Abstract
Mortality is higher in patients with traumatic brain injury (TBI) resuscitated with albumin compared with saline, but the mechanism for increased mortality is unknown. In patients from the Saline vs. Albumin Fluid Evaluation (SAFE) study with TBI who underwent intracranial pressure (ICP) monitoring, interventional data were collected from randomization to day 14 to determine changes in ICP (primary outcome) and in therapies used to treat increased ICP. Pattern mixture modelling, designed to address informative dropouts, was used to compare temporal changes between the albumin and saline groups, and 321 patients were identified, of whom 164 (51.1%) received albumin and 157 (48.9%) received saline. There was a significant linear increase in mean ICP and significantly more deaths in the albumin group compared with saline when ICP monitoring was discontinued during the first week (1.30±0.33 vs. −0.37±0.36, p=0.0006; and 34.4% vs. 17.4%; p=0.006 respectively), but not when monitoring ceased during the second week (−0.08±0.44 vs. −0.23±0.38, p=0.79; and 18.6% vs. 12.1%; p=0.36 respectively). There were statistically significant differences in the mean total daily doses of morphine (−0.42±0.07 vs. −0.66±0.0, p=0.0009), propofol (−0.45±0.11 vs. −0.76±0.11; p=0.034) and norepinephrine (−0.50±0.07 vs. −0.74±0.07) and in temperature (0.03±0.03 vs. 0.16±0.03; p=0.0014) between the albumin and saline groups when ICP monitoring ceased during the first week. The use of albumin for resuscitation in patients with severe TBI is associated with increased ICP during the first week. This is the most likely mechanism of increased mortality in these patients.
Key words: albumin, ICP, resuscitation, saline, TBI
Introduction
Fluid resuscitation to restore the systemic and cerebral circulations is a fundamental component in the hemodynamic management of patients with traumatic brain injury (TBI).1 We previously demonstrated that patients with severe TBI resuscitated in the intensive care unit (ICU) with 4% albumin had a significant (19.6%) increase in death at 2 years compared with patients resuscitated with 0.9% saline.2 In order to identify potential biological mechanisms for these observations, we hypothesized that the increase in mortality associated with albumin was primarily related to the development of increased intracranial pressure (ICP), and/or adverse effects of therapies used to treat increased ICP. A second hypothesis was that albumin may have caused a coagulopathy resulting in secondary intracranial hemorrhage.
Methods
Study design
This study was an additional post-hoc analysis of a subgroup of patients with TBI who were randomized into a prospective, blinded randomized controlled trial (the Saline vs Albumin Fluid Evaluation [SAFE] Study).3
In brief, the SAFE study was a 6997 patient, double-blind, randomized, controlled trial conducted in multidisciplinary ICUs in Australia and New Zealand between November 2001 and June 2003. Eligible adult patients were randomly assigned to receive either 4% albumin (Albumex ®, CSL, Melbourne, Australia) or normal (0.9%) saline for all fluid resuscitation in the ICU until death, discharge, or 28 days after randomization. Randomization was stratified by a diagnosis of trauma. TBI was defined as a diagnosis of trauma plus a Glasgow Coma Scale (GCS) score of ≤134 at first hospital presentation plus an abnormality on a cranial computed tomographic (CT) scan consistent with TBI.
We had previously identified 460 patients with TBI from the SAFE study database and prospectively determined their mortality 2 years after randomization into the SAFE study (SAFE-TBI).2 For this additional study, we identified patients from the SAFE-TBI data set who underwent ICP monitoring, and retrospectively collected additional data from patient records to determine potential mechanisms associated with the development and treatment of raised ICP and with mortality.
The study protocol was approved by the ethics committee of each participating institution.
Outcome measures
The primary outcome measure was the mean change in ICP from randomization to 14 days post-randomization.
Secondary outcome measures were indices of intracranial mechanisms associated with the development of increased ICP (intracranial hemorrhage associated with coagulopathy or progression of diffuse axonal injury on CT appearance); and indices of therapies directed at preventing or treating increased ICP (use of vasopressors, sedatives, and intravenous anesthetic agents; induced hypothermia; osmotherapy and hyperventilation).
Data captured
Data relating to ICP monitors included end-hourly measurements of ICP,5 and daily volumes of cerebrospinal fluid drained from external ventricular drains. Daily measures of coagulation included highest activated partial thromboplastin time (APTT), international normalized ratio (INR), and lowest platelet count. Comparisons of CT scans taken before and after randomization were scored using the Marshall classification6 by a neuroradiologist blinded to treatment allocation.
Data relating to the interventional measures included end-hourly measurements of mean arterial pressure, temperature, arterial carbon dioxide tension, and serum sodium concentration; and total daily doses of sedatives (opiates and benzodiazepines), intravenous anesthetic agents (barbiturates and propofol), vasopressors (norepinephrine), neuromuscular relaxants, and hypertonic saline.
