Abstract
Pigs continue to grow in importance as a tool in neuroscience. However, behavioral tests that have been validated in the rodent model do not translate well to pigs because of their very different responses to behavioral stimuli. We refined metrics for assessing porcine open field behavior to detect a wide spectrum of clinically relevant behaviors in the piglet post-traumatic brain injury (TBI). Female neonatal piglets underwent a rapid non-impact head rotation in the sagittal plane (n=8 evaluable) or were instrumented shams (n=7 evaluable). Open field testing was conducted 1 day prior to injury (day −1) in order to establish an individual baseline for analysis, and at days +1 and +4 after injury. Animals were then killed on day +6 after injury for neuropathological assessment of axonal injury. Injured piglets were less interested in interacting with environmental stimuli and had a lower activity level than did shams. These data were compared with previously published data for axial rotational injuries in neonatal piglets. Acute behavioral outcomes post-TBI showed a dependence on the rotational plane of the brain injury, with animals with sagittal injuries demonstrating a greater level of inactivity and less random usage of the open field space than those with axial injuries. The persistence of axonal injury is also dependent on the rotational plane, with sagittal rotations causing more prolonged injuries than axial rotations. These results are consistent with animal studies, finite element models, and studies of concussions in football, which have all demonstrated differences in injury severity depending upon the direction of head impact rotation.
Key words: behavioral assessments, cognitive function, pediatric brain injury, TBI
Introduction:
Traumatic brain injury (TBI) is a leading cause of death in children, with the highest incidence occurring in young children up to 4 years of age, with 76 hospitalizations and 5 deaths per 100,000 children annually.1,2
The anatomy and development of the pig brain resembles the human brain more closely than does the rodent brain.3–6 Whereas rodent brains are lissencephalic and have little white matter, porcine and human brains are gyrencephalic, and have a similar distribution of white and gray matter. Furthermore, changes in growth and myelination of the porcine brain during development are similar to those of the human brain, making the neonatal piglet a more appropriate model for the infant brain.7–9 Whereas the porcine model has many advantages, there is limited information available regarding immature porcine behavior following TBI. Open field testing has demonstrated that injured piglets have a decreased interest in exploring their environment,10 and that piglets with multiple brain injuries showed deficits in attention and short-term memory in a T-maze task.11 The further characterization of porcine behavior post-TBI will greatly increase the value of this model.
Our goal is to use this infant TBI brain model as a vehicle to investigate differences in pathology and behavior following closed head injury produced by two different planes of rotation (sagittal and axial). Different planes of rotation have been shown to produce differences in intracranial pressure, duration of unconsciousness, and cerebral blood flow in piglets post-injury.12 In a study by Eucker and colleagues,12 sagittal plane injuries were found to have the largest increases in intracranial pressure, the longest durations of unconsciousness, and the biggest decreases in cerebral blood flow of all three planes of rotation; however, sagittal injuries had similar levels of axonal injury (AI) and tissue infarction to the high rotational velocity group of axial plane injuries. Primate studies have shown that injury severity depends upon the head rotation direction as well, but that coronal head accelerations produced the most severe coma and diffuse AI.13,14 A detailed discussion of the differences between the piglet and primate models that may contribute to these findings is given by Eucker and colleagues.12 Humans have also shown differing injury susceptibility based on the plane of head rotation. Analysis of concussion in football players revealed that impacts to the face mask result in concussions at lower head accelerations than do impacts to other portions of the helmet,15 and that finite element modeling of human adult TBI demonstrated that rotational direction affects the cumulative strain and injury risk, with rotations that contained an axial component producing the greatest injury risk.16 These studies present conflicting information about which rotational plane results in the most severe injuries, possibly because of differences in the anatomy of the species under consideration, and the injury outcomes being assessed; however, they all agree that the rotational plane plays an important role in the type and severity of the injury sustained. None of these studies investigated cognitive or behavioral changes following injury in multiple rotational planes, and none looked at longer-term pathological outcomes; therefore, our study is novel, because it links pathological and cognitive outcomes with the direction of head rotation.
Methods
The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all protocols. Only positive conditioning with milk replacer as a reward was conducted, and no aversive conditioning was used.
Acclimation and pre-injury testing
Nineteen female neonatal piglets from five litters were studied in groups of three to four littermates per group. Littermates were housed together throughout the duration of the study. Two days prior to injury (day −2) naïve piglets were placed in an empty test space (1.2 m × 2.4 m) with a bowl of milk replacer (Littermilk, Land O Lakes, Arden Hills, MN) in the center. They were allowed to explore the space freely for 1 h to become acclimated to the testing environment, the research staff, and the food bowl.
Injury
On study day 0, all piglets were anesthetized with 4% isoflurane via a snout mask. Once a pinch reflex was extinguished, animals were intubated with a 3.0 mm endotracheal tube. Buprenorphine (0.02 mg/kg i.m.) was administered to all animals for analgesia. End tidal CO2, oxygen saturation, heart rate, and core body temperature were monitored continuously (Surgivet V9204, Smiths Medical, Dublin, OH) until extubation post-injury or sham, and animals were ventilated as needed (Hallowell AWS, 1-3% isoflurane, Hallowell EMC, Pittsfield, MA). Two to three piglets from each group were randomly designated to the injury group (n=11) and the other one to two piglets in the group were instrumented shams (n=8). Injured animals underwent a rapid non-impact head rotation in the sagittal direction via the HYGE pneumatic actuator system (described previously12,17), which rotated the head rapidly through 58 degrees, with the center of rotation in the cervical spine. Average sagittal angular velocities were 149±9 rad/sec (mean±SD) with angular accelerations of 57,935±4225 rad/sec2. Sham animals were placed on the biteplate but not rotated. Piglets of the same age had been injured under the same protocol with rapid rotations in the axial plane for several previous studies.11,12,18 These data were compared against the current study data to establish the role of rotational direction on pathology and behavior.
