Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is known to be present in small animal veterinary clinical environments. However, a better understanding of the ecology and dynamics of MRSA in these environments is necessary for the development of effective infectious disease prevention and control programs. To achieve this goal, a yearlong active MRSA surveillance program was established at The Ohio State University (OSU) Veterinary Medical Center to describe the spatial and molecular epidemiology of this bacterium in the small animal hospital. Antimicrobial susceptibility testing, staphylococcal chromosomal cassette mec (SCCmec) typing, pulsed-field gel electrophoresis (PFGE) typing, and dendrogram analysis were used to characterize and analyze the 81 environmental and 37 canine-origin MRSA isolates obtained during monthly sampling events. Overall, 13.5% of surfaces were contaminated with MRSA at 1 or more sampling times throughout the year. The majority of the environmental and canine isolates were SCCmec type II (93.8% and 86.5%, respectively) and USA100 (90.1% and 86.5%, respectively). By PFGE analysis, these isolates were found to be closely related, which reflects a low diversity of MRSA strains circulating in the hospital. For 5 consecutive months, 1 unique pulsotype was the most prevalent across the medical services and was recovered from a variety of surfaces and hospital locations. Carts/gurneys, doors, and examination tables/floors were the most frequently contaminated surfaces. Some surfaces maintained the same pulsotypes for 3 consecutive months. Molecular analysis found that incoming MRSA-positive dogs were capable of introducing a new pulsotype into the hospital environment during the surveillance period. Our results suggest that once a MRSA strain is introduced into the hospital environment, it can be maintained and spread for extended periods of time. These findings can aid in the development of biosecurity and biocontainment protocols aimed at reducing environmental contamination and potential exposures to MRSA in veterinary hospital staff, clients, and patients.
Key Words: MRSA, Surveillance, Molecular epidemiology, Veterinary hospitals, Environment
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is known to be a primary pathogen capable of causing severe health problems in both humans (Klein et al. 2007) and animals (O'Mahony et al. 2005, Abbott et al. 2010, Pinto et al. 2011, Pantosti 2012). The increasing number of reported cases (Klein et al. 2007, Abbott et al. 2010, Aklilu et al. 2010, Loeffler et al. 2011) suggests that MRSA represents a growing animal and public health concern. The risk of zoonotic transmission of MRSA between humans and companion animals has been described in households, the community, and healthcare settings (Seguin et al. 1999, Manian 2003, van Duijkeren et al. 2004, O'Mahony et al. 2005, van Duijkeren et al. 2005, Moodley et al. 2006, Weese et al. 2006, Nienhoff et al. 2009, Rutland et al. 2009, van Duijkeren et al. 2010).
MRSA is capable of contaminating and surviving for up to 7 months on inanimate objects and contact surfaces in households and healthcare facilities (Kramer et al. 2006, Otter et al. 2011). Several studies have implicated hospital surfaces contaminated with nosocomial pathogens, including MRSA, in the dissemination of hospital-acquired infections (Rampling et al. 2001, Schultsz et al. 2003, Hota 2004, Kramer et al. 2006, Sexton et al. 2006, Boyce 2007, Weber et al. 2010, Otter et al. 2011). Paralleling these are reports of the increasing role of MRSA in nosocomial infections in veterinary settings (Seguin et al. 1999, Leonard, et al. 2006, Weese et al. 2007, Benedict et al. 2008, McLean and Ness 2008, van Duijkeren et al. 2010). A recent study of veterinary teaching hospitals accredited by the American Veterinary Medicine Association (AVMA) found that MRSA was the second most common pathogen (13/31, 42%) associated with nosocomial outbreaks (Benedict et al. 2008). However, the interaction between the environment, patients, and hospital personnel in the transmission of MRSA in veterinary hospitals has not been described.
