Google Scholar]
- 9.Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu. Rev. Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javitt NB. Oxysterols: novel biologic roles for the 21st century. Steroids. 2008;73:149–157. doi: 10.1016/j.steroids.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Crosignani A, Zuin M, Allocca M, Del Puppo M. Oxysterols in bile acid metabolism. Clin. Chim. Acta. 2011;412:2037–2045. doi: 10.1016/j.cca.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Shibata N, Glass CK. Macrophages, oxysterols and atherosclerosis. Circulation J. 2010;74:2045–2051. doi: 10.1253/circj.cj-10-0860. [DOI] [PubMed] [Google Scholar]
- 13.Diczfalusy U. Analysis of cholesterol oxidation products in biological samples. J. AOAC Int. 2004;87:467–473. [PubMed] [Google Scholar]
- 14.Wu Z, Martin KO, Javitt NB, Chiang JY. Structure and functions of human oxysterol 7α-hydroxylase cDNAs and gene CYP7B1. J. Lipid Res. 1999;40:2195–2203. [PubMed] [Google Scholar]
- 15.Russell DW. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta. 2000;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 16.Brown AJ, Jessup W. Oxysterols: sources, cellular storage and metabolism and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 2009;30:111–122. doi: 10.1016/j.mam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkhem I, Lutjohann D, Diczfalusky U, Stahle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24(S)-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- 18.Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids. 2011;164:457–468. doi: 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.McDonald JG, Smith DD, Stiles AR, Russell DW. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 2012;53:1399–1409. doi: 10.1194/jlr.D022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Sheflin LG, Poter NA, Fliesler SJ. 7-dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith–Lemli–Opitz syndrome. Biochim. Biophys. Acta. 2012;1821(6):877–883. doi: 10.1016/j.bbalip.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haigh WG, Lee SP. Identification of oxysterols in human bile and pigment gallstones. Gastroenterology. 2001;121:118–123. doi: 10.1053/gast.2001.25513. ▪▪ First description of oxysterol species in human bile and pigment gallstones.
- 22. Yoshida T, Matsuzaki Y, Haigh WG, et al. Origin of oxysterols in hepatic bile of patients with biliary infection. Am..J. Gastroenterol. 2003;98:2275–2280. doi: 10.1111/j.1572-0241.2003.07703.x. ▪▪ Comprehensive quantitative analysis of oxysterol species in hepatic bile obtained from patients with biliary tract diseases, showing an association with infected bile.
- 23.Iuliano L, Michelette F, Natoli S, Ginanni Corradini S, et al. Measurement of oxysterols and alpha-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal. Biochem. 2003;312:217–223. doi: 10.1016/s0003-2697(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 24.Kidambi S, Patel SB. Cholesterol and non-cholesterol sterol transporters: ABCG5, ABCG8 and NPC1L1: a review. Xenobiotica. 2008;38:1119–1139. doi: 10.1080/00498250802007930. [DOI] [PubMed] [Google Scholar]
- 25.Staprans I, Pan XM, Rapp JH, Feingold KR. Oxidized cholesterol in the diet is a source of oxidized lipoproteins in human serum. J. Lipid Res. 2003;44:705–715. doi: 10.1194/jlr.M200266-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Liseisen J, Wolfram G. Absorption of cholesterol oxidation products from ordinary foodstuff in humans. Ann. Nutr. Metab. 1998;42:221–230. doi: 10.1159/000012737. [DOI] [PubMed] [Google Scholar]
- 27.Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J. Lipid Res. 2009;50(Suppl.):S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill S, Chow R, Brown AJ. Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog. Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Lange Y, Ory DS, Ye J, Lanier MH, Hsu FF, Seck TL. Effectors of rapid homeostatic responses of endoplasmic reticulum cholesterol and HMG-CoA reductase. J. Biol Chem. 2008;283:1445–1455. doi: 10.1074/jbc.M706967200. [DOI] [PubMed] [Google Scholar]