Open field behavioral testing
Open field testing was conducted 1 day prior to injury (day −1) in order to establish an individual baseline for analysis, and on days +1 and +4 after injury. Each morning of testing, the animals were fasted for 2 h and weighed to monitor growth. The order in which the piglets were tested varied. During the testing of one piglet, the remaining littermates were placed in individual pet carriers in a separate room. All testing was recorded via camera and saved to DVD for later scoring by a naïve evaluator.
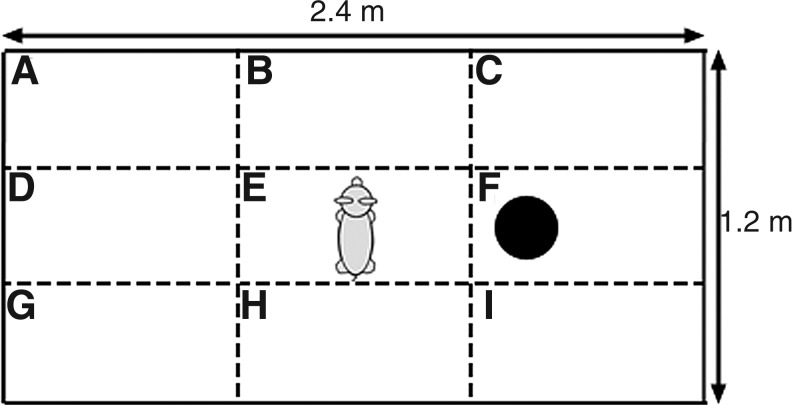
On each testing day, each animal was placed in a 1.2 m × 2.4 m pen with a single toy (19 cm diameter blue ball) at a consistent predetermined location and allowed to explore the space freely for 10 min (Fig. 1). An array of behaviors were tracked for presence or absence during each minute-long epoch and recorded as the number of epochs over which the behavior was observed. The behaviors assessed were: sniffing floor, walls, or toy; running, walking, standing still for >1 sec, lying down, moving the toy, and attempting to escape the test space.
FIG. 1.
Diagram of open field test space and toy placement. Black circle marks the location of the blue ball. Zone designations are labeled with letters.
To assess piglet activity and patterns of space usage, the open field was divided into nine zones (Fig. 1), and the position of the piglet's snout within the open field was marked at 2 sec time intervals, resulting in a 300 character-long position sequence. This zone position sequence was evaluated by four measures adapted from symbolic dynamics: PDIAG, Shannon Entropy, first order mutual information, and normalized Lempel-Ziv Complexity. The definitions and calculations of each measure are given in detail in the analysis section of the methods.
Analysis of open field locomotion
The open field 300-character sequence specifying the zone occupied by the piglet at 2-sec intervals was analyzed using four different measures to quantify the detailed internal structure of the piglet locomotion.
The first measure, PDIAG, quantifies how much a piglet tends to dwell in the same zone. Given a symbol alphabet of Nα distinct elements (Nα=9 for number of zones in this study), PIJ is defined as the probability that zone I will be followed by zone J. PDIAG is defined as the average probability that a symbol will be followed by itself.
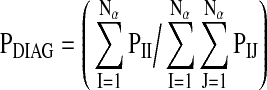 |
(Eq. 1) |
This calculation gives the probability that if a piglet is in a particular zone, it will be in that same zone at the next time point. PDIAG varies between 0 and 1, where a 0 indicates that the piglet changed zones at every time point, and a 1 indicates that the piglet never changed zones.
The second measure, Shannon entropy, evaluated how much of the open field test space was utilized. Defined as:
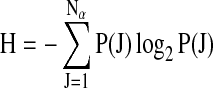 |
(Eq. 2) |
where P(J) is the probability of the J-th zone position symbol. H varies between 0 and 1. If one or a few zones dominate the zone sequence, then the value of H is low. If the zones present in the sequence are uniformly distributed over all possible nine zones (A through I), then H approaches 1. In contrast with measures of complexity introduced later, Shannon entropy is not sensitive to the order of the zone sequence; it is invariant under a random shuffle of the zone sequence.
The third measure, first order mutual information, was used to examine the temporal stability and sequential structure of piglet zone usage. We constructed the n=300 character piglet zone sequence X and shifted it by k=1 time point (i.e., first order) to create Y, defined as:
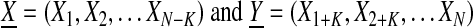 |
(Eq. 3) |
Their mutual information (or nonlinear decorrelation function), denoted I(X, Y), is the average number of bits of message Y that can be predicted by measuring X. The mutual information was calculated using an adaptive XY-plane partition method.19 A high value indicates that the sequential structure of the symbol sequence is stable over a single time point shift. A low value indicates that this structure is rapidly changing through time.