The presence of MRSA in veterinary environments has been reported during outbreaks or limited periods of surveillance (Weese et al. 2004, Loeffler et al. 2005, Heller et al. 2009, Murphy et al. 2010, Hoet et al. 2011). The environment in the equine section of a veterinary teaching hospital was sampled after the recognition of a cluster of MRSA infections in horses and humans (Weese et al. 2004). MRSA was found on multiple surfaces and on equipment throughout the equine hospital. The authors concluded “that the environment may be an important source of MRSA infection” (Weese et al. 2004), which could be the result of the close interaction of the animals with their contaminated surroundings. Nevertheless, no longitudinal reports of the ecology and dynamics of MRSA in veterinary environments in the absence of an outbreak have been reported. Therefore, our objectives were to generate baseline data for the presence and distribution of MRSA contamination on surfaces in a small animal teaching hospital and to describe the temporal dynamics of MRSA in this environment using molecular epidemiological analyses.
Materials and Methods
Active MRSA surveillance program
This targeted surveillance was performed over a 1-year period at the Small Animal Hospital in The Ohio State University Veterinary Medical Center (OSU VMC) between November, 2007, and October, 2008. This is a tertiary referral veterinary medical hospital that accepts patients from smaller veterinary clinics and/or private practices throughout the midwestern United States, but it also operates as a primary care facility for local companion animals. The active MRSA surveillance program had 2 major components—regular sampling of the hospital environment and concurrent sampling of incoming dogs prior to their entering the surveillance areas.
Location, selection, and sample collection of environmental surfaces
On the basis of results obtained in a preliminary study in 2007 (Hoet, et al. 2011), specific services of the small animal hospital were targeted for MRSA monitoring. These were: Community Practice (examination room and treatment area), Dermatology (treatment room and wards), Intensive Care Unit, and Surgery (presurgery room, anesthesia room, surgery suits, and wards). These areas were targeted because they previously tested positively to MRSA contamination (Hoet et al. 2011) and because the presence of this pathogen represents an important risk for nosocomial infection of the patients visiting these services.
Once the areas to be regularly monitored were selected, specific high-contact surfaces were identified for monthly sampling. The number and type of surfaces to be sampled were determined based on the results from the previous pilot surveillance (Hoet et al. 2011). An average of 48 samples were collected each month for 1 year: Community Practice (5 samples/month), Dermatology (11 samples/month), Intensive Care Unit (8 samples/month), and Surgery (21 samples/month). Samples were also obtained from the hall carts/gurneys that traveled among several services, because they were not limited to 1 section in particular they were classified as General (3 samples/month). The preselected surfaces were divided into human and animal-contact surfaces as seen in Table 1. Human-contact surfaces were defined as those surfaces that are regularly touched by multiple people during routine activities, but are typically out of reach for direct contact with animals, such as computers (keyboard and mouse) and phones. In contrast, animal-contact surfaces are those that are mainly touched by multiple animals, such as cages and water bowls.
Table 1.
Human and Canine Contact Surfaces Sampled with Electrostatic Cloths (■) or Sterile Swabs (▴) by Service at the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the MRSA Active Surveillance
| Hospital service | Human contact | Animal contact |
|---|---|---|
| Community practice | Doors■ | Exam tables■a |
| Computers■a,b | Floor (exam)■a | |
| Otoscope▴ | Muzzles▴ | |
| Dermatology | Doors (ward)■ | Cages (ward)■ |
| Exam lights■ | Floors (exam)■ | |
| Fax/phone▴ | Muzzles (ward)▴ | |
| Computer▴b | Water bowls (ward)▴ | |
| Microscope▴ | ||
| Otoscope▴ | ||
| Paper towel dispenser▴a | ||
| Alcohol-gel dispensers▴a | ||
| Intensive care unit | Doors■ | Cages (2)■ |
| IV pumps▴ | Muzzles▴ | |
| Computer▴a,b | Water bowls▴ | |
| Laptop▴ | ||
| Surgery | Clippers▴ | Cages■ |
| Doors■ | Exam tables■ | |
| Drawer handles■ | Muzzles (ward)▴ | |
| Exam lights■a | Oxygen monitors▴ | |
| Light switches■a | Warming pads■c | |
| Table knobs■a | Water bowls▴ | |
| General | NA | Carts/gurney■ (3) |
Samples collected as a pool within the same service.
