Abstract
Purpose
Preclinical studies suggested that the oral anti-fungal agent itraconazole specifically inhibits proliferation, migration, and tube formation of endothelial cells. Itraconazole has potent anti-angiogenic activity and enhances the efficacy of cytotoxic chemotherapy in multiple primary xenograft lung cancer models. Based on these data, we performed an exploratory clinical study assessing the efficacy of itraconazole with cytotoxic chemotherapy in the treatment of patients with advanced lung cancer.
Patients/Methods
The study enrolled patients with progressive non-squamous non-small cell lung cancer after one prior cytotoxic therapy for metastatic disease, randomized 2:1 to pemetrexed 500 mg/m2 IV day 1 with or without itraconazole 200 mg oral daily on a 21-day cycle. Outcome measures included percent progression-free at 3 months, progression-free survival, overall survival, and observed toxicity.
Results
A total of 23 patients were enrolled; the study was stopped early due to increasing use of pemetrexed in the first line setting. Sixty-seven percent of patients were progression-free at 3 months on itraconazole plus pemetrexed vs. 29% on the control arm of pemetrexed alone (p=0.11). Median progression-free survivals were 5.5 months (itraconazole) vs. 2.8 months (control) (hazard ratio (HR)=0.399, p=0.089). Overall survival was longer in patients receiving itraconazole (median 32 months) vs. control (8 months) (HR=0.194, p=0.012). There were no evident differences in toxicity between the study arms.
Conclusion
Itraconazole is well tolerated in combination with pemetrexed. Consistent with our preclinical data, daily itraconazole administration is associated with trends suggestive of improved disease control in patients receiving chemotherapy for advanced lung cancer.
Keywords: Itraconazole, anti-angiogenic, lung cancer
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the United States, responsible for more deaths than colon, breast, and prostate cancers combined.1, 2 More effective treatments for this disease are critically needed. Solid tumor growth and progression is dependent on tumor-associated angiogenesis.3 Tumor expression and circulating levels of angiogenic factors have been correlated with aggressive tumor growth, predilection for metastasis, and prognosis in a wide array of solid tumors, including non-small cell lung cancer.4–6 The only currently approved anti-angiogenic therapy for lung cancer is the monoclonal antibody bevacizumab, directed against the vascular endothelial growth factor (VEGF). A landmark phase III clinical study, the Eastern Cooperative Oncology Group (ECOG) 4599, randomized 878 patients with advanced non-squamous non-small cell lung cancer to a standard chemotherapy doublet with or without bevacizumab.7 This study demonstrated a statistically significant improvement in both progression-free and overall survival in favor of the bevacizumab-containing arm.
While the ECOG 4599 study indicated the potential for anti-angiogenic therapy to improve outcome in solid tumor patients, there were major limitations in these data as well. First, the absolute improvements in progression-free and overall survival were modest (1.7 and 2 months, respectively). Second, there were significant toxicities attributable to bevacizumab, with 15 treatment-related deaths on the bevacizumab arm (vs. 2 on control; p = 0.001), including multiple fatal hemoptyses. The rates of hypertension, proteinuria, bleeding, neutropenia, febrile neutropenia, thrombocytopenia, hyponatremia, rash, and headache were significantly higher on bevacizumab (p < 0.05 for each). Third, the financial cost of bevacizumab, given the limited efficacy and significant toxicity, was seen by many as excessive, with an incremental cost-utility ratio compared to chemotherapy alone of over $500,000 per quality-adjusted year of life.8 A final concern came from a “confirmatory” trial, the AVAiL study, which enrolled 1,043 patients randomized to a cisplatin and gemcitabine with or without bevacizumab.9 This study failed to demonstrate a statistically significant difference in survival.9 In summary, while evidence is strong that angiogenesis is critical to tumor growth, less toxic, less cost-prohibitive, and more effective, therapies are needed.
We performed a high throughput screen for agents with previously unsuspected anti-angiogenic activity, using as an initial assay differential inhibition of endothelial cell proliferation.10 Itraconazole was found to be among the most potent and selective inhibitors of endothelial cell proliferation, with IC50 of 0.16 μM for human umbilical vein endothelial cells, and minimal if any anti-proliferative effects against multiple non-endothelial controls (IC50s > 100 μM). None of the several related antifungal agents had similar activity.