The fourth measure, Lempel-Ziv Complexity, is a model-based, nonprobabilistic, randomness-finding measure of complexity. Lempel-Ziv Complexity can distinguish between two zone sequences with the same number of visits to each zone, such as:
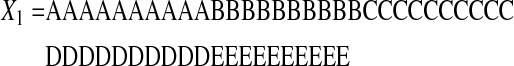 |
and
 |
The Lempel-Ziv Complexity of X1 is 6 bits, and the complexity of X2 is 22 bits, supporting our intuitive understanding that X2 is more complex than X1. The definition of Lempel-Ziv Complexity is the number of steps required to rebuild the original zone sequence, and was computed using a program based on a published pseudocode.20
Because the Lempel-Ziv Complexity is influenced by the length of the zone sequence and its degree of disorder, we normalized it using 499 random surrogates to capture disorder that is independent of the number of observations.21 The normalized complexity varies from ∼ 0 to 1. At the extreme, 0 is obtained when the message consists of a single repeated symbol, and 1 is obtained when the sequence is truly random.
Pathology
The animals were euthanized on study day+6 to evaluate AI. Animals were anesthetized via snout mask with 4% isoflurane. Once a pinch reflex was absent, an intravenous sodium pentobarbital overdose was administered and brain tissue was fixed by transcardiac perfusion using 2 L of normal saline followed by 3 L of 10% unbuffered formalin (Spectrum Chemical, Gardena, CA). Brains were removed and post-fixed for 1 week at room temperature, then stored in 1×phosphate-buffered saline (PBS). Coronal slices 3 mm thick were taken from the entire rostral-caudal length of the fixed brains, resulting in 15–18 total slices that spanned the cerebrum, brainstem, and high cervical spinal cord. Sections were photographed and examined for visible tissue tears, intracerebral hemorrhage, and subarachnoid hemorrhage. After routine processing, the tissue was embedded in paraffin wax and 6-μm-thick slices were cut from each 3 mm section for microscopic evaluation. Slices were stained with the immunohistochemical markers for AI β-amyloid precursor protein (β-APP), and lightly counterstained with Meyer's hematoxylin. All fields in these 15–18 slides were examined by a blinded neuropathologist (C.S.) at a scanning power of 5–10× magnification, with specific locations examined at 20–40× magnification. Locations of AI were marked on the digital photographs of the coronal sections.
The total brain area for each piglet was calculated by tracing photographs of each slice, measuring the area in Adobe Photoshop, and summing the area of each slice. Likewise, regions of AI marked by the neuropathologist were traced, the areas measured, and summed. Percent AI was obtained by dividing the total area of injury by the total brain area.
Previous study data used for comparison
Open field outcomes at day +1 post-injury from previously published axial rotation data11,18 were analyzed with the current sagittal rotation data set to make comparisons about how rotational plane impacts behavior (Table 1). The previously published studies all used 3-5-day-old piglets and the same rapid rotational injury model as the current study, but with an axial plane rotation. Furthermore, all piglets from these studies underwent similar open field testing 1 day after injury. Videos from this previous open field testing were re-analyzed to obtain the four new locomotion measures. Uninjured shams from all studies were combined into a single sham group for analysis.
Table 1.
Overview of Data Used in Analysis of Locomotion Across Different Planes of Injury
| Friess 2009 | Naim 2010a | Total including this study | |||
|---|---|---|---|---|---|
| Uninjured sham | n=4 | Uninjured sham | n=7 | Uninjured sham | n=19 |
| Single axial injury | n=3 | Single axial injury | n=7 | Axial injury | n=13 |
| Double axial injury 24 h apart | n=3 | Sagittal injury | n=8 | ||
Animals from Naim 2010 were taken from groups not treated with folate.
In order to compare pathology recovery across both sagittal and axial rotational planes, the sagittal injury plane with 6 day survival data from this study was combined with data from previous studies that included sagittal and axial 6 h survival data,12 and axial 6 day survival data.18 These studies all used 3-5-day-old piglets and the same rapid rotational injury model. However, to achieve the same initial AI volume, axial plane injured animals were injured at a higher angular velocity level than sagittal plane injured ones (Table 2). This velocity difference is likely the result of geometrical asymmetry of the piglet brain. Within each rotational plane, the angular velocity load levels were approximately the same between 6 h and 6 day survival studies. This means that all studies being compared had a consistent initial injury severity.
Table 2.
Angular Velocity Levels for Studies Used in Analysis of Axonal Injury Recovery
| |
6 h |
6 days |
||
|---|---|---|---|---|
| Plane | Velocity (rad/s) | n | Velocity (rad/s) | n |
| Sagittal | 166±1.4a | 6 | 149±2.9b | 8 |
| Axial | 198±3.9a | 9 | 194±2.9c | 8 |
Eucker 2011 (Groups SAG and HOR-HIGH)
Data from present study.
Naim 2010 (Group INJ +Saline).
Mean and standard error shown.
Statistical analysis
Data for each behavior and locomotion measure was analyzed for group (injured or sham) and day (+1 or +4) effects and interactions using a two way ANOVA for repeated measures. This was implemented using a mixed-effects model with group and day as the fixed effects and subject nested within group as the random effect. Behavioral measures were analyzed for injury plane (axial or sagittal) effects using a one way ANOVA. Pathology was analyzed for injury plane (axial or sagittal) and pathology time point (6 h post-injury or 6 days post-injury) effects using a two way ANOVA with no repeated measures. Post-hoc analysis was conducted using the Tukey–Kramer method for all analyses. Significance was defined as p<0.05 and all results are reported as mean±standard error unless otherwise noted.