All data collectors and assessors were blinded to treatment assignment.
The study was endorsed by the Australian and New Zealand Intensive Care Society Clinical Trials Group. Trial design, site, and data management and statistical analysis were conducted by the Management Committee in collaboration with methodologists and statisticians at the Australian and New Zealand Intensive Care Research Centre (School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia) and the George Institute for Global Health (Sydney, Australia). The authors accept final responsibility for the integrity of the manuscript.
Statistical analysis
Analyses were conducted on an intention-to-treat basis and were unadjusted, except where indicated. No imputation for missing values was done. All tests were two sided with a nominal value of α=0.05.
Discrete variables were summarized by frequencies and percentages; continuous variables by mean±standard deviation (SD) or median±interquartile range (IQR) where appropriate. Univariate analysis was performed using χ2 tests for equal proportions, Student t tests for normally distributed outcomes, and Wilcoxon tests where appropriate.
Initially, a blind review of individual patient profile plots assigned to each treatment arm, including differentiating patterns for patients who died from those who survived, was conducted. This exercise defined the evolution of individual patients' data, particularly when variables ceased to be collected (the informative dropout period).7
For the primary outcome measure, standard descriptive analyses of changes in mean ICP over time were conducted, followed by repeated measures analysis based on a linear mixed model with random intercept.8 Mean ICP and data associated with treatments on prescribed study days (day 3, 7, 14) were presented as summary data.
Thereafter, pattern mixture models were applied to account for informative dropouts in ICP.7,9,10 Two dropout patterns of the ICP response were identified from individual profile plots classified by the last day of ICP measurement: during days 1–7 and days 8–14 after randomization. Changes in the respective slopes of the unadjusted mean ICP over time were determined, and differences between each of the treatment arms was compared.
The same analyses adjusting for the same covariates described in the SAFE-TBI study (age > 60 years,11 post-resuscitation GCS ≤8,12 pre-randomization mean arterial pressure < 50 mm Hg,13 and CT evidence of traumatic subarachnoid hemorrhage14) and significant differences in baseline were conducted.
For the secondary outcome measures, a similar hierarchy of analyses was conducted, but some variables required a quadratic term to be added to the pattern mixture models when clear patterns from profile plots emerged.7
CT scan scores performed before and after randomization were scored using the Marshall classification (where a score of two indicates swelling, three indicates compression, and four indicates midline shift in the absence of intracranial mass lesions).6 A three level outcome of the score indicating no change (stability), an improvement in score (regression), and deterioration (progression) was determined by logistic regression and modelled by ordinal logistic regression. A common odds ratio (OR) for progression versus stability/regression and stability/progression versus regression was determined.15
Results
From the SAFE-TBI database of 460 patients, 321 (69.7%) patients underwent ICP monitoring and were included in this study. Of these, 164/321 (51.1%) were assigned to receive albumin and 157/321 (48.9%) were assigned to receive saline.
There were no statistically significant differences in baseline demographics between the two groups (Table 1).
Table 1.
Baseline Characteristics of the Patients
| Variable | Albumin (n=164) | Saline (n=157) |
|---|---|---|
| Age - years | 37.8±17.4 | 36.0±15.8 |
| Age>55 years (%) | 18.9 | 12.1 |
| Male (%) | 75.6 | 70.7 |
| Injury severity | ||
| APACHE II - median (IQR) | 21.0 (17–25) | 20.0 (16–24) |
| Abbreviated ISS | 29.8±10.2 | 29.4±10.3 |
| Physiological measures | ||
| Mean arterial pressure – mmHg | 82.3±12.9 | 84.8±14.2 |
| Heart rate – beats/min | 84.8±19.8 | 86.8±19.9 |
| Central venous pressure – mmHg | 7.2±3.2 | 7.1±3.2 |
| Serum albumin – g/L | 30.5±7.6 | 31.7±6.8 |
| Glasgow Coma Scores (GCS) | ||
| Median (IQR) | 6 (4–8) | 6 (4–8) |
| Motor score median (IQR) | 4 (2–5) | 4 (2–5) |
| GCS 3–8 (%) | 77.4 | 77.1 |
| GCS 9–12 (%) | 17.1 | 15.9 |
| GCS 12–13 (%) | 5.5 | 7.0 |
| CT Scan scores (Marshall et al., 1992) | ||
| Diffuse injury II (swelling) (%) | 42.1 | 42.7 |
| Diffuse injury III (cisternal compression) (%) | 14.6 | 16.6 |
| Diffuse Injury IV (midline shift) (%) | 4.9 | 3.8 |
| Non-evacuated mass lesion (%) | 25.6 | 23.6 |
| Evacuated mass lesion | 4.9 | 4.5 |
| Traumatic subarachnoid hemorrhage (%) | 47.0 | 47.1 |
| Intracranial pressure measurements | ||
| Pre-randomization ICP>20mmHg –n/N (%) | 11/108 (10.2) | 11/113 (9.7) |
| ICP on insertiona – mmHg | 15.0±12.9 | 12.5±7.2 |
Data are presented as mean±standard deviation, unless specified otherwise.