We subsequently conducted a series of preclinical analyses of itraconazole using both in vitro and in vivo model systems.11 Itraconazole inhibited endothelial cell proliferation in response to known angiogenic drivers, including both VEGF and fibroblast growth factor (FGF), and inhibited phosphorylation of the primary angiogenic receptors for these factors. Itraconazole led to a dose-dependent suppression of VEGF- and FGF-mediated endothelial cell migration and VEGF- and FGF-mediated endothelial tube formation. Oral itraconazole administration to animals bearing primary xenograft NSCLCs resulted in tumor growth inhibition similar to that achieved with cisplatin, and in combination with cisplatin resulted in even more marked suppression of tumor growth. Use of itraconazole in vivo was associated with tumor hypoxia, as shown by induction of tumor-specific expression of HIF1α, as well as decreased tumor microvessel density and tumor vascular area.11
Taken together, these data support that itraconazole may have substantial promise as a novel anti-angiogenic agent. In contrast to bevacizumab, itraconazole is an inexpensive oral agent, off patent and currently available in a generic formulation. Itraconazole has been safely administered to thousands of patients, including patients receiving high dose cytotoxic therapy for allogeneic bone marrow transplantation.12
As a first study to evaluate the potential anti-cancer activity of itraconazole in conjunction with standard chemotherapy for patients with lung cancer, we initiated a randomized study of standard dose pemetrexed as second-line therapy for non-squamous NSCLC, given with or without itraconazole at a standard anti-fungal dose of 200 mg daily. In the 571 patient phase III trial that supported the United States Food and Drug Administration registration of pemetrexed in the second line setting for metastatic non-small cell lung cancer, 3-month event-free survival was approximately 40%, median progression-free survival was 2.9 months, and median overall survival was 8.3 months.13 We hypothesized that the anti-angiogenic activity of itraconazole might lead to improved disease control with minimal additional toxicity in patients with recurrent and progressive non-squamous non-small cell lung cancer.
METHODS
Patient Population
Eligible candidates for this study (NCT00769600) were adults with histologically or cytologically confirmed non-squamous non-small cell lung cancer, with progressive disease after one and only one prior chemotherapy regimen for metastatic disease. Patients with known EGFR mutation were allowed to have received prior oral EGFR tyrosine kinase inhibitor therapy. Eligible patients had an ECOG performance status of ≤ 1, and had adequate bone marrow, renal, and hepatic function. Patients were excluded if they had underlying or predisposing condition of bleeding (history of non-chemotherapy induced thrombocytopenia with bleeding within 1 year, active peptic ulcer or hemorrhagic esophagitis/gastritis, active immune thrombocytopenic purpura, etc.).
This study was conducted according to the Declaration of Helsinki and with approval from Institutional Review Boards of all participating study sites. All participants provided written informed consent before participating.
Study Design
This was an open-label, randomized two arm phase 2 study of second-line therapy for metastatic non-squamous non-small cell lung cancer. On both arms, patients received standard dose and schedule pemetrexed (500 mg/m2 IV on day 1 of a 21-day cycle). On Arm A only, patients also received 200 mg itraconazole daily, starting on day 1 of cycle 1. This dose of itraconazole was chosen for this initial exploratory study based on an expectation that this would be well tolerated as this reflects standard daily dosing recommendations for prolonged treatment of fungal infection. Subjects could remain on therapy indefinitely, until disease progression or intolerable toxicity. Although this study was not completed, the original statistical design called for a total of 112 patients, with the intent to assess the proportion of patients alive and progression-free at 3 months in each arm. The null hypothesis based on historical controls was that 3-month progression-free survival would be approximately 40%. With a target 3-month progression-free survival of 60%, a one-stage Fleming phase II design yielded an alpha of 0.10 (one sided) and a power of 0.80 using A’Hern’s exact binomial probabilities.14, 15
Assessments
Safety
Safety assessments included history and physical examinations, vital signs, ECOG performance status, adverse event assessment, blood chemistry and complete blood counts with differential. Safety assessments were performed at screening and at least once in each cycle of therapy, typically on day 1. Adverse event severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0.16 All adverse effects grade 3 or higher were tabulated, regardless of attribution to study drug. Relationships of adverse events to therapy (definitely, probably, possibly, unlikely, or unrelated) were assessed by the principal investigator.
Efficacy
Tumor response was assessed using standard Response Evaluation Criteria in Solid Tumors (RECIST) after every 2 cycles of therapy.17 The planned primary endpoint was percent progression-free on each arm at 3 months. Additional prespecified efficacy variables included progression-free and overall survival assessed by Kaplan-Meier curves, with statistical differences assessed by log-rank.
