Abstract
Natural history studies suggest increased risk for kidney function decline with HIV infection, but few studies have made comparisons with HIV-uninfected women. We examined whether HIV infection treated with highly active antiretroviral therapy (HAART) remains associated with faster kidney function decline in the Women's Interagency HIV Study. HIV-infected women initiating HAART with (n=105) or without (n=373) tenofovir (TDF) were matched to HIV-uninfected women on calendar and length of follow-up, age, systolic blood pressure, hepatitis C antibody serostatus, and diabetes history. Linear mixed models were used to evaluate differences in annual estimated glomerular filtration rate (eGFR). Person-visits were 4,741 and 11,512 for the TDF-treated and non-TDF-treated analyses, respectively. Mean baseline eGFRs were higher among women initiated on TDF-containing HAART and lower among those on TDF-sparing HAART compared to their respective HIV-uninfected matches (p<0.05 for both). HIV-infected women had annual rates of eGFR changes similar to HIV-uninfected matches (p-interaction >0.05 for both). Adjusting for baseline eGFR, mean eGFRs at 1 and 3 years of follow-up among women initiated on TDF-containing HAART were lower than their uninfected matches (−4.98 and −4.26 ml/min/1.73 m2, respectively; p<0.05 for both). Mean eGFR of women initiated on TDF-sparing HAART was lower versus uninfected matches at 5 years (–2.19 ml/min/1.73 m2, p=0.03). HAART-treated HIV-infected women had lower mean eGFRs at follow-up but experienced rates of annual eGFR decline similar to HIV-uninfected women. Tenofovir use in HIV-infected women with normal kidney function did not accelerate long-term kidney function decline relative to HIV-uninfected women.
Introduction
Prior to effective antiretroviral therapy in 1996, natural history studies of HIV infection demonstrated that HIV-infected individuals were at high risk of kidney function decline.1 The initial reports of HIV-related kidney disease described a rapidly progressive condition of what is now referred to as HIV-associated nephropathy (HIVAN).2 Prior to the more widespread use of highly active antiretroviral therapy (HAART), HIVAN was the third leading cause of end-stage renal disease among younger African-American adults.1 With initiation of HAART at earlier stages of HIV infection and greater longevity of HIV-infected persons, HIVAN is increasingly uncommon,3 and other causes of kidney disease have emerged as important contributors to kidney disease in this patient population. Kidney disease, however, remains a strong risk factor for death among HIV-infected individuals.4
Although some contemporary observational studies have shown that HAART is effective in stabilizing or even reversing the previously unrelenting course of HIVAN,3,5 the effect of HAART use on other forms of kidney disease, such as immune-complex glomerulonephritis, is unknown. Moreover, concerns for detrimental renal effects of HAART-related metabolic changes and direct renal toxicity of certain antiretroviral drugs exist.6–8 For example, tenofovir (TDF), one of the most common components of modern HAART regimens, has been variably associated with declines in longitudinal kidney function.7,9–12 To determine whether HAART-treated HIV infection is associated with faster decline in kidney function, we compared longitudinal estimates of glomerular filtration rate (eGFR) between HAART-treated HIV-infected women and HIV-uninfected women with similar baseline risk factors for kidney disease in the Women's Interagency HIV Study (WIHS).
Materials and Methods
Study population
The WIHS is an ongoing, multicenter prospective cohort study of HIV-infected and HIV-uninfected women with similar sociodemographic characteristics. The study was designed to evaluate the natural and treated history of HIV infection and has been previously described in detail.13,14 Briefly, the six study sites are located in Bronx and Brooklyn, New York; Chicago, Illinois; Los Angeles and San Francisco, California; and Washington, District of Columbia. Women were enrolled from October 1994 through November 1995 and from October 2001 through September 2002. Participants undergo interviews, physical examinations, and laboratory tests according to standardized protocols. The study is approved by all local institutional review boards.