Includes some post-randomization measurements.
APACHE II, Acute Physiology and Chronic Health Evaluation (Knaus et al., 1985); ISS, Injury severity score (Baker et al., 1974); GCS, Glasgow Coma Score; ICP, intracranial pressure; IQR, interquartile range.
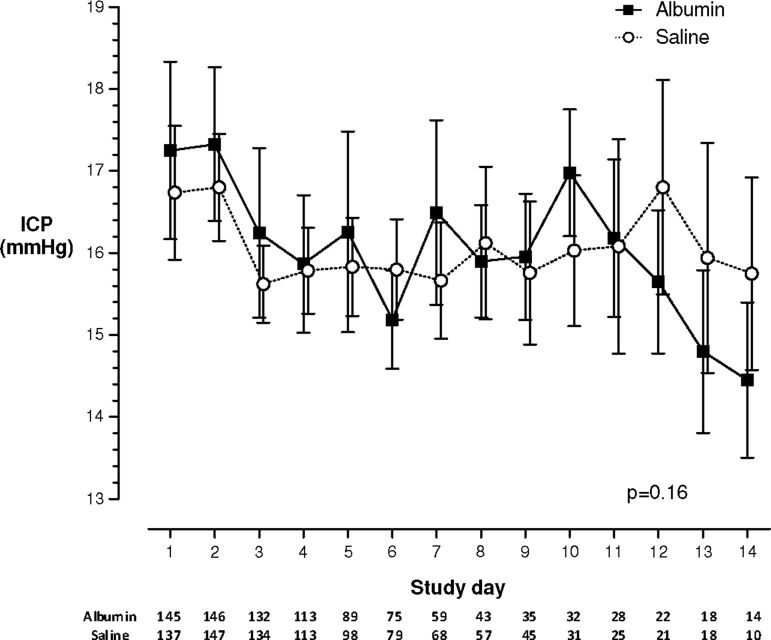
Mean daily ICP measurements for the whole cohort are shown in Figure 1. No significant differences between the albumin or saline groups were demonstrated from randomization to 14 days post-randomization (p=0.16).
FIG. 1.
Changes in mean±standard error of intracranial pressure from randomization to 14 days post-randomization for entire cohort, without correction for dropouts. Table below study day is number of patients per group for that day.
Least-square mean ICP values based on the missing-at-random analysis demonstrated a statistically significant difference between albumin and saline at the end of day 7 (19.2±1.07 vs. 15.4±1.06 mm Hg, p=0.01), but no difference at day 3, at day 14, or overall was observed.
Of the 321 patients, ICP monitoring was discontinued during the first week (days 1–7) in 116 patients in the albumin group and on 92 patients in the saline group; of these 40/116 (34.4%) and 16/92 (17.4%) had died in the albumin and saline groups, respectively (relative risk [RR] 1.98, 95% confidence interval [CI] 1.22–3.22, p=0.006). Of these patients, 27/40 in the albumin group and 10/16 in the saline group had ICP measurements >20 mm Hg (RR 1.08, 95% CI 0.70–1.67, p=0.72).
ICP monitoring was discontinued during the second week (days 8–14) in 43 patients in the albumin group and 58 patients in the saline group; of these 8/43 (18.6%) and 7/58 (12.1%) died in each group (RR 1.54, 95% CI 0.61–3.90, p=0.36).
