Abstract
Purpose
This study was undertaken to assess historical trends in the use of radiation therapy (RT) for pediatric cancers over the past 4 decades.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results database of the 9 original tumor registries (SEER9) was queried to identify patients aged 0–19 years with acute lympholytic leukemia (ALL), acute myeloid leukemia (AML), bone and joint, brain and other nervous system, Hodgkin’s lymphoma (HL), neuroblastoma, non-Hodgkin’s lymphoma (NHL), soft tissue, Wilms tumor, or retinoblastoma from 1973 to 2008. Patients were grouped into 4 year time epochs. Number and percentage of patients who received RT as a part of initial treatment were calculated per epoch by each diagnosis group from 1973–2008.
Results
RT usage for ALL, NHL, and retinoblastoma declined sharply from 57%, 57%, and 30% in 1973–76 to 11%, 15%, and 2% in 2005–08, respectively. Similarly, smaller declines in RT usage were also seen in brain (70% to 39%), bone (41% to 21%), Wilms tumors (75% to 53%), and neuroblastoma (60% to 25%). RT usage curves for Wilms tumors and neuroblastoma were nonlinear with nadirs in 1993–96 at 39% and 19%, respectively. There were minimal changes in RT use for HL, soft tissue cancers, or AML, roughly stable at 72%, 40%, and 11%, respectively. Almost all patients treated with RT were given exclusively external beam radiation therapy (EBRT). However, from 1985–2008, treatments involving brachytherapy, radioisotopes, or combination therapy increased in frequency, comprising 1.8%, 4.6%, and 11.9% of RT treatments in brain cancer, soft tissue cancer, and retinoblastoma, respectively.
Conclusions
The use of RT is declining over time in seven out of ten pediatric cancer categories. A limitation of this study is a potential underascertainment of radiotherapy usage in the SEER9 database including the delayed use of RT.
Keywords: History, pediatric oncology, radiation oncology, SEER
Introduction
Approximately 12,000 new cases of childhood cancer are diagnosed in the United States each year for patients 19 years or younger1. The most common childhood cancers include leukemia, brain and CNS tumors, and lymphomas, with neuroblastomas, Wilms tumors, and sarcomas being less common1. Over the past 20 years, incidence rates for invasive cancers have increased from 11.5 per 100,000 in 1975 to 14.5 per 100,000 in 20042. However, the 5-year survival rate for childhood cancers has increased from 58.1 % to 79.6%2. The majority of the improvement in survival has been seen in leukemias and lymphomas, with 5 year survival rates for acute lymphoblastic leukemia (ALL) increasing from 61% to 88% and Non-Hodgkin’s Lymphoma (NHL) increasing from 45% to 88% in that time period. This improvement can be attributed to advances in chemotherapy adapted to prognostic or risk stratification, but secondarily to enhanced surgical and radiotherapy techniques. Equally important, the large number of clinical trials and randomized control trials carried out in the past 50 years has led to more effective treatment regimens involving the aforementioned modalities despite the relative rarity of pediatric tumors.
The use of radiotherapy has historically been one of the great successes in the treatment of pediatric cancers, particularly in ALL, HL, rhabdomyosarcoma, Wilms, and Ewing’s sarcoma3. Beginning in the 1960s, St. Jude Children’s Research Hospital was a pioneer in some of the earliest trials for ALL. These “Total Therapy” trials greatly increased the five-year survival rate of ALL with the use of increased craniospinal irradiation. The following decade, the National Wilms Tumor Society initiated a series of randomized studies and established the use of RT in late stage Wilms tumor. However, despite these successes, radiation therapy (RT) is fraught with challenges and adverse side effects that have resulted in its diminished use in treating pediatric cancers over the past 20 years. Although RT is cytotoxic against tumor cells, it also has deleterious effects towards normal tissue. One of the most significant side effects for pediatric patients treated with RT has been growth and developmental failure4. Other late effects include gastrointestinal dysfunction, pulmonary and cardiac abnormalities, neurocognitive defects, infertility, and secondary cancers. As the late effects of radiotherapy in pediatric cancer have been well documented, there has been a concerted effort to reserve its use, in tandem with other therapies, to clinical scenarios in which the benefits are well documented and outweigh the risks5. However, to retain a favorable therapeutic ratio, there has been particular attention to optimizing benefit with RT dose and volume reductions where feasible.