RESULTS
Patient Characteristics and Study Drug Dosing
The study was stopped early after a total of 23 patients had enrolled, due to slow accrual associated with increasingly frequent use of pemetrexed as first-line and/or maintenance treatment of patients with metastatic non-squamous non-small cell lung cancer at Johns Hopkins. Fifteen patients were randomized to receive pemetrexed and itraconazole, and 8 patients to pemetrexed alone. The majority of patients were of performance status 1. A summary of patient demographics is shown in Table 1. All patients were started at the planned dose and schedule. The most common reason for study discontinuation on both arms was disease progression.
Table 1.
Patient Demographics
| Demographic category | Pemetrexed/Itraconazole N = 15 | Pemetrexed N = 8 | |
|---|---|---|---|
| Median age (range) | 60 (49 – 75) | 59 (48 – 72) | |
|
| |||
| Female, n (%) | 7 (47%) | 5 (63%) | |
|
| |||
| ECOG PS, n (%) | 0 | 6 (40%) | 4 (50%) |
| 1 | 8 (53%) | 4 (50%) | |
| 2 | 1 (7%) | 0 | |
|
| |||
| Smoking history | Never smoker | 4 (27%) | 3 (37%) |
| Former smoker | 11 (73%) | 5 (63%) | |
| Pack-year, median (range) | 25 (0 – 80) | 13 (0 – 70) | |
|
| |||
| Driver mutations* | KRAS | 3/11 (27%) | 3/8 (37%) |
| EGFR | 2/11 (18%) | 2/8 (25%) | |
| not tested | 4 | 0 | |
One patient on Arm A with CTNNB1 mutation
Safety and Tolerability
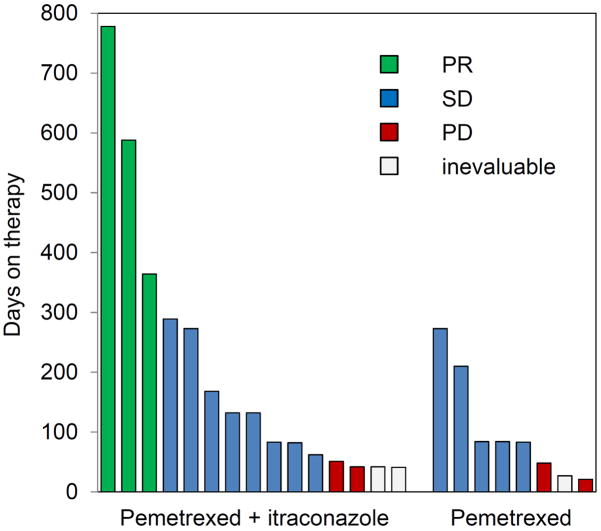
Therapy was well-tolerated on both arms, with the spectrum of adverse effects reflective of the known toxicities of pemetrexed, and disease-related complications of advanced lung cancer. The most common grade 3 toxicity on both arms was lymphopenia (2 of 8 on pemetrexed, and 3 of 15 on the combination). One patient on the combination of pemetrexed and itraconazole experienced transient grade 4 neutropenia not associated with fever, and grade 3 leukopenia. Another patient on the combination arm experienced an intratumoral bleed in a large adrenal metastasis within the first 30 days after going off study; this was considered possibly therapy-related. All other grade 3 or higher toxicities were considered not or unlikely to be therapy-related, and are summarized in Table 2. The median number of treatment cycles for patients on the combination of pemetrexed and itraconazole was 6 (range, 1 – 35) and the median treatment duration was 4.3 months (range, 1 – 26) (Figure 1). The median number of treatment cycles on pemetrexed alone was 3.5 (range, 1 – 13) and the median treatment duration was 2.7 months (range, 1 – 9).
Table 2.
Grade 3 or higher adverse events.
| Toxicity | Pemetrexed/Itraconazole (N = 15) | Pemetrexed (N = 8) | ||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Lymphopenia | 3 * | 2 * | ||
| Neutropenia | 1* | |||
| Leukopenia | 1* | |||
| Cough | 1 | 1 | ||
| Dyspnea | 1 | |||
| Pain | 1 | |||
| Vertigo | 1 | |||
| Pulmonary embolus | 1 | |||
| Intratumoral bleed | 1*, ** | |||
possibly, probably, or definitely therapy-related
occurred after discontinuation of study
Figure 1. Duration of therapy on study.