- 30.Porter JA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 31.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 32.Stottmann RW, Turbe-Doan A, Tran P, et al. Cholesterol metabolism is required for intracellular hedgehog signal transduction in vivo. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002224. e1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kha HT, Basseri B, Shouhed D, et al. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J. Bone Miner. Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 35.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl Acad. Sci. USA. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachtergaele S, Mydock LK, Krishnan K, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat. Chem. Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannedouche S, Zhang J, Yi T, et al. Oxysterols direct B cell and T cell migration via EBI2. Nature. 2011;475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Yang XV, Wu J, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 39.Ngo MH, Colbourne TR, Ridgway ND. Functional implications of sterol transport by the oxysterol-binding protein gene family. Biochem. J. 2010;429:13–24. doi: 10.1042/BJ20100263. [DOI] [PubMed] [Google Scholar]
- 40.Lehto M, Olkkonen VM. The OSBP-related proteins: a novel protein family involved in vesicle transport, cellular lipid metabolism and cell signaling. Biochim. Biophys. Acta. 2003;1631:1–11. doi: 10.1016/s1388-1981(02)00364-5. [DOI] [PubMed] [Google Scholar]
- 41.Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu. Rev. Cell Dev. Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan E, Chopra J, McCarthy FO, Maguire AR, O’Brien NM. Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol and oxidation products with their corresponding cholesterol oxidation products. Br. J. Nutr. 2005;94:443–451. doi: 10.1079/bjn20051500. [DOI] [PubMed] [Google Scholar]
- 43.Bjorkhem I, Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 2002;22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 44.Kudo K, Emmons GT, Casserly EW, et al. Inhibitors of sterol synthesis. Chromatography of acetate derivatives of oxygenated sterols. J. Lipid Res. 1989;30:1097–1111. [PubMed] [Google Scholar]
- 45.Fuda H, Javitt NB, Mitamura K, Ikegawa S, Strott CA. Oxysterols are substrates for cholesterol sulfotransferase. J. Lipid Res. 2007;48:1343–1352. doi: 10.1194/jlr.M700018-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Bai Q, Rodriguez-Agudo D, Hylemon P, et al. Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholeserol and 25-hydroxycholesterol-3-sulfate. Lipids. 2010;45:821–832. doi: 10.1007/s11745-010-3451-y. [DOI] [PubMed] [Google Scholar]
- 47.Bai Q, Xu L, Kakiyama G, Runge-Morris MA, Hylemon P, et al. Sulfation of 25-hydroxycholesterol by SULF2B1b decreases cellular lipids via the LXR/SREBP-1c signaling pathway in human aortic endothelial cells. Atherosclerosis. 2011;214:350–356. doi: 10.1016/j.atherosclerosis.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Shen S, Ma Y, et al. 25-hydroxycholesterol-3-sulfate attenuates inflammatory response via PPARγ signaling in human THP-1 macrophages. Am. J. Physiol. Endocrinol. Metab. 2012;302:e788–e799. doi: 10.1152/ajpendo.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai Q, Zhang X, Xu L, et al. Oxysterol sulfation by cytosolic sulfotransferase suppresses liver X receptor/sterol regulatory element binding protein-1c signaling pathway and reduces serum and hepatic lipids in mouse models of nonalcoholic fatty liver disease. Metabolism. 2012;61:836–845. doi: 10.1016/j.metabol.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko CW, Lee SP. Gallstone formation. Local factors. Gastroenterol. Clin. North Am. 1999;28:99–115. doi: 10.1016/s0889-8553(05)70045-5. [DOI] [PubMed] [Google Scholar]
- 51.Stokes CS, Krawczyk M, Lammert F. Gallstones: environment, lifestyle and genes. Dig. Dis. 2011;29:191–201. doi: 10.1159/000323885. [DOI] [PubMed] [Google Scholar]
- 52.Lee DK, Tarr PI, Haigh WG, Lee SP. Bacterial DNA in mixed cholesterol gallstones. Am. J. Gastroenterol. 1999;94:3502–3506. doi: 10.1111/j.1572-0241.1999.01614.x. [DOI] [PubMed] [Google Scholar]
- 53.Haigh WG, Wong T, Lee SP. The production of oxysterols in bile by activated human leukocytes. Biochem. Biophys. Res. Comm. 2006;34:467–469. doi: 10.1016/j.bbrc.2006.02.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oda D, Lee SP, Hayashi A. Long-term culture and partial characterization of dog gallbladder epithelial cells. Lab. Invest. 1991;64:682–692. [PubMed] [Google Scholar]
- 55. Seo DW, Choi H-S, Lee SP, Kuver R. Oxysterols from human bile induce apoptosis of canine gallbladder epithelial cells in monolayer culture. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1247–G1256. doi: 10.1152/ajpgi.00013.2004. ▪ Cultured dog gallbladder epithelial cells were found to undergo apoptosis via a mitochondrial pathway when exposed to model bile solutions containing cholest-4-en-3-one, cholesta-4,6-dien-3-one, and 5β-cholestan-3-one, three oxysterol species identified in bile.