Based on the current use of RT for modern pediatric cancer patients, and the concern for delayed irradiation treatment-related side effects, it is worthwhile to examine the historical usage of RT in the past 25 years. The use of radiotherapy in the clinic has varied over time with the advent of effective chemotherapeutics and enhanced surgical techniques. In this paper we examined trends in the use of radiation therapy for common childhood cancers from 1973–2008.
Methods
The Surveillance, Epidemiology, and End Results (SEER) registry of the National Cancer Institute (NCI) collects data on all patients diagnosed within each SEER region and is largely representative of the general U.S. population with regard to poverty and education level, though with more urban and foreign-born persons6. The original 9 SEER registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Ohio) contain epidemiologic data as early as 1973 (except for Seattle-Puget Sound, and Atlanta, which have data from 1974 and 1975, respectively). The SEER-9 registry data was queried to identify patients aged 0–19 years with acute lymphoblastic leukemia, acute myeloid leukemia, bone and joint cancers, cancers of the brain and nervous system, Hodgkin’s lymphoma, neuroblastoma, non-Hodgkins lymphoma, soft tissue cancers, Wilms tumor, or retinoblastoma from 1973 to 2008. Patients were grouped into 4 year time epochs. Number and percentage of patients who received radiation therapy were calculated per epoch by each diagnosis group from 1973–2008.
Cancer incidence relative to population-matched US Census data was analyzed with SEER*Stat (National Cancer Institute, Bethesda, MD). All other statistical analyses were performed with STATA/SE 9.2 (Stata Corporation, College Station, Tex).