Included keyboard and mouse.
Multiple warming pads located in the same room were sampled as a pool.
IV, intravenous; NA, not applicable.
Dry electrostatic cloths/Swiffer® (for larger surfaces) or sterile premoistened cotton swabs (for smaller surfaces) were used to sample the different contact surfaces, either as individual or pooled samples, as previously described (Hoet et al. 2011). Immediately after sampling, electrostatic cloths were folded and placed into sterile bags, and 90 mL of BPW (BD BBL™ Buffered Peptone Water, Becton, Dickinson and Company, Sparks, MD) was added before incubating them at 35°C for 24 hours. Similarly, the individual swabs were placed in tubes with TSB (BD BBL™ Trypticase Soy Broth, Becton, Dickinson and Company) and incubated at 35°C for 24 h. Negative and positive controls were included in each sampling.
Canine population and sample collection
Parallel to the environmental sampling, incoming dogs admitted to the hospital services being monitored were sampled upon their arrival as previously described (Hoet et al. 2013). Briefly, only dogs, either referrals or OSU VMC patients that had not been in the hospital in the last 6 months, were included in the study. Canines from the Community Practice and Intensive Care Unit (n=148), Dermatology Service (n=145), and Surgery Service (n=142) were recruited on a monthly basis, averaging 36–37 dogs per month during the surveillance year. Upon arrival, a signed consent form was obtained from the dog's owner for inclusion into the study, and the animal was sampled prior to any clinical examination. To screen for the presence of MRSA, sterile premoistened cotton swabs in TSB were used. Samples were collected from the nasal cavity, ear canals, external surface of the perianal area, and any skin lesions (if present) from each canine. To increase the likelihood of identifying MRSA, these specific anatomical locations were selected based on previous studies demonstrating positive carriage in these sites (Morris et al. 2006; Boost et al. 2008).
MRSA isolation and identification
All samples collected (environmental and animal) were processed in the Diagnostic and Research Laboratory for Infectious Diseases (DRLID) at OSU, College of Veterinary Medicine, for isolation of S. aureus and further testing for resistance to methicillin. As previously described (Hoet et al. 2011), samples were incubated at 35°C in pre-enrichment medium for 24 h and then streaked onto mannitol salt agar (BD BBL™ Mannitol Salt Agar, Dickinson and Company) with 2 μg/mL of oxacillin. After 24–48 h, 1–3 colonies per sample were selected and plated on trypticase soy agar with 5% sheep blood agar plates (Remel®, Blood Agar [trypticase soy agar, TSA, with 5% sheep blood], Lenexa, KS) for further testing. Identification of S. aureus was performed by standard colony morphology (including size, pigmentation, and hemolysis pattern) and biochemical tests reactions that included mannitol fermentation, gram stain, catalase, tube coagulase, anillin fermentation, Polymyxin B susceptibility, acetoin production (Vogues–Proskauer test), and latex agglutination (Sure-Vue® Color Staph ID, Biokit USA, Inc., Lexington, MA) (Hoet et al. 2011). Phenotypic MRSA confirmation was performed by growth on Oxacillin Screen Agar® (OSA) plates containing 6 μg/mL of oxacillin supplemented with NaCl (BD BBLTM, Becton Dickinson and Company) following the Clinical Laboratory Standards Institute protocols (CLSI 2008).