In this longitudinal substudy, HIV-infected women who initiated HAART were matched to HIV-uninfected women on calendar and follow-up time, age±5 years, systolic blood pressure±20 mm Hg, hepatitis C antibody serostatus, and history of diabetes mellitus at the visit prior to HAART initiation (pre-HAART visit). If data were missing at the visit prior to HAART initiation, values no more than 1 year prior to HAART initiation were used for matching. We excluded women who (1) were receiving HAART or had previously received HAART at enrollment (n=487); (2) could not be matched to an HIV-uninfected woman based upon chronic kidney disease (CKD) risk factors at the pre-HAART visit (n=824); (3) did not have a kidney function estimate or data on matching factors available at the pre-HAART visit (n=259); or (4) had eGFR below 15 ml/min/1.73 m2 (n=13). HIV-infected women were categorized according to whether or not their initial HAART regimen included TDF, and matching was stratified by this initial regimen type such that the same HIV-uninfected woman could contribute as a match to women in both groups. Women were followed from the pre-HAART visit to April 1, 2010.
Kidney function and other variables
Serum creatinine was measured biannually using the modified Jaffe method.15 Glomerular filtration rate (eGFR) was estimated at the pre-HAART visit, then semiannually using the abbreviated Modification of Diet in Renal Disease (MDRD) equation.16 There were 629 values in which eGFR exceeded 140 ml/min/1.73 m2; we reset these values to 140 ml/min/1.73 m2. The definition of HAART was guided by the U.S. Department of Health and Human Services treatment guidelines.17 Diabetes history was defined by self-report, insulin or oral hypoglycemic medication use, a fasting glucose of 126 mg/dl or greater, or hemoglobin A1c of greater than 6.5%. CD4+ lymphocyte count and HIV-1 RNA levels were measured semiannually in HIV-infected women.
Statistical analysis
The individual longitudinal eGFR trajectories were analyzed using linear mixed effect models to account for the repeated assessments of the outcome for each individual. Interaction terms with time were used to evaluate differences in the rate of eGFR decline by HIV serostatus. All analyses were stratified by type of initial HAART regimen (TDF regimen or non-TDF regimen) and adjusted for pre-HAART eGFR and race. HIV-infected women who initiated on TDF-containing HAART regimens were considered TDF exposed for the duration of the follow-up to evaluate the residual effect of TDF on kidney function. All women were censored at the last available visit or death. In addition, HIV-infected women initiated on TDF-sparing HAART were also censored along with their uninfected matches upon switching to a TDF-containing HAART regimen (n=443). To adjust for potential bias as a result of differential loss to follow-up of those with more rapid progression of kidney dysfunction, we applied inverse probability of censoring weights to our models.18 Weights were constructed at each time point for each individual using pooled logistic regression to predict the probability of remaining in the study from the following covariates: CD4+ cell counts, HIV-1 viral load, injection drug use, age at visits, and comorbidity. By up-weighting the contribution of the observation by the probability of remaining in the study, observations from individuals with a high likelihood of exiting the study are given larger weight to counter their underrepresentation in the study. All statistical analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
At the time of this analysis, WIHS had enrolled 2,791 HIV-infected and 953 HIV-uninfected women. A total of 1,561 HIV-infected women initiated HAART during follow-up. For 105 of the 162 HIV-infected women who initiated TDF-containing HAART, we found 474 matches with similar characteristics at the pre-HAART visit. We also identified 740 HIV-uninfected women with matching characteristics at the pre-HAART visit for 373 of 1,159 HIV-infected women who initiated TDF-sparing HAART. Compared with HIV-infected women excluded from the study, HIV-infected women included in the study were younger (mean age of 39 vs. 37 years old, respectively; p=0.05), less likely to be hepatitis C antibody positive (45% vs. 29%, respectively; p<0.001), and more likely to have a history of diabetes (6% vs. 11%, respectively; p<0.001). Conversely, they had a similar proportion of African-American women and similar levels of CD4+ cells, HIV-1 RNA, systolic blood pressure, and kidney function. In total, 4,741person-visits were included in the analysis of the TDF-treated group. Among the group initiated on TDF-sparing HAART, 11,512 person-visits were included in the analysis.