Results
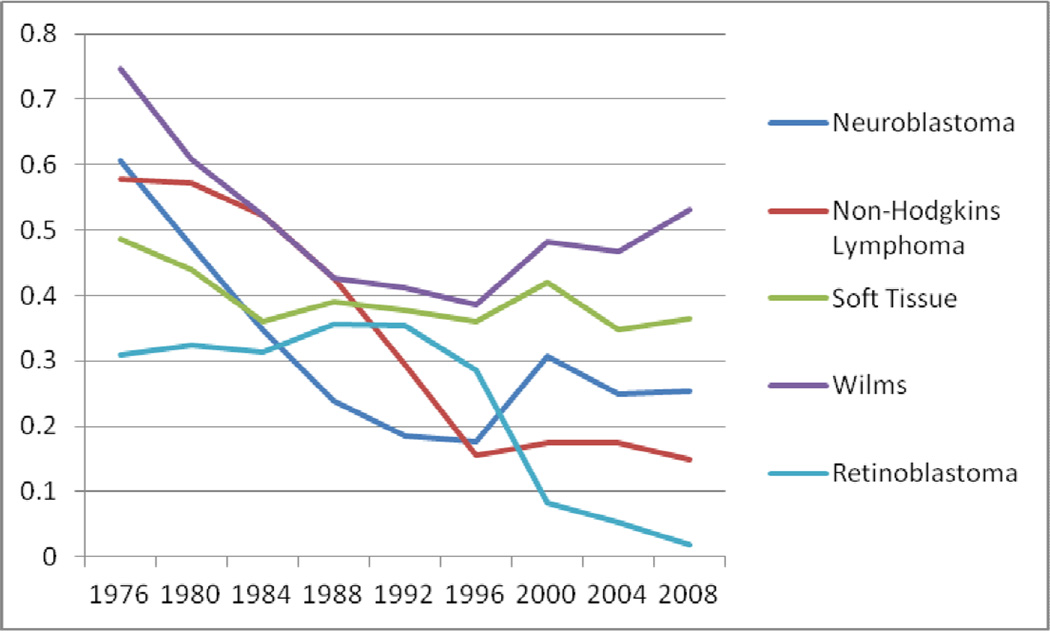
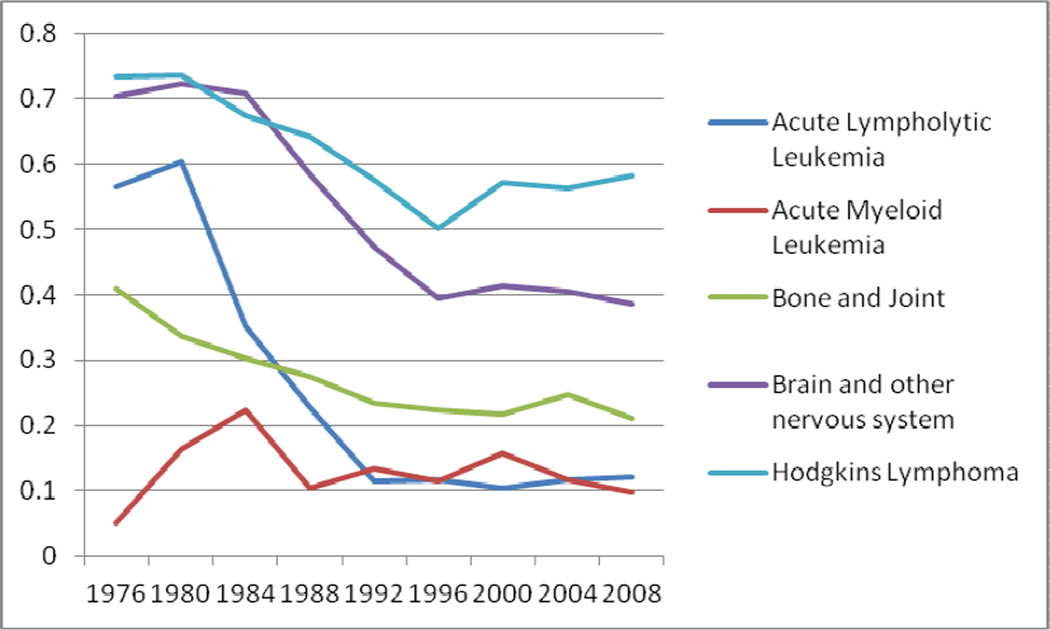
RT usage for acute lymphocytic leukemia, NHL, and retinoblastoma declined sharply from 57%, 57%, and 30% in 1973–76 to 11%, 15%, and 2% in 2005–08, respectively (Figure 1 and 2). Similarly, smaller declines in RT were also seen in brain cancers (70% to 39%), bone cancers (41% to 21%), Wilms tumor (75% to 53%), and neuroblastoma (60% to 25%). RT usage curves for Wilms tumor and neuroblastoma were nonlinear with nadirs in 1993–96 at 39% and 19%, respectively. There were minimal changes in frequency of RT use for HL and soft tissue cancers, roughly stable at 72% and 40%, respectively. The role of RT in AML remained overall low at 11% of cases. (Figures 1 and 2).
Figure 1.
Fraction of Patients Receiving RT as a part of initial treatment for Pediatric Cancers from 1973–2008
Figure 2.
Fraction of Patients Receiving RT as a part of initial treatment for Pediatric Cancers from 1973–2008
For ALL, NHL, bone cancers, Wilms tumor, neuroblastoma, HL, and AML, amongst those who received any RT, the irradiation modality was exclusively External Beam Radiation Therapy (EBRT). The usage of brachytherapy, radioisotopes, and combination therapy increased in frequency beginning in the mid-1980s. From 1985–2008, EBRT in combination with brachytherapy or radioisotopes accounted for 1.8% of all RT for patients with brain tumors. During this period, brachytherapy and combination therapy comprised 4.6% of all RT for patients with soft tissue cancers, whereas brachytherapy alone represented 11.9% of all RT for retinoblastoma patients.