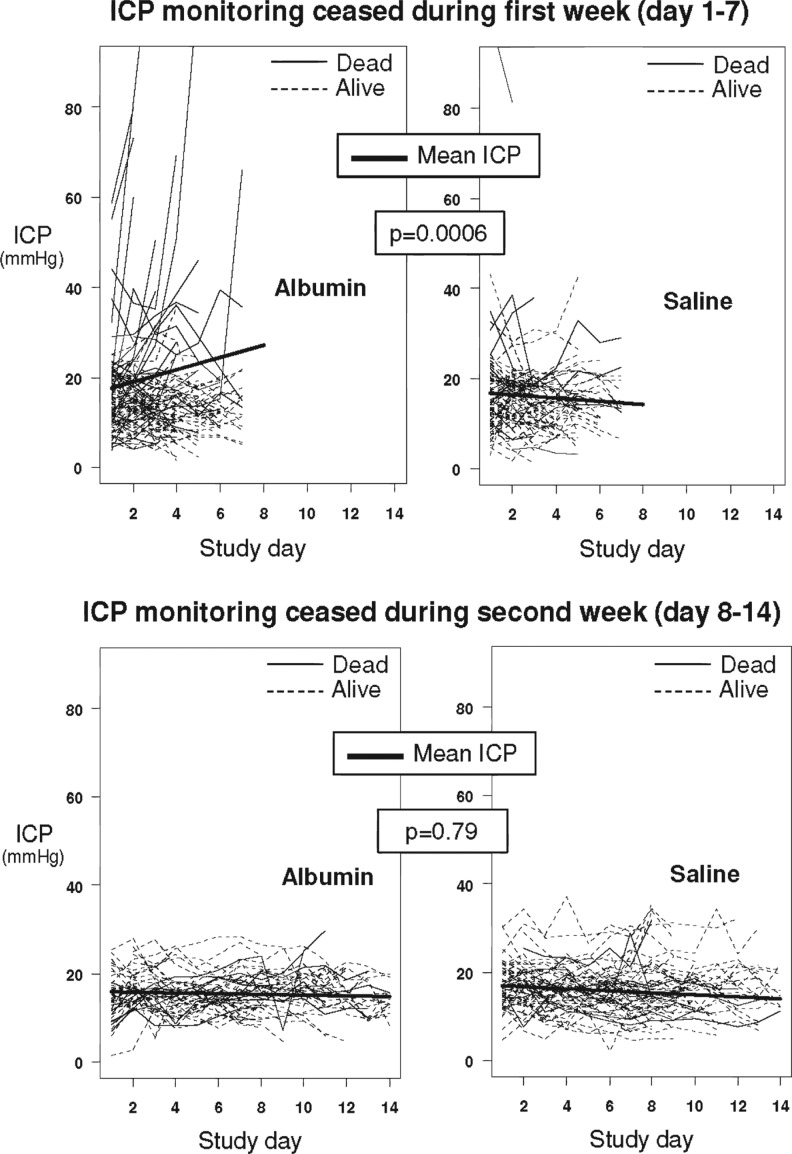
For patients for whom ICP monitoring had ceased during the 1st and 2nd weeks, profile plots displaying changes in individual patient and mean ICP over time for each treatment arm are shown in Figure 2. There was no difference in the mean ICP at baseline (represented by the intercepts on the graphs) between the groups in which ICP monitoring had ceased during the 1st week (16.6±1.01 vs. 17.0±1.14 mm Hg; p=0.76) or during the 2nd week (16.0±1.56 vs. 17.22±1.35 mm Hg; p=0.55). However, there was a statistically significant increase in the slope of mean ICP in the albumin group compared with the saline group in patients for whom ICP monitoring had ceased during the 1st week (1.30±0.33 vs. −0.37±0.36; p=0.0006), but not during the 2nd week (−0.08±0.44 vs. −0.23±0.38; p=0.79).
FIG. 2.
Individual profile plots of patients are presented in light lines, with solid lines for those who died and dashed lines for those who were alive after ICP monitoring was discontinued during the 1st week (top panel) and 2nd week (bottom panel). Pattern-mixture analysis showing temporal changes in unadjusted mean intracranial pressure (ICP) for each group and time period are presented by the heavy line; p value relate to the difference in slopes of mean ICP between albumin and saline groups.
Adjusting the means for age, GCS, mean arterial pressure, and CT evidence of traumatic subarachnoid hemorrhage did not alter the difference of the slopes between the two groups in patients for whom ICP monitoring had ceased during the 1st week (1.31±0.33 vs. −0.37±0.36; p=0.0007).
There were no significant differences between the intercepts of any of the secondary outcome variables between the two groups (Table 2).
Table 2.