The study population was comprised of young, predominantly African-American, normotensive women with normal kidney function (Table 1). While the mean baseline eGFR for HIV-infected women initiated on TDF-containing HAART was higher than their uninfected matches (103 vs. 97 ml/min/1.73 m2, respectively; p=0.03), the mean baseline eGFR for HIV-infected women initiated on TDF-sparing HAART was lower than their uninfected matches (95 vs. 98 ml/min/1.73 m2, respectively; p=0.02). One-fourth to nearly one-third of women were hepatitis C antibody positive. Approximately 18% of HIV-infected women receiving TDF-containing HAART had diabetes at the pre-HAART visit, while 9% of HIV-infected women who initiated TDF-sparing HAART were diabetic. Among HIV-infected women, the median CD4+ counts were modestly low, and the range of HIV-1 RNA levels was wide.
Table 1.
Study Population Characteristics at Pre-Highly Active Antiretroviral Therapy Visit
| Characteristics | HIV+ with TDF (n=105) | Matched HIV− (n=474) | HIV+ without TDF (n=373) | Matched HIV− (n=740) |
|---|---|---|---|---|
| Median age, years (IQR) | 38 (34–47) | 38 (33–46) | 37 (31–43) | 37 (31–42) |
| Black, % | 59 | 64 | 60 | 59 |
| Median systolic blood pressure, mm Hg (IQR) | 112 (104–126) | 113 (110–123) | 113 (102–128) | 116 (108–124) |
| Hepatitis C antibody positive, % | 24 | 24 | 29 | 29 |
| Diabetic, % | 18 | 18 | 9 | 9 |
| Median CD4+ cell count, cells/mm3 (IQR) | 287 (200–381) | — | 281 (148–424) | — |
| Median HIV-1 RNA level, copies/ml (IQR) | 27,000 (1,700–65,000) | — | 17,500 (4,000–865,000) | — |
| Mean estimated GFR, ml/min/1.73 mm3 (SD) | 103 (23) | 97 (20) | 95 (25) | 98 (21) |