Discussion
There are two major findings from our analysis. The first is that the use of radiation therapy to treat childhood cancers has generally declined from 1973–2008. The trends observed in RT use confirm what has been seen in the literature over time. RT use for patients with HL and soft tissue cancers showed minimal changes. RT use for AML has been stable, but only at a relatively low level. RT use for patients with neuroblastoma, Wilms tumor, brain cancers, and bone cancers saw moderate declines, while RT use for patients with NHL, ALL, and retinoblastoma saw sharp declines. We also found that EBRT was the dominant form of RT administered to pediatric patients. However, starting in the mid-1980s, the use of brachytherapy, radioisotopes, or both in conjunction with EBRT increased in frequency for patients with brain tumors, soft tissue cancers, and retinoblastomas.
A major success story in the history of oncology and particularly radiation oncology has been the use of subtotal or total nodal irradiation in both children and adults with Hodgkin’s Lymphoma, employing relatively large, “extended” fields to doses of 36–40 Gy. Following the development of combination chemotherapy programs for relapse or advanced stage disease, pediatric oncology adopted combined modality strategies in the late 1970s through mid-1980s with the introduction of low dose (21–25 Gy) involved field RT well before similar changes in adult practice. Initially this was motivated to reduce musculoskeletal toxicities, but later was found to be important to reduce other toxicities such as secondary cancers and cardiopulmonary effects. Improved imaging and clinical staging, along with risk stratification, have led to corresponding fine tuning of the intensity of therapy to match risk7. For HL, this continued use of radiotherapy in the pediatric population is likely due to the 90% cure rate that has been achieved7 with combined modality treatment (CMT) that includes RT. Although involved-field radiation therapy (IF-RT) has largely replaced subtotal nodal irradiation (STNI), thereby reducing many devastating late effects, secondary cancers remain a problem in pediatric HL7. However, clinical trials have shown that low dose IF-RT may not be deleted without an increase in recurrences. CCG 5942 is the latest example of the importance of retaining low dose RT8. In the future as clinical trials of pediatric HL show that low risk patients or those with an early response to chemotherapy do not require RT, the proportion of pediatric HL patients getting RT will likely decrease; however, this is not yet an observable trend in this present SEER study.
Similar to HL, RT use in soft tissue cancers has stayed proportionately the same over time. This category includes both rhabdomyosarcoma (40% of pediatric soft tissue tumors) and other non-rhabdo soft tissue sarcomas (roughly 60% of pediatric soft tissue tumors). In rhabdomyosarcomas, RT has been a part of the standard treatment in nearly all cases except when the tumor is completely resected with favorable histology, due to the need to maintain good local tumor control rates9–10. Non-rhabdo soft tissue sarcomas (NRSTS) also use RT in the treatment of high grade, bulky, and unresectable tumors11. Despite findings of subsets that respond well to chemotherapy and improved surgical techniques, the proportion of sarcomas requiring RT for local control has remained steady. Moreover, recent studies suggest that radiotherapy remains important for disease control for these high risk sarcomas12.
The other disease category showing stability in RT usage is AML. While, pediatric AML has remained a challenging disease to cure over the decades with disease free survival staying in the 40–50% range, approximately 5–15% of patients present with CNS involvement that often requires the use of RT for palliation or to complement chemotherapy. Subsequent CNS relapse remains a problem, but prophylactic cranial RT never gained widespread use in practice as it did in ALL. Studies from St. Jude13 and other groups demonstrated low rates of relapse using intrathecal chemotherapy. Thus, RT has rarely if ever been employed in the upfront treatment of AML and its use has stayed consistently low, as systemic and IT chemotherapy have been deemed moderately sufficient. However, its use has continued for palliation of CNS and extramedullary disease.