Antimicrobial susceptibility testing (phenotyping)
A total of 161 MRSA isolates (90 from the environment and 71 from dogs) were tested for susceptibility to 18 antimicrobial drugs important to veterinary and human medicine. Susceptibility to 17 of these drugs was tested using the Kirby–Bauer Disc Diffusion technique following protocols described by CLSI (CLSI 2008). Antimicrobials included were: amikacin 30 μg, ampicillin 10 μg, amoxicillin with clavulanic acid 30 μg, cefovecin 30 μg, cefpodoxime 10 μg, cephalotin 30 μg, chloramphenicol 30 μg, ciprofloxacin 2 μg, clindamycin 2 μg, doxycycline 30 μg, enrofloxacin 5 μg, erythromycin 15 μg, gentamicin 1 μg, oxacillin 1 μg, sulfamethoxazole trimethoprim 25 μg, and tetracycline 30 μg. The last antimicrobial, vancomycin, was tested using Vancomycin Screen Agar plates (6 mg/L) (BD BBL™ Vancomycin Screen Agar, Dickinson and Company). For quality control purposes, the following strains were included: S. aureus (ATCC 43300), S. aureus (ATCC 29213), S. aureus (ATCC 25923), Enterococcus faecalis (ATCC 23212), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853). Isolates resistant to 3 or more classes of antimicrobials (including β-lactams after confirmation of the mecA gene) were considered to be multidrug resistant (MDR). Inducible clindamycin resistance was tested by placing the erythromycin disc next to the clindamycin disc during the phenotyping process and evaluating the bacterial growth pattern consistent with the D-test (Fiebelkorn et al. 2003).
mecA confirmation and staphylococcal chromosome cassette mec typing
On the basis of the phenotyping results, 118 unique isolates (81 from the environment and 37 from the dogs) were selected for further molecular testing. Because 1–3 colonies were selected per plate during the isolation and identification process, selection was performed by ruling out isolates coming from the same sample source with identical morphology and phenotypic results in all the antimicrobials tested. Methicillin-resistant status was confirmed by detection of the mecA gene and typing of the staphylococcal chromosomal cassette mec (SCCmec) simultaneously. Briefly, DNA was extracted using a boiling method, where a 100 μL bacterial suspension was heated for 7 min at 95°C (Kilic et al. 2006). A multiplex PCR was performed (Milheirico et al. 2007) with a few modifications. Each PCR mixture with a final volume of 25 μL contained 2 μ of DNA template, 12.5 μL of 2×Multiplex PCR Master Mix (Qiagen®, Foster City, CA) containing HotStartTaq Polymerase, 3 mM MgCl2, and deoxyribonucleotide triphosphates (dNTPs). Primer concentrations were doubled compared to what had been previously reported (Milheirico et al. 2007). The PCR products (10 μL) were resolved in a 3% Seakem LE (Cambrex, Rockland, ME) agarose gel with 1×Tris-acetate-EDTA buffer (Promega Corporation, Madison, WI) at 100 V for 2 h, and were detected with ethidium bromide. All assays were performed in a Gradient Thermocycler (Eppendorf, Hamburg, Germany). The cycling conditions were the following: 95°C for 15 min; 35 cycles of 94°C for 30 s, 57°C for 90 s, and 72°C for 90 s; and a final extension of 72°C for 10 min.
Pulsed-field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE) of SmaI-digested chromosomal DNA was performed according to the protocol established by Centers for Disease Control and Prevention (CDC)/Pulse-Net. Restriction fragments were separated using a CHEF Mapper system (Bio-Rad Laboratories, Nazareth, Belgium). The resulting band patterns were analyzed by BioNumerics® software (version 6.6, Applied Maths, Ghent, Belgium) to determine the relatedness between strains by using Dice coefficient and unweighted pair group method using arithmetic averages (UPGMA) to achieve dendrograms with a 1% band position tolerance (Murchan et al. 2003). Dendrogram interpretation was performed as follows: Each band pattern represented a pulsotype; isolates with≥98% similarity were considered indistinguishable and characterized as the same pulsotype. These types were grouped in clusters of≥80% similarity that were considered to be closely related (Singh et al. 2006). Designation of USA types was performed comparing our isolates to a CDC database containing 100 S. aureus strains with the most typical band patterns for each USA type, using≥80% similarity as the cutoff point. The Salmonella serotype Branderup strain H9812 was digested with XbaI and used as a molecular size marker. Three different dendrograms were built—environmental isolates alone, canine isolates alone, and a combination of both environmental and canine isolates.