TDF, tenofovir; IQR, interquartile range; GFR, glomerular filtration rate; SD, standard deviation.
Kidney function in HIV-infected women initiated on HAART with TDF and matched HIV-uninfected women
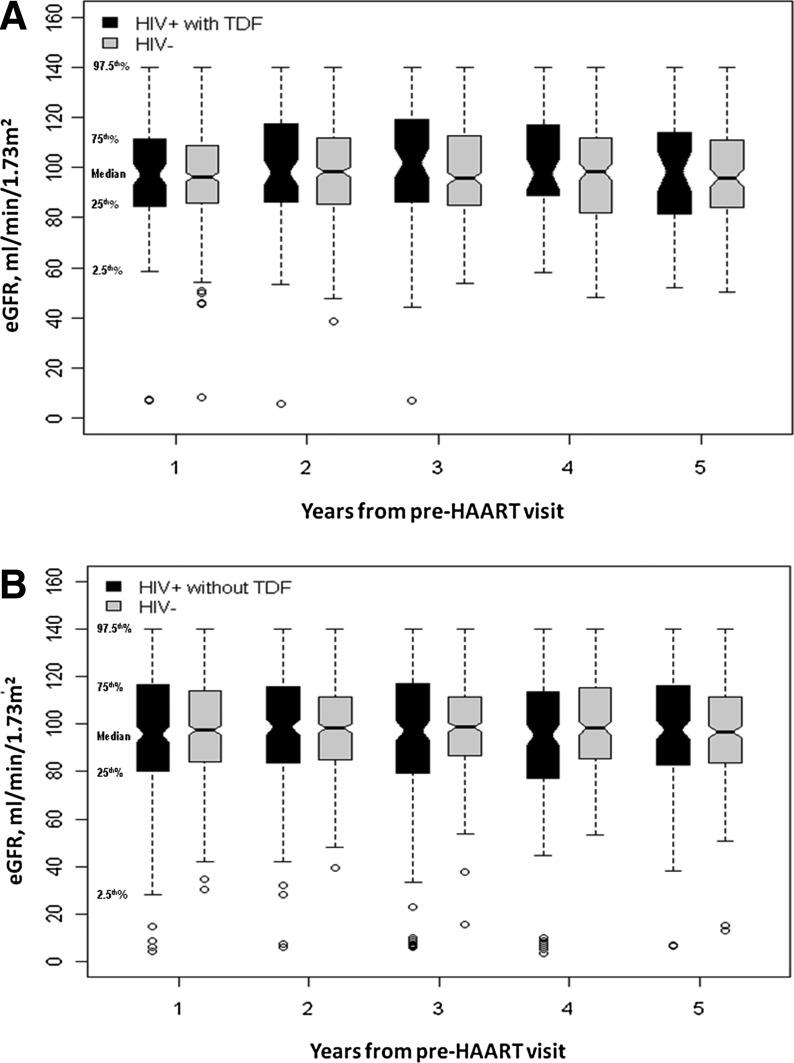
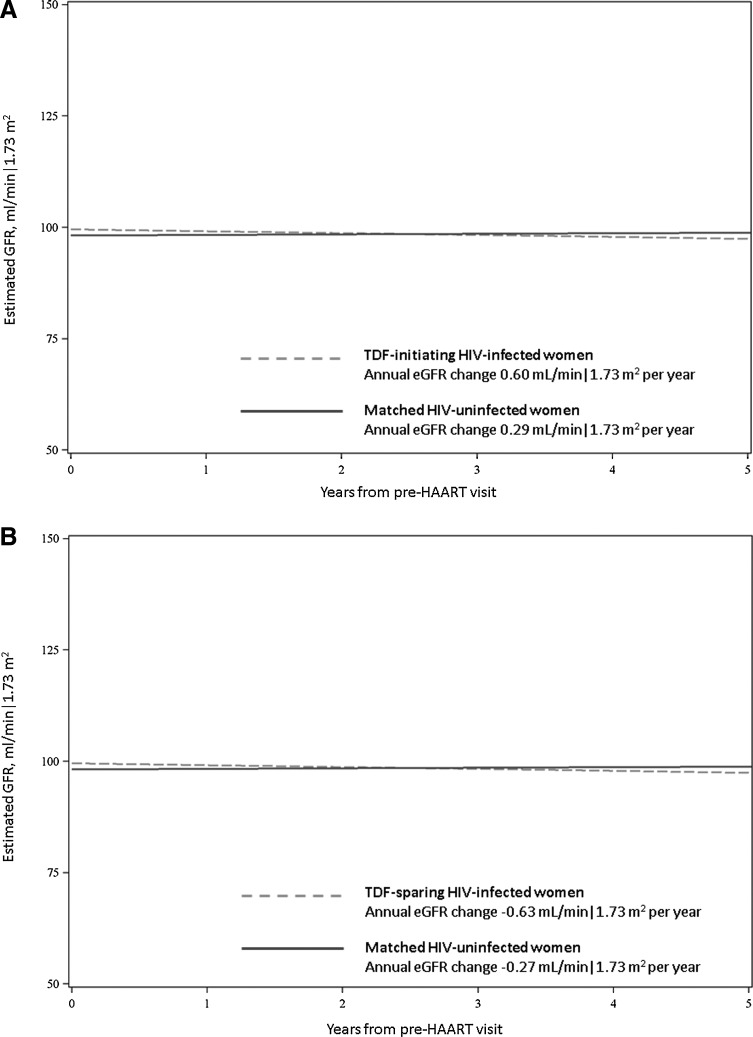
The distribution of eGFR values during 5 years of follow-up among HIV-infected women who initiated on TDF-containing HAART and their HIV-uninfected matches is displayed in Fig. 1A. In general, eGFR values were widely distributed, and the median eGFR values were similar between the two groups. Figure 2A displays the nonweighted trends in longitudinal kidney function in HAART-initiated HIV-infected women and their HIV-uninfected matches, stratified by TDF status at HAART initiation. Women initiating on TDF-containing HAART appeared to have a slight decline in kidney function longitudinally while their matched HIV-uninfected women appeared to have stable kidney function. In weighted models, the mean annual change in eGFR was similar between TDF-treated HIV-infected women [0.60 ml/min/1.73 m2 per year, 95% confidence interval (CI): −0.24, 1.44] and HIV-uninfected matches (0.29 ml/min/1.73 m2 per year, 95% CI: −0.11, 0.69) (p for interaction=0.51). Estimated GFRs at 1 and 3 years following HAART initiation in TDF-treated HIV-infected women were lower than those of matched HIV-uninfected women (mean difference of −4.98 and −4.26 ml/min/1.73 m2, respectively, p<0.05 for both). This difference was slightly smaller at 5 years of follow-up (–3.74 ml/min/1.73 m2), but no longer reached statistical significance (p=0.07) (Table 2).
