Abstract
Over the last several years we have developed a rapidly-expanding suite of genetically-encoded reagents (e.g., ChR2, Halo, Arch, Mac, and others) that, when expressed in specific neuron types in the nervous system, enable their activities to be powerfully and precisely activated and silenced in response to light. If the genes that encode for these reagents can be delivered to cells in the body using gene therapy methods, and if the resultant protein payloads operate safely and effectively over therapeutically important periods of time, these molecules could subserve a set of precise prosthetics that use light as the trigger of information entry into the nervous system, e.g. for sensory replacement. Here we discuss the use of ChR2 to make the photoreceptor-deprived retina, as found in diseases such as retinitis pigmentosa, sensitive to light, enabling restoration of functional vision in a mouse model of blindness. We also discuss arrays of light sources that could be useful for delivering patterned sensory information into the nervous system.
I. Introduction
In 2005, we reported that expression of the algal light-gated cation channel channelrhodopsin-2 (ChR2), a membrane protein from C. reinhardtii, in neurons, enabled the neurons to fire electrical action potentials in response to brief pulses of blue light [1]. In 2007, we reported the use of the archaeal light-driven chloride pump halorhodopsin (Halo/NpHR) from N. pharaonis to hyperpolarize neurons in response to yellow light [2]. Three years later, we reported a second class of light-driven neural silencer, the light-driven outward proton pump, which could support extremely powerful neural silencing, an order of magnitude greater than that mediated by the original halorhodopsins, as exemplified by the molecule Arch from H. sodomense [3], which can result in ~100% shutdown of neural activity in the awake brain in response to green or yellow light. Other light-driven outward proton pumps, such as the molecule Mac from L. maculans, can be used to silence neurons in response to blue light [3]. These molecules require no chemical co-factors in the mammalian brain, and operate at high enough speeds to enable driving or deletion of individual action potentials. We have distributed these tools to approximately 400 groups around the world, where they are used in animals (either engineered to be transgenic, or expressing the genes in defined neurons after viral delivery), to study the roles that defined neurons play in normal and abnormal brain computations.
If these genes that encode for these reagents can be delivered to cells in the body using gene therapy methods, and if the resultant protein payloads operate safely and effectively over therapeutically important periods of time, these molecules could subserve a set of precise prosthetics that use light as the trigger of information entry into the nervous system, e.g. for sensory replacement.
Earlier, we reported the first use of ChR2 in the non-human primate brain, providing evidence of safe expression and function of ChR2 in a brain similar to the human brain [5]. Here we discuss the use of ChR2 to make the photoreceptor-deprived retina, as found in diseases such as retinitis pigmentosa, sensitive to light, enabling restoration of functional vision in a mouse model of blindness. We also discuss arrays of light sources that could be useful for delivering patterned sensory information into the brain and peripheral nervous system.
II. BLINDNESS
Previous work established retinal expression of channelrhodopsin-2 (ChR2), an algal cation channel gated by light, restored physiological and behavioral visual responses in otherwise blind rd1 mice. However, a viable ChR2-based human therapy must meet several key criteria: (i) ChR2 expression must be targeted, robust, and long-term, (ii) ChR2 must provide long-term and continuous therapeutic efficacy, and (iii) both viral vector delivery and ChR2 expression must be safe. We have demonstrated the development of a clinically relevant therapy for late stage retinal degeneration using ChR2, in a recent study [4]. We achieved specific and stable expression of ChR2 in ON bipolar cells using a recombinant adeno-associated viral vector (rAAV) packaged in a tyrosine-mutated capsid. Targeted expression led to ChR2-driven electrophysiological ON responses in postsynaptic retinal ganglion cells and significant improvement in visually guided behavior for multiple models of blindness up to 10 months postinjection (Fig. 1). Light levels to elicit visually guided behavioral responses were within the physiological range of cone photoreceptors. Finally, chronic ChR2 expression was nontoxic, with transgene biodistribution limited to the eye. No measurable immune or inflammatory response was observed following intraocular vector administration. Together, these data indicate that virally delivered ChR2 can provide a viable and efficacious clinical therapy for photoreceptor disease-related blindness.
Figure 1. Expression of ChR2-GFP in retinal on bipolar cells leads to improved visual performance.