Strain characterization
Each isolate was characterized and classified as a strain if it possessed a unique combination of phenotypic profile, SCCmec type, and pulsotype.
Statistical analysis
Surface location, date of collection, culture, and molecular strain characterization results were organized and stored. Further analysis was performed using the statistical software STATA (Small Stata 12.0, StataCorp LP, College Station, TX). Chi-squared coefficients were calculated to make comparisons between types of contact surfaces (human vs. animal) and also to compare between hospital services (Community Practice, Dermatology, Surgery, and Intensive Care Unit). To be able to establish seasonality, the year was divided into 4 groups, each with 3 months (January to March, April to June, July to September, and October to December), and chi-squared coefficients were also used to make comparisons between them. Relationships were considered significant when their p value was equal to or less than 0.05.
Results
General prevalence, phenotyping, and molecular characterization of environmental isolates
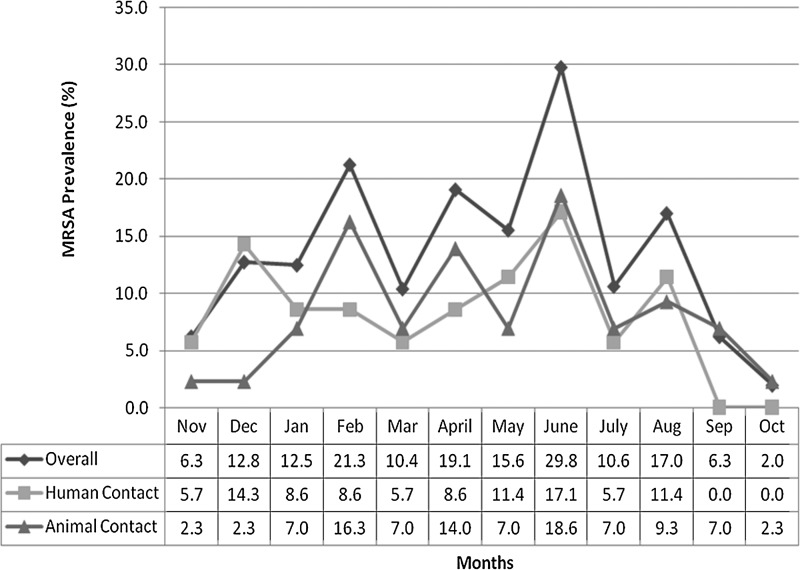
A total of 569 environmental samples were collected during the active MRSA surveillance; 77/569 (13.5%) were positive for MRSA. The monthly prevalence varied from as low as 2.0% to as high as 29.8% (Fig. 1). The presence and distribution of MRSA on the human- and animal-contact surfaces in the different services are summarized in Table 2. From 77 positive environmental samples, 81 MRSA isolates were recovered, indicating that some surfaces (e.g., computer keyboards, doors, and examination tables/floors) were contaminated with 2 distinctive MRSA strains at the time of sampling.
FIG. 1.
Distribution of methicillin-resistant Staphylococcus aureus (MRSA) prevalence on human- and canine-contact surfaces at the Small Animal Hospital in The Ohio State University Veterinary Medical Center Section during the MRSA Active Surveillance.
Table 2.