FIG. 1.
Distribution of eGFR values during 5 years of follow-up among highly active antiretroviral therapy (HAART)-initiated HIV-infected women versus HIV-uninfected women. (A) The estimated glomerular filtration rate (eGFR) distribution among HIV-infected women initiated on tenofovir (TDF)-containing HAART versus matched HIV-uninfected women. (B) The eGFR distribution among HIV-infected women initiated on TDF-sparing HAART versus matched HIV-uninfected women. Open circles represent outliers.
FIG. 2.
Longitudinal kidney function among HAART-initiated HIV-infected women versus HIV-uninfected women. (A) The longitudinal kidney function among HIV-infected women initiated on TDF-containing HAART versus matched HIV-uninfected women. (B) The longitudinal kidney function among HIV-infected women initiated on TDF-sparing HAART versus matched HIV-uninfected women. The estimated glomerular filtration rate (GFR) for HIV-infected women is shown by the dashed line and for HIV-uninfected women is shown by the solid line.
Table 2.
Mean Estimated Glomerular Filtration Rate and Annual Change in Estimated Glomerular Filtration Rate in HIV-Infected and Matching HIV-Uninfected Women
| TDF-initiating HIV-infected women | Matched HIV-uninfected women | p-value | TDF-sparing HIV-infected women | Matched HIV-uninfected women | p-value | |
|---|---|---|---|---|---|---|
| Mean estimated GFRa after HAART initiation (years) | ||||||
| 1 year | 94.92 (92.54, 97.29) | 99.90 (98.80, 101.00) | <0.001 | 98.68 (97.08, 100.29) | 99.42 (98.27, 100.56) | 0.47 |
| 3 years | 96.12 (93.55, 98.69) | 100.48 (99.29, 101.68) | 0.003 | 97.41 (95.93, 98.89) | 98.87 (97.81, 99.93) | 0.12 |
| 5 years | 97.33 (93.69, 100.96) | 101.07 (99.36, 102.78) | 0.07 | 96.14 (94.51, 97.98) | 98.33 (97.13, 99.54) | 0.03 |
| Mean change in estimated GFR per yearb | ||||||
| 0.60 (−0.24, 1.44) | 0.29 (0.11, 0.69) | 0.51 | −0.63 (−0.96, −0.31) | −0.27 (−0.52, −0.02) | 0.08 | |
In ml/min/1.73 m2 (95% confidence interval).
In ml/min/1.73 m2 per year (95% confidence interval).
Model adjusted for baseline estimated GFR and race.
Kidney function in HIV-infected women initiated on HAART without TDF and matched HIV-uninfected women
Figure 1B shows the distribution of eGFR values during 5 years of follow-up among HIV-infected women who initiated on TDF-sparing HAART and their HIV-uninfected matches. Overall, the eGFR distribution and median values were similar between the two groups. Women initiating TDF-sparing HAART and their HIV-uninfected matches appeared to have slow declines in kidney function (Fig. 2B). In weighted models, the mean annual change in eGFR was −0.63 ml/min/1.73 m2 in HIV-infected women initiated on TDF-sparing HAART (95% CI: −0.96, −0.31). This was similar to the mean annual change in eGFR in matched HIV-uninfected women (–0.27 ml/min/1.73 m2 per year; 95% CI: −0.52, −0.02) (p for interaction=0.08). Estimated GFRs at 1 and 3 years following HAART initiation were similar between these two groups. In contrast, HIV-infected women initiated on TDF-sparing HAART had lower eGFR at 5 years than their HIV-uninfected matches; however, this difference was small (difference −2.19 ml/min/1.73 m2; p=0.03).