Results
Mortality
Eleven animals underwent a rapid head rotation in the sagittal plane on day 0 of the study and eight were instrumented shams. Three injured animals were euthanized within hours of injury. These animals never regained full consciousness following injury and had large subdural hematomas on necropsy. The angular velocity levels of the animals that were euthanized early (138.6–158 rad/sec) were within the same range as the animals that survived (137.8–160 rad/sec). Furthermore, one sham animal that arrived with low body weight and poor circulation had difficulty tolerating anesthesia. Despite attempts to treat with subcutaneous saline and supplemental oral feedings, it was found dead in the housing facility the morning after the sham anesthesia. All further analysis considers only animals that survived the duration of the study, which includes n=8 injured and n=7 sham.
Open field behavior
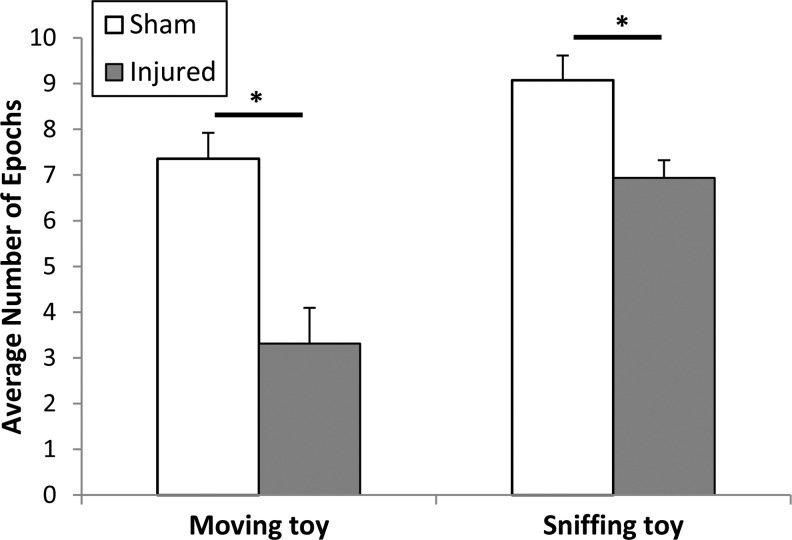
Analysis of the behavior exhibited by the piglets in the open field demonstrated that injured piglets were less interested in interacting with environmental stimuli (Fig. 2). Both sniffing the toy and moving the toy showed significant injury effects when considering both post-injury days combined (sniffing toy: F-ratio=9.64 p<0.01; moving toy: F-ratio=12.25 p<0.01.). Injured animals sniffed the toy during an average of 6.9 epochs of the open field test and moved the toy during an average of 3.3 epochs, which is significantly lower than for sham animals, who exhibited these behaviors during 9.1 and 7.4 epochs respectively. Although the reduction in sniffing the toy after injury did not reach significance in the post-hoc analysis at either post-injury day of study, the moving the toy behavior was significantly reduced in injured animals compared with shams at day +4 (p=0.0241).
FIG. 2.
Data from both post-injury days combined. Moving toy p<0.01, sniffing toy p<0.01. Mean and standard error shown.
There was also a significant day and injury interaction effect in running and escaping behaviors (running: F-ratio=6.0, p=0.03; escaping: F-ratio=5.18, p=0.04), indicating that despite the lack of significant differences in these measures with injury, injured and sham animals exhibited significantly different trends in how the measure changed with day. Injured animals showed an increase in epochs with running from day +1 (0.63±0.5) to day +4 (2.4±0.9), whereas sham animals showed a decrease (3.7±1.5 to 2±1.3). Similarly, injured animals had an increase in the escaping behavior from day +1 to +4 (0±1.1 to 1.6±0.8), whereas shams had a decrease (1.1±1 to 0.4±0.4). The other open field behaviors tracked showed no significant effects.
Open field locomotion
Analysis of piglet locomotion during the open field test demonstrated that injured piglets also had a lower activity level than shams. Whereas the number of epochs spent walking and standing still did not reveal this change, by using more sensitive measures, we were able to see changes in piglet locomotion with injury (Fig. 3). PDIAG had a significant injury effect (F-ratio=5.15, p=0.04) indicating that injured animals were more stationary than shams. Furthermore, the increase in PDIAG from 0.44 on day −1 to 0.61 on day +1 in injured animals was significant (p=0.03), whereas there were no significant differences between sham animals across each study day or between injured and sham animals on day −1. Shannon entropy demonstrated no differences between injured and sham animals, but did have a significant day effect (F-ratio=7.68, p=0.02, df=1), with day +1 values (0.9±0.2) higher than day +4 values (0.82±0.3); however, neither were significantly different from day −1 (0.87±0.2). This decrease in entropy from day +1 to day +4 indicates that both groups of piglets went from visiting all zones of the open field with almost the same frequency to visiting a subset of zones with greater frequency than other zones. First order mutual information demonstrated no significant differences between injured and sham animals or changes over study day. This shows that the temporal stability of the position sequence was not affected by injury (sham: 2.72±0.07; injured: 2.79±0.06) or study day (day −1: 2.74±0.07; day +1: 2.87±0.05; day +4: 2.67±0.1). Lempel-Ziv Complexity showed no significant effects with the ANOVA, but there was a trend in injured animals to less random motion on day +1(0.55±0.06) compared with their day −1 values (0.64±0.03) and compared with shams on day +1 (0.67±0.04).
FIG. 3.
Box-and-whisker plot that shows median, 1st and 3rd quartile as the box and the minimum and maximum values as the whiskers. PDIAG Day −1 to Day +1 p=0.03.