Overall Prevalence of MRSA Contamination of Human- and Animal-Contact Surfaces Distributed by Services at the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the MRSA Active Surveillance
| Service | Human-contact MRSA/samples collected | Animal-contact MRSA/samples collected | Total contact MRSA/samples collected |
|---|---|---|---|
| Community practicea | 6/37 (16.2%) | 7/24 (29.2%) | 13/61 (21.3%) |
| Dermatologyb | 1/86 (1.2%) | 8/48 (16.7%) | 9/134 (6.7%) |
| Intensive Care Unitc | 8/48 (16.7%) | 5/49 (10.2%) | 13/97 (13.4%) |
| Surgeryd | 19/108 (17.6%) | 8/135 (5.9%) | 27/243 (11.1%) |
| Generale | N/A | 15/34 (44.1%) | 15/34 (44.1%) |
| Total | 34/279 (12.2%) | 43/290 (14.8%) | 77/569 (13.5%) |
Included examination and treatment area of the service.
Included treatment area and ward of the service.
Included treatment area of the service.
Included pre-surgery, surgery rooms and wards of the service.
Included the carts that transport patients from service to service; since they do not belong to one specific service.
MRSA, methicillin-resistant Staphylococcus aureus.
Genotypically, 93.8% (76/81) of the isolates were SCCmec type II (Table 3). A dendrogram of only the environmental samples based on PFGE results revealed 2 major clusters (C1 and C2) and 30 pulsotypes were identified (P1–P30). Of the 81 isolates, 86.4 % (70/81) were grouped in the same cluster.
Table 3.
Molecular Characterization of Environmental MRSA Isolates from the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the MRSA Active Surveillance
| |
By sample (total n=77) |
By isolate (total n=81) |
||
|---|---|---|---|---|
| Environment | MRSA | % | MRSA | % |
| SCCmec typing | ||||
| Type II | 73/77 | 94.8% | 76/81 | 93.8% |
| Type III | 2/77 | 2.6% | 2/81 | 2.5% |
| Type IV | 3/77 | 3.9% | 3/81 | 3.7% |
| PFGE | ||||
| USA 100 | 71/77 | 92.2% | 73/81 | 90.1% |
| USA 300 | 2/77 | 2.6% | 2/81 | 2.5% |
| USA 500 | 1/77 | 1.3% | 1/81 | 1.2% |
| USA 800 | 1/77 | 1.3% | 1/81 | 1.2% |
| Iberian | 1/77 | 1.3% | 1/81 | 1.2% |
| Novel type | 2/77 | 2.6% | 2/81 | 2.5% |
| No typea | 1/77 | 1.3% | 1/81 | 1.2% |
One isolate that did not match to any USA type.
MRSA, methicillin-resistant Staphylococcus aureus; SCCmec, staphylococcal chromosomal cassette mec PFGE, pulsed-field gel electrophoresis.
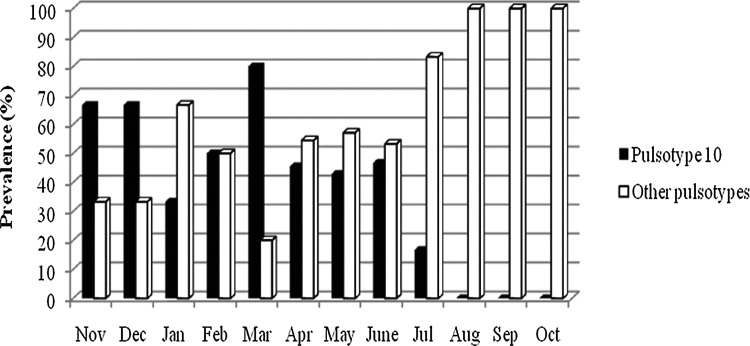
Ninety percent (73/81) of the isolates were classified as USA100 (Table 3). The majority of isolates fell into 2 pulsotypes; Pulsotype 10 (P10) had the highest number of isolates (33 isolates) followed by pulsotype 27 (P27) with 8 isolates. The predominant pulsotype P10 was observed in the hospital environment from November, 2007, until July, 2008. A comparison of the prevalence of P10 with the combined prevalence of the other 29 pulsotypes is shown in Figure 2. Even though a predominant pulsotype was found during more than half of the year's surveillance, other unique pulsotypes were intermittently introduced in the hospital environment. These pulsotypes (other than P10) were sporadically observed while P10 was present, but from June to October the number of isolates representing them gradually increased. By the end of the surveillance (last 3 months), a complete shift had occurred in the molecular characteristics of the isolates present in the hospital environment, circulating mostly new strains not present at the beginning of the surveillance.