Discussion
In this diverse, well-characterized cohort of HIV-infected and HIV-uninfected women, we observed that HIV-infected women generally had slightly lower mean eGFRs during follow-up compared with HIV-uninfected women; however, annual rates of eGFR decline were similar between HAART-treated HIV-infected women and HIV-uninfected women with comparable risk factor profiles for CKD. Furthermore, TDF-containing primary HAART regimens did not appear to increase the annual rate of eGFR decline. This study shows that younger HIV-infected individuals who initiate HAART with normal kidney function can attain an eGFR trajectory similar to HIV-uninfected persons over a 5-year period.
The general effect of HAART on longitudinal rates of kidney function decline has been variable across observational studies likely due to differences in sociodemographic and clinical characteristics across study populations. In the Study of the Consequences of the Protease Inhibitor Era (SCOPE) cohort, Choi and colleagues showed that HAART attenuated kidney function decline among predominantly antiretroviral-exposed patients, but in general patients continued to show significant loss of renal function even among those who attained long-lasting viral suppression.6 In a large study of predominantly white HIV-infected persons who were HAART exposed, cumulative exposures to TDF, indinavir, and/or atazanavir were associated with increased risk for CKD7; however, only 3.3% of participants developed CKD over a median follow-up of 3.7 years. Compared with our study, however, these study populations were generally older with reported mean and median ages of 43 to 47 years across the studies.6,7
Studies of longitudinal kidney function among HAART-naive individuals have been largely limited to those evaluating TDF and provide conflicting results. In the SCOPE cohort, the rate of eGFR change improved by approximately +2.8 ml/min/1.73 m2 per year following HAART initiation in a subgroup analysis of 82 HAART initiators; however, this study did not include HIV-uninfected individuals, precluding determination of whether the eGFR trajectory improved to levels comparable to HIV-uninfected persons.6 In a study by Horberg et al., HIV-infected individuals who initiated HAART experienced significant declines in kidney function over a follow-up period of 2 years. In that study, the decline was more pronounced among those with a baseline eGFR of more than 80 ml/min/1.73 m2 and among those who initiated TDF-containing HAART.19 In a more recent large study of HAART-naive HIV-infected U.S. veterans, TDF was associated with a 33% increased risk for CKD for each year of exposure.12 These previous studies, however, consisted of older individuals (mean age 43 and 47 years, respectively) compared with women included in our study. Moreover, in the case of the study among U.S. veterans, women comprised only 2.2% of the study population.12
In contrast, prior studies that consisted of younger, HAART-naive HIV-infected individuals as in our study showed minimal if any association between TDF and longitudinal kidney function. In the predominantly African-American Johns Hopkins HIV Clinical Cohort with a mean age of 40 years, Gallant and Moore demonstrated no significant changes in eGFR among HIV-infected patients initiating TDF-containing or TDF-sparing primary regimens during 2 years of follow-up beyond an initial eGFR decline observed at 6 months post-HAART initiation.9 Secondary analysis of a randomized controlled trial of TDF among antiretroviral-naive HIV-infected participants with a mean age of 35 years has also shown minimal effect of TDF on kidney function.10 Our study is consistent with these two latter studies. We demonstrated that HIV-infected women who initiated their primary HAART regimen with TDF at normal levels of kidney function did not have faster annual declines in eGFR compared to matched HIV-uninfected women. The disparate observations of the associations between HAART and longitudinal kidney function across studies highlight that the risk for kidney function decline with HAART differs depending on individual characteristics such as age and HIV history.