The moderate declines in RT use for patients with neuroblastoma, Wilms tumor, brain cancers, and bone cancers reflect the impact of positive and negative revelations regarding the use of RT in pediatric patients. In the 1980’s and 1990’s, neuroblastoma patients used to uniformly receive 20–40 Gy until late irradiation-related side effects were noticed, such as scoliosis and other growth abnormalities14. RT was then abandoned as molecular biology insights facilitated new prognostic groupings with surgery alone in select favorable patients, reserving chemotherapy for relapses. In other patients, chemotherapy was intensified allowing for RT to be dropped out despite earlier studies suggesting a benefit similar to HL when chemotherapy intensity was low. In the highest risk patients, intensive chemotherapy culminated in an autologous hematologic transplant along with newer systemic agents such as cis-retinoic acid or antiganglioside antibodies, which have been shown to be of benefit15–16. Myeloablative therapy involving total-body irradiation in high risk patients may account for some of the RT use in neuroblastoma patients. Moreover, recent randomized controlled trials of high risk patients have suggested a benefit of low to moderate dose RT as an important adjunct either in consolidating local control of the tumor after surgical resection or in treating resistant metastatic tumor sites17–19. This likely explains the relative plateauing of RT use in patients with neuroblastomas at roughly 25–28%.
As for bone tumors, radiotherapy is rarely used for osteosarcomas. Ewing’s sarcoma may be effectively managed with either surgery, radiotherapy, or both as local modalities. With concerns for secondary osteosarcomas from radiotherapy, improved surgical techniques, and a preference for surgery over RT for local control, a general decline in RT for Ewings sarcoma and bone tumors in general is not unexpected.
Brain tumors have shown a marked decrease in the use of RT during the period studied. While RT remains a mainstay of treatment for many brain tumors including medulloblastomas, germ cell tumors, ependymomas, and high grade gliomas, low grade gliomas have been found to be managed well initially with surgery and chemotherapy. In addition, the use of RT has diminished over time for optic chiasm tumors due to concern for late irradiation-related side effects, including strokes, pituitary and hypothalamic dysfunction, and second cancers, particularly in patients with the Neurofibromatosis-1 gene20. Radiotherapy may still be effective, but with concerns for neurocognitive dysfunction and other late effects, RT has been relegated to management of relapse of low grade gliomas, which is not recorded in the SEER database. As 30–35% of pediatric brain tumors may fall into this group of grade 1–2 astrocytomas, the deferral of RT over the last several decades largely accounts for the observed pattern of decreased RT usage from 70% to 39%.
Amongst other brain tumors in infants, there has been a push to eliminate radiation therapy given concerns for profound developmental toxicities. This has been especially the case in medulloblastomas, which is felt to be a chemotherapy sensitive tumor. However, Timmerman et al.21 conducted a study in which they examined two groups of patients – 14 receiving RT and 15 not receiving RT. They found the 3-year overall survival (OS) rate for the RT group was 28.6% compared to the OS rate for the non-RT group, 6.7%. Furthermore, the only significant predictive factor for OS was administration of radiotherapy. On the other hand, young children with the desmoplastic variant of medulloblastoma may be adequately managed with surgery and chemotherapy without craniospinal RT. Advances in the use of RT in ependymoma, another brain tumor afflicting very young children, has shown that postoperative RT using conformal techniques such as IMRT to limit neurocognitive damage has allowed for improved outcomes22 and more opportunities for radiation oncologists to participate in the care of pediatric brain tumors in a beneficial manner.
Treatment of Wilms tumor with RT has decreased moderately, mainly due to results from a series of randomized trials by the National Wilms Tumor Study group in the 1970s and 80s. Their findings showed that RT was unnecessary for children with stage I and II Wilms tumors of favorable histology and yet was effective at low doses to stage III patients23.