FIG. 2.
Prevalence comparison between pulsotype 10 (P10) and other pulsotypes combined by month during the methicillin-resistant Staphylococcus aureus (MRSA) Active Surveillance at the Small Animal Hospital in The Ohio State University Veterinary Medical Center.
Of the 81 environmental isolates characterized in this study, 44 unique MRSA strains were identified on the basis of their combination of phenotypic profile, SCCmec type, and PFGE pulsotype. Phenotypically, 11 different antimicrobial susceptibility profiles were found. All of the strains (100%) were classified as MDR MRSA. In addition to the expected resistance to β-lactams, 97.7% (43/44) of the environmental MRSA strains were resistant to ciprofloxacin, and 95.5% (42/44) were resistant to erythromycin and enrofloxacin. Also, inducible clindamycin resistance was detected in 65.9% of the strains (29/44) obtained from the environment. All of the strains (100%) were susceptible to doxycycline, chloramphenicol, and vancomycin.
Human- versus animal-contact surfaces
The animal-contact surfaces (43/290, 14.8%) had a MRSA prevalence similar to the human-contact surfaces (34/279, 12.2%) (Tables 2 and 4). The distribution of MRSA by type of contact surface over time is presented in Figure 1. Table 4 summarizes the prevalence of MRSA on each type of human- and animal-contact surfaces. Doors were the predominant human-contact surface, with 22.7% (22/97) of samples positive. Of the animal-contact surfaces, carts/gurneys were the most prevalent, with 44.1% (15/34) MRSA positive.
Table 4.
Prevalence of MRSA on Human- and Animal-Contact Surfaces at the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the MRSA Active Surveillance
| Contact surface | MRSA/samples collected | Prevalence per location |
|---|---|---|
| Human-contact aurfaces | ||
| Doors | 22/97 | 22.7% |
| Drawer handles | 2/12 | 16.7% |
| IV pumps | 2/12 | 16.7% |
| Computersa | 7/48 | 14.6% |
| Paper towel and alcohol-gel dispensers | 1/12 | 8.3% |
| Clippers | 0/12 | 0.0% |
| Exam lights (dermatology) | 0/12 | 0.0% |
| Exam lights, light switches, table knobs | 0/24 | 0.0% |
| Fax/phone | 0/12 | 0.0% |
| Microscope | 0/12 | 0.0% |
| Otoscope | 0/24 | 0.0% |
| Miscellaneous locationsb | 0/2 | 0.0% |
| Total | 34/279 | 12.2% |
| Animal-contact surfaces | ||
| Hall carts | 15/34 | 44.1% |
| Exam tables and floors | 15/48 | 31.3% |
| Water bowls | 5/36 | 13.9% |
| Muzzles | 4/60 | 6.7% |
| Cages | 4/73 | 5.5% |
| Oxygen monitor | 0/15 | 0.0% |
| Warming pads | 0/24 | 0.0% |
| Total | 43/290 | 14.8% |
Included all the keyboards and mice, as well as the laptop in the intensive care unit.
A few extra locations were suggested for sampling during the duration of the study
MRSA, methicillin-resistant Stapylococcus aureus; IV, intravenous.