Intercepts and Slopes of Linear Random Effects Time Trends (Pattern Mixture Modelling) for Secondary Outcomes in Patients Classified by Day of Ceasing ICP Monitoring: Day 1–7 (Top) and Day 8–14 (Bottom)
| |
ICP monitoring ceased day 1–7 |
|||||
|---|---|---|---|---|---|---|
| |
Intercept |
Slope |
||||
| Albumin | Saline | p | Albumin | Saline | p | |
| APTT | 32.1 (0.9) | 33.3 (0.9) | 0.34 | 0.09 (0.2) | −0.17 (0.2) | 0.43 |
| INR | 1.26 (0.0) | 1.26 (0.0) | 0.99 | −0.02 (0.0) | −0.04 (0.0) | 0.07 |
| Platelets | 159.8 (14.4) | 183.0 (15.6) | 0.27 | 9.48 (4.5) | 5.44 (4.5) | 0.52 |
| CSF drainagea | 38.1 (25.2) | 39.8 (26.9) | 0.96 | −2.68 (1.95) | −6.46 (2.06) | 0.33 |
| TDD morphine | 3.56 (0.27) | 4.13 (0.3) | 0.16 | −0.42 (0.07) | −0.66 (0.07) | 0.02 |
| TDD norepinephrine | 3.97 (0.3) | 4.75 (0.3) | 0.07 | −0.5 (0.07) | −0.74 (0.07) | 0.02 |
| TDD propofol | 4.28 (0.5) | 4.81 (0.5) | 0.45 | −0.45 (0.11) | −0.76 (0.11) | 0.05 |
| TDD midazolam | 2.53 (0.3) | 2.75 (0.3) | 0.65 | −0.3 (0.07) | −0.41 (0.07) | 0.26 |
| Temperature | 37.1 (0.1) | 36.7 (0.1) | 0.10 | 0.03 (0.03) | 0.16 (0.03) | 0.001 |
| Sodiuma | 144.8 (1.1) | 142.1 (1.1) | 0.08 | −0.13 (0.08) | −0.26 (0.08) | 0.34 |
| PaCO2a | 35.0 (0.9) | 35.1 (0.9) | 0.94 | −0.26 (0.08) | −0.16 (0.08) | 0.31 |
| |
ICP monitoring ceased day 8–14 |
|||||
|---|---|---|---|---|---|---|
| |
Intercept |
Slope |
||||
| Albumin | Saline | p | Albumin | Saline | p | |
| APTT | 35.3 (0.5) | 32.6 (0.5) | 0.20 | −0.33 (0.07) | −0.1 (0.07) | 0.42 |
| INR | 1.26 (0.02) | 1.2 (0.02) | 0.20 | 0.01 (0.0) | 0.01 (0.0) | 0.07 |
| Platelets | 50.3 (8.4) | 69.1 (8.1) | 0.11 | 34.3 (1.8) | 35.3 (1.8) | 0.67 |
| CSF drainagea | 56.9 (22.4) | 60.9 (19.5) | 0.89 | −2.35 (0.29) | −2.96 (0.29) | 0.82 |
| TDD morphine | 4.95 (0.17) | 5.26 (0.17) | 0.19 | −0.30 (0.02) | −0.37 (0.02) | 0.33 |
| TDD norepinephrine | 4.5 (0.19) | 4.76 (0.32) | 0.33 | 0.32 (0.02) | −0.37 (0.02) | 0.09 |
| TDD propofol | 3.64 (0.29) | 3.61 (0.28) | 0.93 | −0.12 (0.03) | −0.07 (0.03) | 0.20 |
| TDD midazolam | 4.12 (0.21) | 4.52 (0.20) | 0.17 | −0.28 (0.02) | −0.33 (0.02) | 0.08 |
| Temperature | 36.9 (0.09) | 36.8 (0.09) | 0.49 | 0.07 (0.01) | 0.08 (0.01) | 0.45 |
| Sodiuma | 145.3 (0.61) | 145.3 (0.59) | 0.99 | 0.01 (0.01) | 0.01 (0.01) | 0.72 |
| PaCO2a | 36.6 (0.46) | 35.9 (0.43) | 0.31 | −0.03 (0.01) | −0.00 (0.01) | 0.29 |
Data are shown as mean (standard deviation).
Quadratic term was included in the model for these variables.
Units for intercepts: activated partial thromboplastin time (APTT): seconds; international normalized ratio (INR); platelet count: ×109/L; cerebrospinal fluid (CSF) drainage: mL/day; total daily dose (TDD) of drugs transformed by log, temperature: oC; sodium: mmol/L; arterial carbon dioxide tension (PaCO2): mmHg.
Slopes are expressed as positive for increase, negative for decrease.
ICP, intracranial pressure.
In patients for whom ICP monitoring ceased during the 1st week, there was a lesser decrease in the slopes (indicating increased dose) in the albumin group for total daily dose of morphine (−0.42±0.07 vs. −0.66±0.07; p=0.017) and propofol (−0.45±0.11 vs. −0.76±0.11; p=0.053). This difference was more marked after adjustment for the four severity indices (p=0.0009 and 0.034 respectively). There was also a lesser decrease in the slopes in the albumin group for the total daily dose of norepinephrine (−0.50±0.07 vs. −0.74±0.07; p=0.02) and temperature (0.03±0.03 vs. 0.16±0.03; p=0.0014) (Table 2).
There was no significant difference in the slopes of the other variables in patients for whom ICP monitoring ceased during the 1st week or in any secondary outcome variable during the 2nd week (Table 2).
A total of 191/321 (59.5%) patients with ICP monitoring had pairs of CT scans that were available for comparison. No differences in changes in CT score between the albumin or saline groups were found where ICP monitoring was discontinued during the 1st week (OR 1.10, 95% CI 0.56–2.27; p=0.77) or during the 2nd week (OR 1.30, 95% CI 0.53–3.18, p=0.57) (Table 3).