Several cancer types have shown a sharp decline in the use of RT in pediatric patients including NHL, ALL, and retinoblastoma. The sharp decline in RT use to treat patients with NHL reflects the findings from a clinical trial in 198724 that showed no benefit from adjuvant radiotherapy in advanced-stage aggressive NHL. Most NHL presents as a high-grade tumor, like Burkitt’s Lymphoma or lymphoblastic lymphoma, and responds well to chemotherapy alone. Early-stage, indolent NHL, however, does respond well to radiotherapy, and likely explains the majority of RT use in pediatric NHL. Similarly, the use of RT in ALL has decreased dramatically over the past 40 years. A major advance in the management of ALL in the 1970s was the introduction of cranial RT with intrathecal therapy to combat the common problem of CNS relapse, for which prednisone and vincristine were the mainstays of systemic therapy. St. Jude Children’s Research Hospital and cooperative group trials dramatically showed an improved 50–60% survival rate compared to 3–4% with the prior use of first and second generation chemotherapy alone programs as evidenced by the St. Jude and pediatric cooperative group trials25. However, cognitive, endocrine and secondary cancer toxicities from 24 Gy cranial irradiation became apparent in follow-up5. Improvements in risk stratification and intensification of chemotherapy, including agents crossing the blood-brain barrier, have led to the curtailed use of RT in ALL. Investigators at St. Jude no longer use any RT in the upfront management of ALL, while other cooperative groups internationally have migrated to highly selective use of RT in high risk cases along with a reduction in irradiation doses afforded by improved systemic therapy to help limit toxicities26. RT use in retinoblastoma (Rb) has been declining and is currently prescribed infrequently due to the efficacy of chemotherapy in primary management27 as well as the concern for secondary malignancies, especially in hereditary Rb. However, an interesting trend that was confirmed in our analysis was the rising use of plaque brachytherapy as an effective form of local control of this disease28–30, comprising 12% of all RT treatments delivered for Rb between 1985–2008.
In the future, trends indicate that RT will remain important for the treatment of the pediatric population, although with more caution about its implementation compared to earlier decades. It is likely that RT will continue to be used in CMT along with surgery and chemotherapy. However, its use will be limited to high-risk subsets of malignant diseases. Improvements on current technologies will hopefully lead to lower cumulative doses delivered to smaller volumes, resulting in fewer side effects from treatment.
Conclusions
The use of RT is declining over time in seven out of ten pediatric cancer categories. However, it is likely that RT will still play an important role on some pediatric tumors for the foreseeable future. Furthermore, these data have implications for specialized treatment technologies and radiation oncology training needs referable to pediatric radiation oncology.
SUMMARY.
In children, the use of radiotherapy (RT) may be associated with developmental toxicity and secondary cancers. Increasingly, pediatric oncology trials have shifted to risk stratification that has sought to restrict the use, volume, or dose of RT to reduce late effects. Using the Surveillance, Epidemiology, and End Results database of the 9 original tumor registries (SEER9), the authors found a decrease in RT usage in seven out of ten pediatric cancer subtypes from 1973–2008.
Acknowledgements
None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors report no conflicts of interest, financial or otherwise.