Each vertical bar represents an individual patient on study. Height of the bar represents time on therapy, and color represents RECIST response.
Efficacy
Three RECIST responses were seen, all on the combination arm (response rate 3/15 or 20%) (Figure 1). The duration of response ranged from approximately one year to over two years.
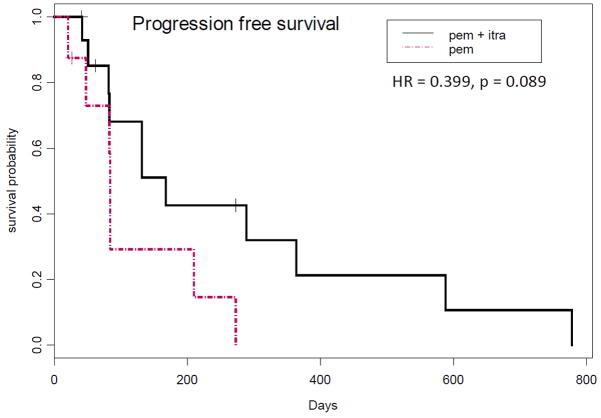
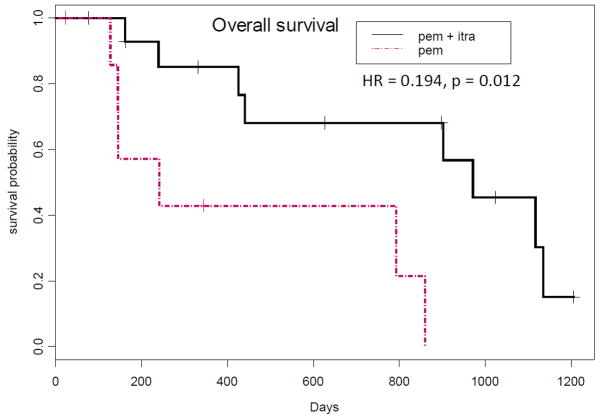
The primary objective of this study was percentage progression-free at 3 months, with the underlying hypothesis being that itraconazole administration would be associated with an improvement in 3-month progression-free survival from 40% to 60%. The actual percentage progression-free at 3 months was 67% on the combination of pemetrexed and itraconazole, and 29% on single agent pemetrexed. Median progression-free survival was 5.5 months on the combination of pemetrexed and itraconazole vs. 2.8 months on single agent pemetrexed (Figure 2; HR=0.399, p=0.089). Median overall survival of patients treated with pemetrexed on this study was 8 months; in contrast, median overall survival of patients treated with the combination of pemetrexed and itraconazole was 32 months (Figure 3; HR=0.194, p=0.012).
Figure 2. Progression-free survival.
Kaplan-Meier curves of progression-free survival by study arm are shown.
Figure 3. Overall survival.
Kaplan-Meier curves of progression-free survival by study arm are shown.
DISCUSSION
Anti-angiogenic therapy as a strategy for the treatment of solid tumors is attractive in many respects: tumors are tenuously hypoxic and highly dependent on ongoing neovascularization for continued growth, and the endothelial cells and associated stromal elements may be more genetically stable and less likely to develop acquired resistance to targeted therapies than cancer cells. There have been successes in anti-angiogenic treatment of cancer using both targeted monoclonal antibodies and small molecule receptor tyrosine kinase inhibitors.18 Nonetheless, the progress to date in the development of potent and durable inhibitors of tumor-associated angiogenesis has not lived up to some of the initial exceptionally high expectations.19
Most targeted anti-angiogenic agents studied in cancer patients to date are highly selective targeted agents directed against key ligand-receptor interactions implicated in cancer-associated angiogenic drive. Like these molecularly targeted agents, itraconazole has minimal if any cytotoxic effect on cancer cells directly, but has potent inhibitory activity against endothelial cell proliferation. While the mechanism of action of itraconazole as a selective inhibitor of endothelial cell proliferation has not been fully defined, it appears to be a “dirtier” inhibitor, active against multiple angiogenic stimuli, in a variety of contexts. In multiple primary xenograft models of non-small cell lung cancer, the efficacy of oral itraconazole in suppressing tumor growth was similar to that of cisplatin.11
Based on this promising but surprising preclinical data, we initiated a clinical trial to explore the efficacy of itraconazole in patients being treated with standard pemetrexed as second line treatment for metastatic non-squamous non-small cell lung cancer. The control arm of this study, pemetrexed alone, performed essentially as expected, with progression-free and overall survival medians of 2.8 and 8 months respectively (compared to the expected 2.9 and 8.3 months based on historical control). In contrast, the cohort receiving the addition of itraconazole experienced median progression-free and overall survivals of 5.5 and 32 months respectively, with the overall survival difference achieving statistical significance.