Table 3.
Changes in Computerized Tomography Scores (Marshall Et Al., 1992) between Last Scan before Randomization and the First Scan Post-Randomization in Patients for whom ICP Monitoring was Discontinued during the 1st Week (Days 1–7) and 2nd Week (Days 8–14)
| |
ICP monitoring ceased day 1–7 (n=117) |
ICP monitoring ceased day 8–14 (n=74) |
||||||
|---|---|---|---|---|---|---|---|---|
| Change n (%) | Albumin (n=63) | Saline (n=54) | OR (95%CI)a | p | Albumin (n=31) | Saline (n=43) | OR (95%CI)a | p |
| Progression | 19 (30.2) | 20 (37.0) | 1.10 (0.56 to 2.21) | 0.77 | 7 (22.6) | 10 (23.35) | 1.30 (0.53 to 3.18) | 0.57 |
| Stability | 35 (55.6) | 24 (44.4) | 14 (45.2) | 24 (55.8) | ||||
| Regression | 9 (14.3) | 10 (18.5) | 10 (32.3) | 9 (20.9) | ||||
OR, odds ratio adjusted for the pre-randomization score by ordinal logistic regression with 95% confidence intervals (CI).
ICP, intracranial pressure.
Discussion
Statement of principal findings
Our study demonstrated that resuscitation with albumin was associated with increased ICP and with associated interventions used to treat increased ICP, in particular sedatives, analgesics, and vasopressors in patients during the 1st week after injury. During the same week, more patients who received albumin died compared with those who received saline. These data suggest that increased cerebral edema leading to increased ICP is the most likely mechanism for increased death observed in TBI patients in the ICU resuscitated with albumin compared with those resuscitated with saline.
Strengths and weaknesses of the study
A strength of this study is that we recognized the statistical challenges in analyzing these data from the outset. A detailed, hierarchical, statistical analysis plan was approved by the authors prior to data analysis and before treatment assignments were unblinded. The analysis plan was designed to address missing data when measurements were stopped after patients ceased to be included in the study (“dropouts”). Missing data may occur for divergent reasons that may be random or non-random (deliberate) events. Censoring of data at the time of dropout, therefore, requires clarification (informative censoring), for which a number of statistical models are used.
Mixed-model repeated-measures analyses alone rely on the missing-at-random assumption and are likely to be biased because subjects with complete data may have more influence at later time points when subjects with incomplete data have dropped out from the analysis. This is particularly true when ICP monitoring cessation is the result of death, as the dropout process itself then is no longer independent of the outcome. Pattern mixture models modify mixed-model analyses by evaluating the model by time of dropout (pattern) and assessing the effect of treatment within that pattern and overall (mixture). Pattern-mixture models therefore adjust for bias that may occur by unbalanced patient withdrawals irrespective of the reason for dropout, and identify appropriate patterns over time.10,16
In our study, values of ICP became unavailable for different reasons, including death, clinical improvement, or technical difficulties associated with the ICP monitor. Pattern-mixture models identified two distinct patterns of ICP in patients for whom ICP monitoring ceased during the 1st week: there was a linear increase in ICP in patients who received albumin, which was not demonstrated in patients who received saline. These observations were not evident using the standard comparative analyses of mean ICP, which we presented in Figure 1. The fact that standard comparisons of available ICP values may obscure real differences between groups is an important observation.
As with any post-hoc subgroup analysis, interpretation of these results requires caution, because of the loss of statistical power and the potential for imbalance between groups, even after adjustment for clinically relevant and statistically significant covariates. Multiple comparisons increase the likelihood of spurious findings. Caution is, therefore, required when determining significance, and the standard level of probability (p<0.05) may be too lenient. However, the observed differences in the primary outcome (ICP) were highly significant (p<0.001), suggesting a higher degree of certainty.
Whereas our data do not explain why ICP was increased in patients who received albumin, it appears that coagulopathy was not responsible. Lesser efforts to control ICP in the patients who received albumin were also not responsible, as the increased ICP occurred despite these patients being treated with higher doses of sedatives, analgesics, and vasopressors, and having more temperature control.
Inferences between association and causation require caution: the secondary outcome measures were surrogate endpoints for complex physiological and pathological processes that, therefore, lose specificity. Furthermore, we could not collect detailed biological data such as genotype mapping, that may be an important determinant of susceptibility to raised ICP.