References
- 1.National Cancer Institute. Childhood Cancers. 2008;Vol 2012 [Google Scholar]
- 2.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2012. [Google Scholar]
- 3.O'Leary M, Krailo M, Anderson JR, et al. Progress in Childhood Cancer: 50 Years of Research Collaboration, a Report From the Children's Oncology Group. Seminars in Oncology. 2008;35:484–493. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breneman JC, Narayana A. Principles of Pediatric Radiation Oncology. In: Bast RC Jr, Kufe DW, Pollock RE, editors. Holland-Frei Cancer Medicine. 5 ed. Hamilton, ON: BC Decker; 2000. [Google Scholar]
- 5.Gibbs IC, Tuamokumo N, Yock TI. Role of radiation therapy in pediatric cancer. Hematol Oncol Clin North Am. 2006;20:455–470. doi: 10.1016/j.hoc.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Population Characteristics. 2012;Vol 2012 [Google Scholar]
- 7.Donaldson SS. Finding the Balance in Pediatric Hodgkin's Lymphoma. J Clin Oncol. 2012;29:29. doi: 10.1200/JCO.2012.42.6890. [DOI] [PubMed] [Google Scholar]
- 8.Wolden SL, Chen L, Kelly KM, et al. Long-Term Results of CCG 5942: A Randomized Comparison of Chemotherapy With and Without Radiotherapy for Children With Hodgkin's Lymphoma--A Report From the Children's Oncology Group. J Clin Oncol. 2012;29:29. doi: 10.1200/JCO.2011.41.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuck A, Mattke AC, Schmidt B, et al. Group II rhabdomyosarcoma and rhabdomyosarcomalike tumors: is radiotherapy necessary? J Clin Oncol. 2004;22:143–149. doi: 10.1200/JCO.2004.04.180. [DOI] [PubMed] [Google Scholar]
- 10.Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari A, Miceli R, Rey A, et al. Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer. 2011;47:724–731. doi: 10.1016/j.ejca.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walterhouse DO, Meza JL, Breneman JC, et al. Local control and outcome in children with localized vaginal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma committee of the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:76–83. doi: 10.1002/pbc.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution's experience. Leukemia. 2003;17:2090–2096. doi: 10.1038/sj.leu.2403131. [DOI] [PubMed] [Google Scholar]
- 14.Paulino AC, Fowler BZ. Risk factors for scoliosis in children with neuroblastoma. International Journal of Radiation Oncology*Biology*Physics. 2005;61:865–869. doi: 10.1016/j.ijrobp.2004.07.719. [DOI] [PubMed] [Google Scholar]
- 15.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children's Cancer Group Study. J Clin Oncol. 2000;18:4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 17.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 18.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–1554. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 19.Rich BS, McEvoy MP, LaQuaglia MP, et al. Local control, survival, and operative morbidity and mortality after re-resection, and intraoperative radiation therapy for recurrent or persistent primary high-risk neuroblastoma. J Pediatr Surg. 2011;46:97–102. doi: 10.1016/j.jpedsurg.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Timmermann B, Kortmann RD, Kuhl J, et al. Role of radiotherapy in supratentorial primitive neuroectodermal tumor in young children: results of the German HIT-SKK87 and HIT-SKK92 trials. J Clin Oncol. 2006;24:1554–1560. doi: 10.1200/JCO.2005.04.8074. [DOI] [PubMed] [Google Scholar]
- 22.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DM. The treatment of stages I–IV favorable histology Wilms' tumor. J Clin Oncol. 2004;22:1366–1372. doi: 10.1200/JCO.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell MJ, Harrington DP, Earle JD, et al. Prospectively randomized clinical trial of three intensive chemotherapy regimens for the treatment of advanced unfavorable histology non-Hodgkin's lymphoma. J Clin Oncol. 1987;5:1329–1339. doi: 10.1200/JCO.1987.5.9.1329. [DOI] [PubMed] [Google Scholar]
- 25.Leukemia and Lymphoma Society. Facts. 2012;Vol 2012 2012. [Google Scholar]
- 26.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman DL, Himelstein B, Shields CL, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18:12–17. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- 28.Merchant TE, Gould CJ, Wilson MW, et al. Episcleral plaque brachytherapy for retinoblastoma. Pediatr Blood Cancer. 2004;43:134–139. doi: 10.1002/pbc.20094. [DOI] [PubMed] [Google Scholar]
- 29.Shields CL, Mashayekhi A, Sun H, et al. Iodine 125 plaque radiotherapy as salvage treatment for retinoblastoma recurrence after chemoreduction in 84 tumors. Ophthalmology. 2006;113:2087–2092. doi: 10.1016/j.ophtha.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Shields CL, Shields JA, Cater J, et al. Plaque radiotherapy for retinoblastoma: long-term tumor control and treatment complications in 208 tumors. Ophthalmology. 2001;108:2116–2121. doi: 10.1016/s0161-6420(01)00797-7. [DOI] [PubMed] [Google Scholar]