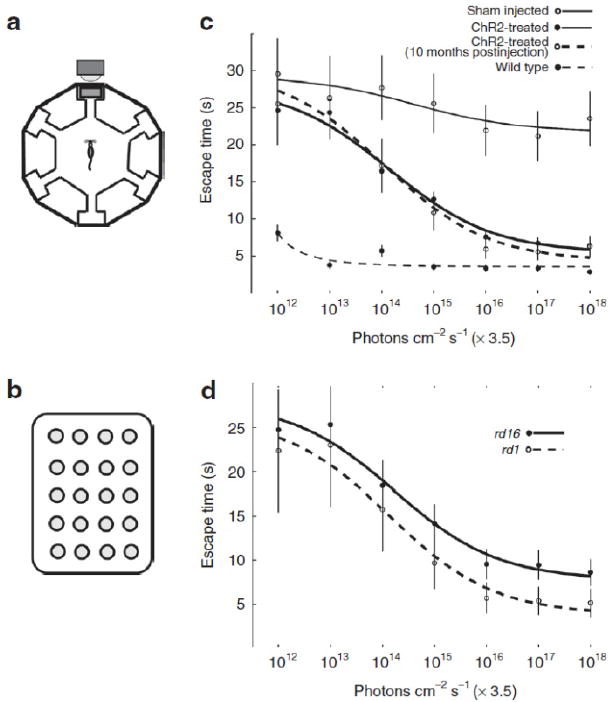
(a) The schematic for the water maze used to measure perceptual threshold (i.e., the minimum light necessary to guide the escape behavior). The escape platform was tethered to a full spectrum 5 × 4 LED array target (b) with a maximum blue light output of 3.5 × 1018 photons/cm−2/s as measured at 470 nm. The ambient light of the room was dim and at least two orders of magnitude below the LED array target output. After 14 training sessions, escape time was measured at different light levels (x axis in c and d). We compared the behavioral performance of rd1 and rd16 mice treated with AAV8-Y733F (mGRM6-SV40-ChR2-GFP) (c) relative to the performance of untreated rd1 mice and normally sighted wild-type C57Bl/6 mice. Generally, both the rd1 and rd16 mouse models of blindness had improved escape times as a function of increasing light intensity, however, rd1 mice appear to perform better than rd16 (d). ChR2, human codon-optimized channelrhodopsin-2; GFP, enhanced green fluorescent protein. Adapted from ref. [4].
III. HARDWARE FOR LIGHT DELIVERY
Optical fibers are commonly inserted into living tissues such as the brain in order to deliver light to deep targets for optogenetics. However, an optical fiber is limited to delivering light to a single target within the three-dimensional structure of the brain. Ideally, for sensory restoration, for example to deliver light to spiral ganglion neurons or to other sensory neurons in deep tissues, it would be possible to deliver light into the nervous system in a 3-d pattern. We recently demonstrated a multi-waveguide probe capable of independently delivering light to multiple targets along the probe axis, thus enabling versatile optical control of sets of distributed brain targets [6]. The 1.45 cm long probe is microfabricated in the form of a 360 micron-wide array of 12 parallel silicon oxynitride (SiON) multi-mode waveguides clad with SiO2 and coated with aluminum; probes of custom dimensions are easily created as well. The waveguide array accepts light from a set of sources at the input end, and guides the light down each waveguide to an aluminum corner mirror that efficiently deflects light away from the probe axis. Light losses at each stage are small (input coupling loss, 0.4 ± 0.3 dB; bend loss, negligible; propagation loss, 3.1 ± 1 dB/cm using the out-scattering method and 3.2 ± 0.4 dB/cm using the cut-back method; corner mirror loss, 1.5 ± 0.4 dB); a waveguide coupled, for example, to a 5 mW source will deliver over 1.5 mW to a target at a depth of 1 cm.
In conclusion, we have explored the principles of optogenetic sensory replacement for the space of blindness. We have also developed a probe for delivery of light into the brain and nervous system, important for sensory restoration for sensory modalities in the body that require central or peripheral light delivery (e.g., auditory, vestibular, somatosensory). Future improvements in molecules, devices, and principles of use of these technologies will continue to advance the use of optogenetics in sensory replacement.
Figure 2. Design and fabrication of a multi-waveguide probe capable of independent light delivery to multiple targets along the probe axis.