Possible mechanisms and implications for clinicians and policy makers
Our study provides novel data that confirms that the selection of resuscitation fluid may have a significant impact on the clinical course and outcome of patients with TBI. Our findings are in keeping with concerns that increased extravasation of albumin from areas of altered blood brain barrier permeability may lead to increased cerebral interstitial colloid osmotic pressure and increased ICP.17 It is also possible that hypotonic stress may contribute, as the albumin preparation used in the SAFE study. Albumex®, (CSL, Melbourne) was relatively hypotonic (260 mOsmol/L) and ∼24 mOsmol/L less than 0.9% saline.
International guidelines for fluid resuscitation in patients with TBI should recommend against the administration of albumin, especially in the 1st week after injury. Whether the findings are specific to albumin or also applicable to synthetic colloids is unanswered by our data. However, until there are comparative data confirming the safety of synthetic colloids, 0.9% saline should be used for fluid resuscitation of patients with TBI.
Conclusion
The use of albumin for resuscitation in patients with severe TBI is associated with increased ICP. This is the most likely mechanism of increased mortality in these patients.
Contributor Information
Collaborators: the SAFE-TBI Investigators, and the Australian and New Zealand Intensive Care Society Clinical Trials Group
Acknowledgments
This study was funded by a project grant (number DP016) from the Victorian Neurotrauma Initiative (grant number DP016), Australia. Associate Professor Heritier is supported by a program grant (number 57281) from the National Health and Medical Research Council of Australia. Statistical analysis was partially funded by the Division of Critical Care and Trauma of the George Institute for Global Health, Sydney, Australia. The Management Committee consisted of D James Cooper, (co-chair), John Myburgh, (co-chair), Michael Bailey, Rinaldo Bellomo, Laurent Billot, Simon Finfer, Parisa Glass, Stephane Heritier, Michael Fitzharris, Alisa Higgins, Daryl Jones, Siouxzy Morrison, Lynette Murray, Alistair Nichol, Gillian Syres, and Shirley Vallance. Associate Professor Michael Bailey assisted with the initial statistical analyses. Associate Professor Dinesh Varma, Alfred Hospital, Melbourne conducted the blind review of cranial CT scans. The Site investigators (Australia unless stated) were Alfred Hospital, Melbourne: D James Cooper, and Shirley Vallance; Austin Hospital, Melbourne: Rinaldo Bellomo, and Donna Goldsmith; Australian and New Zealand Research Centre, Melbourne: Michael Bailey, Rinaldo Bellomo, D James Cooper, Andrew Forbes, Alisa Higgins, Daryl Jones, Siouxzy Morrison, Lynette Murray, Alistair Nichol, and Gillian Syres; Royal Darwin Hospital: Diane Stephens, and Jane Thomas; Fremantle Hospital: David Blythe, and Anna Palermo; John Hunter Hospital, Newcastle: Peter Harrigan, Brett McFadyen, and Agness Tembo; George Institute for Global Health, Sydney: Laurent Billot, Simon Finfer, Michael Fitzharris, Parisa Glass, Stephane Heritier, Serigne Lo, Mathieu Rose, and John Myburgh; Middlemore Hospital, New Zealand: Judy Tai, and Anthony Williams; Nepean Hospital, Sydney: Louise Cole, Iveta Nalos, Ian Seppelt, and Leonie Weisbrodt; Princess Alexandra Hospital, Brisbane: Sally Gipps, Chris Joyce, Ben Mackie, and Bala Venkatesh; Royal Adelaide Hospital: Marianne Chapman, Stephanie O'Connor, and Justine Rivett; Royal Hobart Hospital: Anthony Bell, Kathryn Marsden, and Andrew Turner; Royal Melbourne Hospital: Deborah Barge, Jack Cade, Tania Caf, and Megan Robertson; Royal North Shore Hospital, Sydney: Simon Finfer, Anne O'Connor, Julie Potter, and Naresh Ramakrishnan; St George Hospital, Sydney: Vanessa Dhiacou, Alina Jovanovska, and John Myburgh.
Author Disclosure Statement
Professor Cooper has no competing financial interests in relation to this study. Professor Myburgh has no competing financial interests exist in relation to this study. In relation to ongoing fluid resuscitation research, The George Institute for Global Health and the University of Sydney have received a speaker's fee, unrestricted research grant support, and refund of travel expenses incurred on Professor Myburgh's behalf from Fresenius Kabi. Associate Professor Heritier has no competing financial interests in relation to this study. Professor Finfer has no competing financial interests in relation to this study. The SAFE Study was partially funded by CSL Limited. CSL Limited paid travel expenses for Professor Finfer to present results of the SAFE Study at scientific and industry-sponsored meetings. In relation to ongoing fluid resuscitation research, The George Institute for Global Health and the University of Sydney have received unrestricted research grant support and refund of travel expenses incurred on Professor Finfer's behalf from Fresenius Kabi. Professor Bellomo has no competing financial interests in relation to this study. The SAFE Study was part funded by CSL Limited. CSL Limited paid travel expenses for Professor Bellomo to present results of the SAFE Study at scientific and industry- sponsored meetings. Laurent Billot has no competing financial interests in relation to this study. Lynette Murray has no competing financial interests in relation to this study. Shirley Vallance has no competing financial interests in relation to this study.