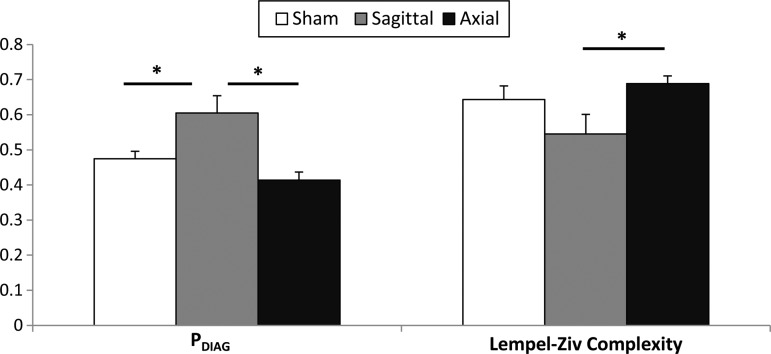
To investigate if different head rotation directions contributed to different behavioral outcomes in the acute phase (day +1 post-injury), data from the open field at day +1 post-injury from two previous axial studies11,18 (Table 1) were re-analyzed to obtain these measures of locomotion. Analysis of the data demonstrated that during the acute phase, sagittal rotational injuries had significantly higher PDIAG values (0.61±0.05) compared with both sham (0.47±0.02) and axial injuries (0.41±0.02), indicating a greater level of inactivity after a sagittal head rotation (p=0.01 vs. sham and p<0.001 vs. axial) (Fig. 4). Sagittal injuries also displayed a significantly lower Lempel-Ziv Complexity (0.55±0.06) than axial ones (0.69±0.02), indicating less random usage of the open field space (p=0.0125).
FIG. 4.
Locomotion at Day +1 for different rotational planes of injury. Mean and standard error shown. PDIAG sagittal to sham p=0.01. PDIAG sagittal to axial p<0.001. Complexity sagittal to sham p=0.08. Complexity sagittal to axial p=0.0125.
Pathology
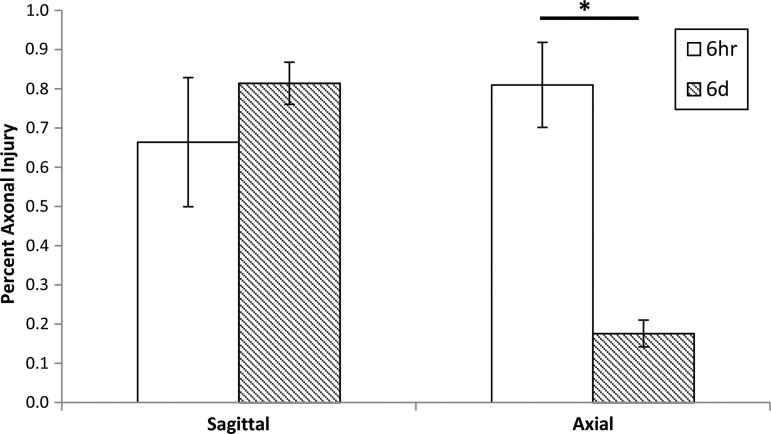
We further compiled AI data from our single rapid rotational studies in the 3–5-day-old piglet with different rotational planes (axial and sagittal) and survival times (6 h and 6 days). Note that to achieve similar volumes of AI at 6 h post-injury (≈0.75% of total brain), significantly higher velocities were used in the axial direction than in the sagittal one (Table 2 and Fig. 5). This is likely because of the biomechanical consequences of the nonaxisymmetric brain shape. Within each plane of rotation, the peak angular velocity was approximately the same for both survival times. Representative coronal sections of a brain from each group stained with β-APP demonstrate the differing persistence of axonal injury depending upon the plane of injury (Fig. 6). For both directions, at 6 h survival there was considerable general upregulation of β-APP, indicating a degree of general ischemia, resulting in a widespread low-level brown discoloration of the sections. However, abnormal axons can be identified because of their more intense brown immunoreaction. By 6 days, the widespread β-APP upregulation had gone, resulting in much less background staining. However, only sagittal plane rotations resulted in marked β-APP staining at 6 days post-injury (Fig. 6C). This qualitative observation was corroborated by our quantitative analysis of β-APP staining (%AI), in which the amount of AI remained unchanged after 6 days for sagittal injuries (0.66%±0.16% at 6 h survival vs. 0.81%±0.05% at 6 day survival), whereas for axial injuries AI was significantly decreased after 6 days (0.81%±0.11% at 6hr survival vs 0.18%±0.03%. p<0.001) (Fig. 5).
FIG. 5.
Percent axonal injury (AI) for each plane of rotation at different time points. Mean and standard error shown. Axial 6 h to 6 days p<0.001.
FIG. 6.