There are important caveats to consider in interpreting these data. First, and most notably, the sample size is small and the estimates of effect sizes are therefore highly unstable. The study was stopped after 23 patients due to concerns for study feasibility: increasingly, patients with good performance status and newly diagnosed metastatic non-squamous non-small cell lung cancer at our center are treated either with a molecularly targeted agent (in the case of an identified driver mutation), or with a pemetrexed-containing doublet; this study was open only to patients after treatment with a prior platinum-doublet, which could not include pemetrexed. We are currently launching a second randomized phase II study of itraconazole in a more feasible clinical context.
Second, this initial exploratory study did not include correlative analyses documenting anti-angiogenic effects in patients treated with itraconazole. Our preclinical work in human tumor xenograft models did convincingly demonstrate marked inhibition of tumor-associated vascularity, with resultant tumor induction of the hypoxia-responsive gene HIF-1α. Our upcoming trial will include serial contrast-enhanced magnetic resonance imaging to assess changes in tumor blood flow. This will be a 70 patient randomized phase II study in the first-line metastatic setting, evaluating a standard regimen of cisplatin and gemcitabine with or without itraconazole, with co-primary endpoints of response rate, and decrease in tumor-associated blood flow.
Despite these inherent limitations, the results from this study are entirely consistent with the strong treatment effects seen in our preclinical modeling of itraconazole in human non-small cell lung cancer primary xenografts. We believe these new clinical data are both provocative and encouraging, and should prompt further clinical evaluation of itraconazole as a novel therapeutic for lung cancer and other solid tumors.
Table 3.
Outcome data summary
| Outcome measure | Pemetrexed/Itraconazole N = 15 | Pemetrexed N = 8 |
|---|---|---|
| RECIST response, N (%) | ||
| PR | 3 (20%) | 0 |
| SD | 8 (53%) | 5 (62%) |
| PD | 2 (13%) | 2 (25%) |
| inevaluable | 2 (13%) | 1 (12%) |
|
| ||
| Progression-free survival | ||
| Median (months) | 5.5 | 2.8 |
| HR (p value) | 0.399 (p = 0.089) | |
|
| ||
| Overall survival | ||
| Median (months) | 32 | 8 |
| HR (95% CI) | 0.194 (p = 0.012) | |
Acknowledgments
Acknowledgement of Research Support: The Flight Attendant Medical Research Institute and National Institutes of Health
This study was supported by grants from the Flight Attendant Medical Research Institute and National Institutes of Health (Johns Hopkins Lung Cancer SPORE; NCI P50CA058184).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 4.Andersen S, Donnem T, Al-Saad S, et al. Angiogenic markers show high prognostic impact on survival in marginally operable non-small cell lung cancer patients treated with adjuvant radiotherapy. J Thorac Oncol. 2009;4:463–471. doi: 10.1097/JTO.0b013e3181991d18. [DOI] [PubMed] [Google Scholar]
- 5.Mineo TC, Ambrogi V, Baldi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seto T, Higashiyama M, Funai H, et al. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53:91–96. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14:836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 10.Chong CR, Xu J, Lu J, et al. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS chemical biology. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 11.Aftab BT, Dobromilskaya I, Liu JO, et al. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764–6772. doi: 10.1158/0008-5472.CAN-11-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471–5489. doi: 10.1200/JCO.2007.12.3851. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 14.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 15.A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events. Version 3.0. Available at: http://ctep.cancer.gov.
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Jayson GC, Hicklin DJ, Ellis LM. Antiangiogenic therapy--evolving view based on clinical trial results. Nat Rev Clin Oncol. 2012;9:297–303. doi: 10.1038/nrclinonc.2012.8. [DOI] [PubMed] [Google Scholar]
- 19.Kolata G. Hope in the lab: a special report; a cautious awe greets drugs that eradicate tumors in mice. The New York TImes. 1998 May 3; [Google Scholar]