References
- 1.Bratton S.L. Chestnut R.M. Ghajar J. McConnell Hammond F.F. Harris O.A. Hartl R. Manley G.T. Nemecek A. Newell D.W. Rosenthal G. Schouten J. Shutter L. Timmons S.D. Ullman J.S. Videtta W. Wilberger J.E. Wright D.W. Guidelines for the management of severe traumatic brain injury. I. Blood pressure and oxygenation. J. Neurotrauma. 2007;24(Suppl. 1):S7–13. doi: 10.1089/neu.2007.9995. [DOI] [PubMed] [Google Scholar]
- 2.SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 3.SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 4.Teasdale G. Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesh B. Garrett P. Fraenkel D.J. Purdie D. Indices to quantify changes in intracranial and cerebral perfusion pressure by assessing agreement between hourly and semi-continuous recordings. Intensive Care Med. 2004;30:510–513. doi: 10.1007/s00134-003-2102-7. [DOI] [PubMed] [Google Scholar]
- 6.Marshall L.F. Marshall S.B. Klauber M.R. Van Berkum C.M. Eisenberg H. Jane J.A. Luerssen T.G. Marmarou A. Foulkes M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma. 1992;9(Suppl. 1):S287–S292. [PubMed] [Google Scholar]
- 7.Molenberghs G. Thijs H. Jansen I. Beunckens C. Kenward M.G. Mallinckrodt C. Carroll R.J. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5:445–464. doi: 10.1093/biostatistics/5.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Vangeneugden T. Laenen A. Geys H. Renard D. Molenberghs G. Applying linear mixed models to estimate reliability in clinical trial data with repeated measurements. Control Clin. Trials. 2004;25:13–30. doi: 10.1016/j.cct.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Pauler D.K. McCoy S. Moinpour C. Pattern mixture models for longitudinal quality of life studies in advanced stage disease. Stat. Med. 2003;22:795–809. doi: 10.1002/sim.1397. [DOI] [PubMed] [Google Scholar]
- 10.van der Heijde D. Klareskog L. Landewe R. Bruyn G.A. Cantagrel A. Durez P. Herrero-Beaumont G. Molad Y. Codreanu C. Valentini G. Zahora R. Pedersen R. MacPeek D. Wajdula J. Fatenejad S. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:3928–3939. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 11.BTF Guidelines. The Brain Trauma Foundation. The American Association of Neurological Surgeons. Age. J. Neurotrauma. 2000;17:573–582. doi: 10.1089/neu.2000.17.573. [DOI] [PubMed] [Google Scholar]
- 12.BTF Guidelines. The Brain Trauma Foundation. The American Association of Neurological Surgeons. Glasgow Coma Scale Score. J. Neurotrauma. 2000;17:563–572. doi: 10.1089/neu.2000.17.563. [DOI] [PubMed] [Google Scholar]
- 13.BTF Guidelines. Brain Trauma Foundation, American Association of Neurological Surgeons: Hypotension. J. Neurotrauma. 2000;17:591–595. doi: 10.1089/neu.2000.17.591. [DOI] [PubMed] [Google Scholar]
- 14.Servadei F. Murray G.D. Teasdale G.M. Dearden M. Iannotti F. Lapierre F. Maas A.J. Karimi A. Ohman J. Persson L. Stocchetti N. Trojanowski T. Unterberg A. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50:261–267. doi: 10.1097/00006123-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chieregato A. Fainardi E. Morselli-Labate A.M. Antonelli V. Compagnone C. Targa L. Kraus J. Servadei F. Factors associated with neurological outcome and lesion progression in traumatic subarachnoid hemorrhage patients. Neurosurgery. 2005;56:671–680. doi: 10.1227/01.neu.0000156200.76331.7a. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub W.S. Spertus J.A. Kolm P. Maron D.J. Zhang Z. Jurkovitz C. Zhang W. Hartigan P.M. Lewis C. Veledar E. Bowen J. Dunbar S.B. Deaton C. Kaufman S. O'Rourke R.A. Goeree R. Barnett P.G. Teo K.K. Boden W.E. Mancini G.B. Effect of PCI on quality of life in patients with stable coronary disease. N. Engl. J. Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 17.Jeffcote T. Ho K.M. Associations between cerebrospinal fluid protein concentrations, serum albumin concentrations and intracranial pressure in neurotrauma and intracranial haemorrhage. Anaesth. Intensive Care. 2010;38:274–279. doi: 10.1177/0310057X1003800208. [DOI] [PubMed] [Google Scholar]