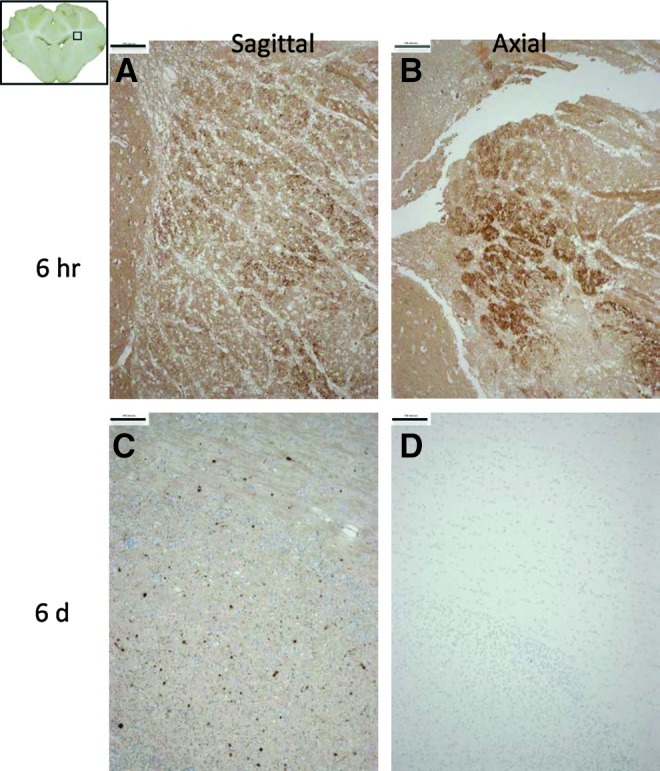
Composite image of representative β-amyloid precursor protein (β-APP) immunostaining from four different animals, but taken from the same region for direct comparison (area outlined in inset, upper left). Two animals were injured in the sagittal direction (A and C) and two in the axial direction (B and D), with post-injury survival times of 6 h (A and B) and 6 days (C and D). Bar=100μm. Color image is available online at www.liebertpub.com/neu
Discussion
Pigs continue to grow in importance as a tool in neuroscience because of their gyrencephalic brain, which resembles the human brain more closely in growth and development than does a rodent model.4–6 This also makes piglets particularly well suited to studies of pediatric populations, and the immature piglet model of TBI has been shown to have many similar findings observed in infant TBI.17,22 Furthermore, this infant piglet model exhibits an injury pattern and severity that is dependent upon rotational plane, similar to experimental and finite element findings in primates and humans.12,14–16 Unfortunately, there is a paucity of validated behavioral tests for the immature piglet model, and those used on rodents do not translate well to pigs because of their different motor abilities, handling needs, and responses to stimuli (e.g., the well-known aversion of mice to light and open spaces is not observed in pigs).4–6,23 As more robust and refined behavioral tests are developed for the porcine TBI model, it will be important to consider the role of the rotational plane on pathological and behavioral outcomes.
Analysis of the open field behaviors showed a significant decrease in exploratory behaviors after a rapid sagittal head rotation (decreased interaction with the toy compared with sham, by ANOVA). Other studies have also analyzed porcine open field behavior for cognitive changes. Donald and colleagues found an increase in exploratory behaviors in pigs treated with azaperone, a drug used to reduce stress in commercial pigs, suggesting that decreased exploration could be associated with stress and fear.24 The decreases in exploratory behavior seen in this study could be indicative of lethargy, fear of novelty, or inability to recognize novel stimuli. It should be noted that on study day +4 there was no difference between the number of epochs of running in injured and sham animals; however, there remained a significant difference between the number of epochs spent moving the toy, which suggests that the decrease in exploratory behavior seen in injured animals is not merely a result of motor deficits or lethargy.
A number of studies have assessed porcine open field locomotion using measures such as number of lines crossed, total distance traveled, number of bouts of movement, and total time of inactivity.24–27 This is the first time that porcine locomotion has been assessed using tools that are sensitive to both the frequency of zone visits and the pattern of space usage. With the exception of PDIAG, which was developed for this study, the other measures have been used to assess behavioral patterns in other situations, such as following administration of psychoactive compounds and following blast exposure.28–32
The data indicated not only differences in open field exploration and locomotion between injured and sham animals in this study, but also differences between animals injured by rotations through the axial and sagittal planes. The previous axial plane studies generally showed decreases in exploratory behaviors as well, but the overall injury effect was not significant.10,11 This indicates that the sagittal plane injury produced greater exploratory deficits than the axial plane injury. The persistence of these exploratory deficits to day +4 post-injury in the sagittally injured animals despite the abatement of motor deficits, indicates that decreases in exploration may arise, at least in part, from cognitive sources, and suggests greater persistent cognitive impairment in sagittal plane injuries than in axial plane injuries. Furthermore, our results indicated two distinct neurofunctional presentations post-TBI. Sagittal plane injured animals exhibited highly patterned (low Lempel-Ziv Complexity) and stationary (high PDIAG) motion in the open field, whereas axial plane injured animals showed more random and frequent movements. This spectrum of locomotion fits with the extremes of apathetic and disinhibited behaviors reported after TBI in human adults and children.33–38 Our animals experiencing sagittal head rotations exhibited apathetic-like behaviors, in contrast to axial plane rotation animals, which exhibited more disinhibited behaviors.
In addition to having different acute behavioral changes post-TBI, the two injury planes demonstrated a different time course of AI recovery. Whereas axial plane injuries exhibited a significant decrease in AI from 6 h to 6 days post-injury, sagittal plane injuries exhibited no change in AI. Because β-APP staining does not necessarily indicate irreversible injury, it is likely that much of the AI seen at 6 h post-injury in the axial group was a transient arrest of APP axonal flow, but not structural axonal damage. In contrast, the sagittal plane injury produced a prolonged disruption to normal axonal function. This difference in underlying AI and time course for recovery could indicate that there will also be differences in long-term behavioral outcomes.