- 56.Yoshida T, Klinkspoor JH, Kuver R, et al. Effects of bile salts on cholestan-3β, 5α, 6β-triol-induced apoptosis in dog gallbladder epithelial cells. Biochim. Biophys. Acta. 2001;1530:199–208. doi: 10.1016/s1388-1981(00)00183-9. [DOI] [PubMed] [Google Scholar]
- 57.Kuver R. The expanding universe of bile acid physiology: delving into the mysteries of dark (green) matter. J. Surg. Res. 2012 doi: 10.1016/j.jss.2012.03.041. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 58.Wang YD, Chen WD, Yu D, Forman BM, Huang W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF–kB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr. Mol. Med. 2010;10:579–595. doi: 10.2174/1566524011009060579. [DOI] [PubMed] [Google Scholar]
- 60.Cipriani S, Mencarelli A, Chini MG, Distrutti E, et al. The bile acid receptor Gpbar-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025637. e25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida T, Klinkspoor JH, Kuver R, Wrenn SP, Kaler EW, Lee SP. Cholestan-3β 5α 6β-triol, but not 7-ketocholesterol, suppresses taurocholate-induced mucin secretion by cultured dog gallbladder epithelial cells. FEBS Lett. 2000;478:113–118. doi: 10.1016/s0014-5793(00)01831-7. [DOI] [PubMed] [Google Scholar]
- 62.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 63.Patel T. Cholangiocarcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 64.Smout MJ, Sripa B, Laha T, et al. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol. Biosyst. 2011;7:1367–1375. doi: 10.1039/c0mb00295j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yongvanit P, Pinlaor S, Bartsch H. Oxidative and nitrative DNA damage: key events in opisthorchiasis-induced carcinogenesis. Parasitol. Int. 2012;61:130–135. doi: 10.1016/j.parint.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Pinlaor S, Sripa B, Ma N, et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J. Gastroenterol. 2005;11:4644–4649. doi: 10.3748/wjg.v11.i30.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thamavit W, Bhamarapravati N, Saphaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 68. Loilome W, Yongvanit P, Wongkham C, et al. Altered gene expression in Opisthorchis viverrin-associated cholangiocarcinoma in hamster model. Mol. Carcinog. 2006;45:279–287. doi: 10.1002/mc.20094. ▪ Differential display PCR on liver tissues from hamsters induced to develop cholangiocarcinoma identified an oxysterol-binding protein, OSBPL-8, among transcripts that were upregulated compared with control tissue.
- 69.Dechakhamphu S, Pinlaor S, Sitthithaworn P, Bartsch H, Yongvanit P. Accumulation of miscoding etheno-DNA adducts and highly expressed DNA repair during liver fluke-induced cholangiocarcinogenesis in hamsters. Mutat. Res. 2010;691:9–16. doi: 10.1016/j.mrfmmm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Dechakhamphu S, Pinlaor S, Sitthithaworn P, Nair J, Bartsch H, Yongvanit P. Lipid peroxidation and etheno DNA adducts in white blood cells of liver fluke-infected patients: protection by plasma alpha-tocopherol and praziquantel. Cancer Epidemiol. Biomarkers Prev. 2010;19:310–318. doi: 10.1158/1055-9965.EPI-09-0849. [DOI] [PubMed] [Google Scholar]
- 71. Loilome W, Wechagama P, Namwat N, et al. Expression of oxysterol binding protein isoforms in opisthorchiasis-associated cholangiocarcinoma: a potential molecular marker for tumor metastasis. Parasitol. Int. 2012;61:136–139. doi: 10.1016/j.parint.2011.07.003. ▪ In human cholangiocarcinoma tissue, increased levels of OSBPL-7 mRNA and protein were found compared with non-tumor liver tissues. Higher levels of OSBP2 and OSBPL-7 mRNA were found in blood samples from cholangiocarcinoma patients compared with normal controls.
- 72. Jusakul A, Loilome W, Namwat N, et al. Liver fluke-induced hepatic oxysterols stimulate DNA damage and apoptosis in cultured human cholangiocytes. Mutat. Res. 2012;731:48–57. ▪▪ In the hamster model of cholangiocarcinoma induced by Opisthorchis viverrini and NMDA, two oxysterols (cholestan-3β 5α 6β-triol and 3-keto-cholesta-4, 6-diene) were found at higher levels in tumor tissues compared with livers from control animals. These two oxysterols were found to induce DNA adduct formation and induce apoptosis in cultured cholangiocytes.
- 73.Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517–10524. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 74.Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732–738. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 75.Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120:190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 76.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric-oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 77.Braconi C, Huang N, Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology. 2010;51:881–890. doi: 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol. Sci. 2006;27:143–149. doi: 10.1007/s10072-006-0618-7. [DOI] [PubMed] [Google Scholar]
- 79.Campbell DJ, Dumur CI, Lamour NF, Dewitt JL, Sirica AE. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol. Res. 2012 doi: 10.1111/j.1872-034X.2012.01026.x. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]