Our study has several limitations to consider. The study population represents a select subset of HIV-infected and HIV-uninfected women; this limits the generalizability of our findings, particularly to individuals who are older, receive TDF as part of secondary HAART regimens, or have preexisting kidney disease. Our findings, however, still have relevance among those who initiate TDF as an element of an initial HAART regimen, a growing proportion of HIV-infected individuals. Due to our sample size, we were unable to conduct subanalyses of the TDF-initiated HIV-infected women by whether or not they were receiving concurrent ritonavir-boosted protease inhibitors. Urine samples to assess for subclinical TDF renal toxicity or standardized serum creatinines were not available for this study. In addition, eGFR is known to be less precise and accurate at levels above 60 ml/min/1.73 m2;20 however, our analysis evaluated repeated, longitudinal estimates of kidney function in which the bias on the level of estimated GFR is less of a factor. As we had fewer participants at later follow-up years, our estimates of annual eGFR changes are less reliable at later visits. We utilized inverse probability weighting to minimize the effect of drop-out on eGFR estimates. Due to the lack of urinary protein measurements or urinary dipstick results in WIHS, we were unable to evaluate for subclinical renal disease. Studies are underway to study these subclinical manifestations of HAART-related renal effects.
In conclusion, young HIV-infected women with normal kidney function who initiate HAART with or without TDF had slightly lower levels of kidney function during follow-up but had annual rates of kidney function decline similar to HIV-uninfected women. Our study suggests that this subset of HIV-infected individuals is at low risk for kidney disease complications related to HAART. Whether the lower mean kidney function at follow-up will impact kidney function trajectory over a longer period of time, however, needs further evaluation. Furthermore, additional studies are needed to differentiate between HIV-infected individuals who are at low risk versus high risk for HAART-related renal disease progression and to identify who would most benefit from routine renal function monitoring.
Acknowledgments
Data in this article were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI grant UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. M.M.E. is supported by NIH-NIDDK grant K23DK081317.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Winston JA. Klotman PE. Are we missing an epidemic of HIV-associated nephropathy? J Am Soc Nephrol. 1996;7:1–7. doi: 10.1681/ASN.V711. [DOI] [PubMed] [Google Scholar]
- 2.Rao TK. Filippone EJ. Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 3.Lucas GM. Eustace JA. Sozio S. Mentari EK. Appiah KA. Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: A 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 4.Estrella MM. Parekh RS. Abraham A, et al. The impact of kidney function at highly active antiretroviral therapy initiation on mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2010;55:217–220. doi: 10.1097/QAI.0b013e3181e674f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atta MG. Gallant JE. Rahman MH, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant. 2006;21:2809–2813. doi: 10.1093/ndt/gfl337. [DOI] [PubMed] [Google Scholar]
- 6.Choi AI. Shlipak MG. Hunt PW. Martin JN. Deeks SG. HIV-infected persons continue to lose kidney function despite successful antiretroviral therapy. AIDS. 2009;23:2143–2149. doi: 10.1097/QAD.0b013e3283313c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mocroft A. Kirk O. Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 8.Flandre P. Pugliese P. Cuzin L, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011;6:1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE. Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971–1975. doi: 10.1097/QAD.0b013e32832c96e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzedine H. Hulot JS. Vittecoq D, et al. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant. 2005;20:743–746. doi: 10.1093/ndt/gfh658. [DOI] [PubMed] [Google Scholar]
- 11.Izzedine H. Isnard-Bagnis C. Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS. 2004;18:1074–1076. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 12.Scherzer R. Estrella M. Li Y. Deeks SG. Grunfeld C. Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan SE. Melnick SL. Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 14.Bacon MC. von Wyl V. Alden C, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osberg IM. Hammond KB. A solution to the problem of bilirubin interference with the kinetic Jaffe method for serum creatinine. Clin Chem. 1978;24:1196–1197. [PubMed] [Google Scholar]
- 16.Levey AS. Bosch JP. Lewis JB. Greene T. Rogers N. Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services; 2009. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents; pp. 1–161. [Google Scholar]
- 18.Curtis LH. Hammill BG. Eisenstein EL. Kramer JM. Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 19.Horberg M. Tang B. Towner W, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53:62–69. doi: 10.1097/QAI.0b013e3181be6be2. [DOI] [PubMed] [Google Scholar]
- 20.Rule AD. Larson TS. Bergstralh EJ. Slezak JM. Jacobsen SJ. Cosio FG. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]