Limitations
This study is limited in that in order to investigate the differences in pathology and behavior with rotational plane, we have combined data from several different, though comparable, previously published studies. However, several differences among the studies being compared could be confounders. First, none of the axial behavior studies incorporated day −1 testing. This additional day of testing could have contributed to habituation in the sagittal plane injured animals that axial plane injured animals did not experience. Habituation is unlikely, however, as only one of our behavioral measures showed a significant day effect (Shannon entropy), and, consequently, we did not compare this measure with the axial plane studies. Second, the open field in the axial plane injured animals had more than one toy, whereas the current study employed an open field with just a single toy. The single toy chosen for the current study was the one preferred by most of the animals in the previous study, and is less likely to influence our analysis. Third, the Friess 2009 study11 included animals with single and double head rotations before day +1 testing, which could produce a broader spectrum of injury severity. Finally, we do not know how the pathology between axial and sagittal plane injuries compares at 24 h post- injury when they demonstrated different open field locomotion. Whereas the injuries were comparable at 6 h post-injury, we do not know how they may have changed by 24 h. Unpublished data from sagittal plane injuries with the same rotational velocity as this study indicate that axonal injury is significantly increased from 6 h to 24 h post-injury (n=11, average %AI=1.35%, SD=0.22%). However, similar 24 h post-injury pathology is unavailable for axial plane rotations.
Other limitations of the study include the limited post-injury time interval (4 days), which does not capture longer-term deficits and recovery. Future work should look at longer survival times to increase the ability of the research to be translated to long-term outcomes in the pediatric population. Finally, because our study goal was to create a narrow range of initial neuropathology rather than a broad spectrum, a correlation of each behavioral metric to AI cannot be performed in a meaningful manner. Further studies should include the addition of animals with both milder and more severe injuries in one of the rotational planes, to evaluate how well the behavior outcomes correlate to severity or distribution of neuropathology.
Conclusion
In summary, we present refined metrics for assessing porcine behavior that allow us to detect a full spectrum of clinically relevant behaviors in the piglet post-TBI, from lethargic and apathetic to kinetic and anxious. Second, we have shown that acute behavioral outcomes post-TBI are dependent upon the rotational plane of the brain injury. Furthermore, the time course of recovery for AI is also dependent upon the rotational plane, with sagittal rotations exhibiting more prolonged injuries than the axial plane. We emphasize the importance of validating neurofunctional assessments across a range of injury models in the development of robust outcome measures.
Acknowledgments
The authors acknowledge Benjamin Bruins for his contribution in conducting the assessments. This study was made possible by the support of National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS039679, U01NS069545, and K08NS064051. Dr. Rapp acknowledges support of the Traumatic Injury Research Program of the Uniformed Services University of the Health Sciences and the Defense Medical Research and Development Program. The opinions and assertions contained herein are the private opinions of the authors and are not to be construed as official or reflecting the views of their respective commands, the United States Navy, or the Department of Defense.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Langlois J.A. Rutland-Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Faul M. Xu L. Wald M.M. Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2010. [Google Scholar]
- 3.Hagberg H. Ichord R. Palmer C. Yager J.Y. Vannucci S.J. Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci. 2002;24:364–366. doi: 10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- 4.Lind N.M. Moustgaard A. Jelsing J. Vajta G. Cumming P. Hansen A.K. The use of pigs in neuroscience: modeling brain disorders. Neurosci. Biobehav. Rev. 2007;31:728–751. doi: 10.1016/j.neubiorev.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Gieling E.T. Schuurman T. Nordquist R.E. van der Staay F.J. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr. Top. Behav. Neurosci. 2011;7:359–383. doi: 10.1007/7854_2010_112. [DOI] [PubMed] [Google Scholar]
- 6.Kornum B.R. Knudsen G.M. Cognitive testing of pigs (Sus scrofa) in translational biobehavioral research. Neurosci. Biobehav. Rev. 2011;35:437–451. doi: 10.1016/j.neubiorev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Dobbing J. Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 8.Fang M. Li J. Gong X. Antonio G. Lee F. Kwong W.H. Wai S.M. Yew D.T. Myelination of the pig's brain: a correlated MRI and histological study. Neurosignals. 2005;14:102–108. doi: 10.1159/000086292. [DOI] [PubMed] [Google Scholar]
- 9.Flynn T.J. Developmental changes of myelin-related lipids in brain of miniature swine. Neurochem. Res. 1984;9:935–945. doi: 10.1007/BF00964525. [DOI] [PubMed] [Google Scholar]
- 10.Friess S.H. Ichord R.N. Owens K. Ralston J. Rizol R. Overall K.L. Smith C. Helfaer M.A. Margulies S.S. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp. Neurol. 2007;204:234–243. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friess S.H. Ichord R.N. Ralston J. Ryall K. Helfaer M.A. Smith C. Margulies S.S. Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma. 2009;26:1111–1121. doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eucker S.A. Smith C. Ralston J. Friess S.H. Margulies S.S. Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 2011;227:79–88. doi: 10.1016/j.expneurol.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gennarelli T.A. Thibault L.E. Adams J.H. Graham D.I. Thompson C.J. Marcincin R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 14.Gennarelli T.A. Thibault L. Tomei G. Wiser R. Graham D. Adams J. Directional Dependence of Axonal Brain Injury due to Centroidal and Non-Centroidal Acceleration. 1987. SAE Technical Paper 872197.