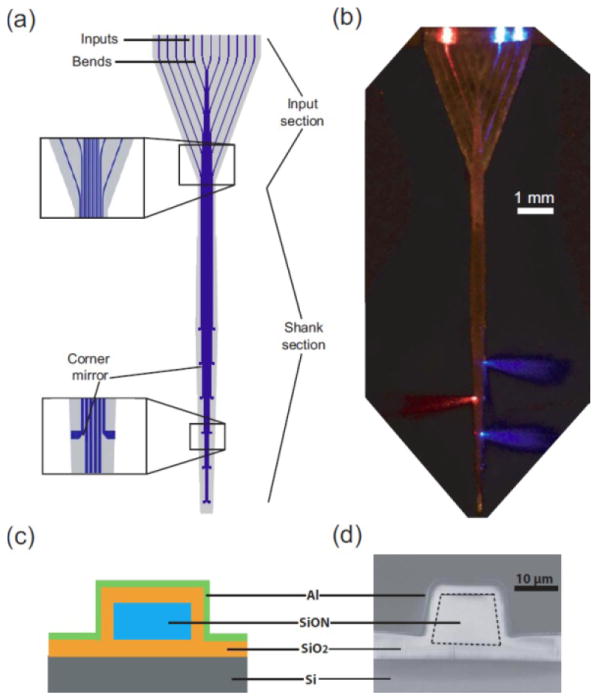
(a) A schematic of an example waveguide probe, 360 microns wide, and containing 12 waveguides, with inputs, bends, shanks, and corner mirrors labeled. (b) A photomicrograph of a waveguide probe fabricated according to the design in (a), with light coupled into three of the twelve waveguides and immersed in a scattering medium, showing emission of 473 nm and 632 nm light out three separate ports. (c) Cross section of a single waveguide, taken through the shank section of the probe. (d) SEM of a single waveguide, for the cross section shown in (c). Adapted from ref. [6].
Acknowledgments
The probe research was funded by the MIT McGovern Institute Neurotechnology Program, the Paul Allen Family Foundation, and NIH. The retinal research was funded by NIH grants EY019201, EY13729, EY11123, and EY08571; The Wallace H. Coulter Foundation; The Baxter Foundation; The Macular Vision Research Foundation; Foundation Fighting Blindness; Hope for Vision; Research to Prevent Blindness. We thank Constance Cepko (Harvard University) for kindly providing the use of her regulatory sequence.
Footnotes
M.M.D. is an employee of Eos Neuroscience, Inc. and holds equity; K.P.G. is consultant to Eos Neuroscience, Inc. and holds equity; E.S.B. is a cofounder of Eos Neuroscience, Inc. and holds equity; W.W.H. is on the scientific advisory board of Eos Neuroscience, Inc. and holds equity in AGTC, Inc. that may, in the future, commercialize some aspects of this work; A.H. is an employee and cofounder of Eos Neuroscience, Inc. and holds equity.
Contributor Information
M. Mehdi Doroudchi, Eos Neuroscience, Inc., Los Angeles, California, USA.
Kenneth P. Greenberg, Eos Neuroscience, Inc., Los Angeles, California, USA and the Division of Neurobiology, University of California Berkeley, Berkeley, California, USA.
Anthony N. Zorzos, MIT Media Lab Synthetic Neurobiology Group, Departments of Brain and Cognitive Sciences and Biological Engineering, MIT McGovern Institute, MIT Microsystems Technology Laboratory, MIT Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA 02139.
William W. Hauswirth, Email: hauswrth@ufl.edu, Department of Ophthalmology, University of Florida (UF), Gainesville, Florida.
Clifton G. Fonstad, Email: fonstad@mit.edu, MIT Microsystems Technology Laboratory, MIT Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139.
Alan Horsager, Email: horsager@usc.edu, Eos Neuroscience, Inc., Los Angeles, California, USA, and the Institute for Genetic Medicine, University of Southern California (USC), Los Angeles, California, USA.
Edward S. Boyden, Email: esb@media.mit.edu, Media Lab, McGovern Institute, and Depts. Of Brain and Cognitive Sciences and Biological Engineering, at the Massachusetts Institute of Technology, Cambridge, MA 02139, and Eos Neuroscience, Inc., Los Angeles, California, USA.
References
- 1.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically-targeted optical control of neural activity. Nature Neuroscience. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 2.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered Channelrhodopsin-2 Safely and Effectively Restores Visual Function in Multiple Mouse Models of Blindness. Molecular Therapy. 2011 doi: 10.1038/mt.2011.69. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han X, Qian X, Bernstein JG, Zhou H-H, Talei Franzesi G, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron. 2009;62(2):191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zorzos AN, Boyden ES, Fonstad CG. A Multi-Waveguide Implantable Probe for Light Delivery to Sets of Distributed Brain Targets. Optics Letters. 2010;35(24):4133–5. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]