- 15.Pellman E.J. Viano D.C. Tucker A.M. Casson I.R. Committee on Mild Traumatic Brain Injury, National Football League. Concussion in professional football: location and direction of helmet impacts-Part 2. Neurosurgery. 2003;53:1328–1341. doi: 10.1227/01.neu.0000093499.20604.21. [DOI] [PubMed] [Google Scholar]
- 16.Weaver A.A. Danelson K.A. Stitzel J.D. Modeling brain injury response for rotational velocities of varying directions and magnitudes. Ann. Biomed. Eng. 2012;40:2005–2018. doi: 10.1007/s10439-012-0553-0. [DOI] [PubMed] [Google Scholar]
- 17.Raghupathi R. Margulies S.S. Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- 18.Naim M.Y. Friess S. Smith C. Ralston J. Ryall K. Helfaer M.A. Margulies S.S. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci. 2010;32:466–479. doi: 10.1159/000322448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cellucci C.J. Albano A.M. Rapp P.E. Statistical validation of mutual information calculations: comparison of alternative numerical algorithms. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005;71:066208. doi: 10.1103/PhysRevE.71.066208. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T.A. Cellucci C.J. Kohegyi E. Bashore T.R. Josiassen R.C. Greenbaun N.N. Rapp P.E. The algorithmic complexity of multichannel EEGs is sensitive to changes in behavior. Psychophysiology. 2003;40:77–97. doi: 10.1111/1469-8986.00009. [DOI] [PubMed] [Google Scholar]
- 21.Rapp P.E. Cellucci C.J. Gilpin A.M. Jiménez-Montaño M.A. Korslund K.E. Communication patterns in a psychotherapy following traumatic brain injury: a quantitative case study based on symbolic dynamics. BMC Psychiatry. 2011;11:119. doi: 10.1186/1471-244X-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duhaime A.C. Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 2006;28:380–387. doi: 10.1159/000094164. [DOI] [PubMed] [Google Scholar]
- 23.Gieling E.T. Nordquist R.E. van der Staay F.J. Assessing learning and memory in pigs. Anim. Cogn. 2011;14:151–173. doi: 10.1007/s10071-010-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donald R.D. Healy S.D. Lawrence A.B. Rutherford K.M. Emotionality in growing pigs: is the open field a valid test? Physiol. Behav. 2011;104:906–13. doi: 10.1016/j.physbeh.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Kanitz E. Tuchscherer M. Puppe B. Tuchscherer A. Stabenow B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav. Immun. 2004;18:35–45. doi: 10.1016/s0889-1591(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 26.Emack J. Matthews S.G. Effects of chronic maternal stress on hypothalamo-pituitary-adrenal (HPA) function and behavior: no reversal by environmental enrichment. Horm. Behav. 2011;60:589–598. doi: 10.1016/j.yhbeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 27.van der Staay F.J. Pouzet B. Mahieu M. Nordquist R.E. Schuurman T. The d-amphetamine-treated Gottingen miniature pig: an animal model for assessing behavioral effects of antipsychotics. Psychopharmacology (Berl) 2009;206:715–729. doi: 10.1007/s00213-009-1599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulus M.P. Geyer M.A. Gold L.H. Mandell A.J. Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proc. Natl. Acad. Sci. U. S. A. 1990;87:723–727. doi: 10.1073/pnas.87.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulus M.P. Geyer M.A. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- 30.Paulus M.P. Geyer M.A. Quantitative assessment of the microstructure of rat behavior: I, f(d), the extension of the scaling hypothesis. Psychopharmacology (Berl) 1993;113:177–186. doi: 10.1007/BF02245695. [DOI] [PubMed] [Google Scholar]
- 31.Paulus M.P. Callaway C.W. Geyer M.A. Quantitative assessment of the microstructure of rat behavior: II. Distinctive effects of dopamine releasers and uptake inhibitors. Psychopharmacology (Berl) 1993;113:187–198. doi: 10.1007/BF02245696. [DOI] [PubMed] [Google Scholar]
- 32.Rapp P.E. Quantitative characterization of animal behavior following blast exposure. Cogn. Neurodyn. 2007;1:287–293. doi: 10.1007/s11571-007-9027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serebro–Sorek K. Shakhar G.B. Hoofien D. Orienting responses and habituation among persons with traumatic brain injury: distinctive aspects of apathetic and disinhibited behaviours. Brain Inj. 2007;21:583–591. doi: 10.1080/02699050701426840. [DOI] [PubMed] [Google Scholar]
- 34.Kant R. Duffy J.D. Pivovarnik A. Prevalence of apathy following head injury. Brain Inj. 1998;12:87–92. doi: 10.1080/026990598122908. [DOI] [PubMed] [Google Scholar]
- 35.Kim E. Agitation, aggression, and disinhibition syndromes after traumatic brain injury. NeuroRehabilitation. 2002;17:297–310. [PubMed] [Google Scholar]
- 36.Ciurli P. Formisano R. Bivona U. Cantagallo A. Angelelli P. Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J. Head Trauma Rehabil. 2011;26:116–126. doi: 10.1097/HTR.0b013e3181dedd0e. [DOI] [PubMed] [Google Scholar]
- 37.Ylvisaker M. Feeney T. Pediatric brain injury: social, behavioral, and communication disability. Phys. Med. Rehabil. Clin. N. Am. 2007:133–144. doi: 10.1016/j.pmr.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 38.McKinlay A. Grace R.C. Horwood L.J. Fergusson D.M. MacFarlane M.R. Long-term behavioural outcomes of pre-school mild traumatic brain injury. Child Care Health Dev. 2010;36:22–30. doi: 10.1111/j.1365-2214.2009.00947.x. [DOI] [PubMed] [Google Scholar]