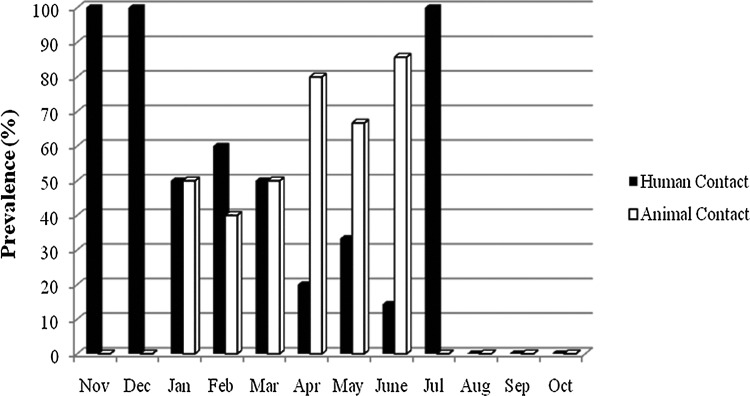
Genotypically, 17 pulsotypes were found on human-contact surfaces and 19 pulsotypes were found on animal-contact surfaces. Six of these, including the most prevalent pulsotypes (P10 and P27), were present on both human- and animal-contact surfaces. Interestingly, P10 was present only on human-contact surfaces for the first 2 months of surveillance (Fig. 3). Then, between January and March, 2008, it was gradually transferred to the animal-contact surfaces. By April, 2008, it was mainly found on animal-contact surfaces, except in July when only 1 sample was positive with this pulsotype and it was a human-contact surface (Fig. 3).
FIG. 3.
Prevalence of pulsotype 10 (P10) between human- and animal-contact surfaces by month at the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the methicillin-resistant Staphylococcus aureus (MRSA) Active Surveillance.
It is noteworthy to highlight that some surfaces were contaminated by the same pulsotype for 2–3 consecutive months. This was the case for 4 different surfaces contaminated with the most prevalent pulsotype (P10). These surfaces were a cart/gurney that was contaminated for 3 consecutive months; the access doors in a ward, the water bowls (from the same ward as the doors), and 1 of the examination floors were contaminated for 2 consecutive months with the same pulsotype. In contrast, 4 types of surfaces were contaminated with distinct and different pulsotypes each month for 2–4 consecutive months, which shows a continuous cycling of strains on those surfaces. The surfaces with such persistent contamination with multiple pulsotypes over time were 2 of the carts/gurneys, computers, multiple doors, and examination tables and floors.
Hospital services
MRSA prevalence for each hospital service (overall and by type of contact surface) is shown in Table 2. The service with the highest MRSA prevalence was Community Practice with 21.3% (13/61 samples), followed by Intensive Care Unit (13/97, 13.4%), Surgery (27/243, 11.1%), and Dermatology (9/134, 6.7%) (p=0.03). It is very important to highlight that MRSA was not detected in any of the surgical suites, with the exception of the doors providing access to that area. All MRSA isolates obtained from the surgery section were found in the presurgery area and in their wards.
Pulsotypes belonging to cluster 2 were present in all the services. In particular, P10 (the most prevalent pulsotype) was found in all the services, and it was highly prevalent in the carts/gurneys (considered as general and not linked to any specific service). The only 3 isolates with SCCmec type IV were found in Community Practice, Intensive Care Unit, and Surgery.
Seasonality
When analyzing the data by months, June had the highest prevalence at 29.8% (14/47) MRSA positive (Fig. 1), followed by February and April both with 21.3% (10/47), August with 17% (8/47), and May with 15.6% (7/45). During March, May, and July (months immediately following the top 3 most prevalent months), it was observed that MRSA prevalence in the environment decreased. Group 1 included from January to March (14.7%, 21/143), group 2 from April to June (21.6%, 30/139), group 3 from July to September (11.3%, 16/142), and group 4 from October to December (6.9%, 10/145). The analysis showed that the season of the year was associated with the prevalence of MRSA (p=0.002), with the highest prevalence in the spring months (April to June).
General prevalence, phenotyping, and molecular characterization of canine isolates
A total of 435 dogs were sampled during the MRSA surveillance period, and 25 (5.7%) were found to be MRSA positive. Epidemiological data regarding these dogs was discussed elsewhere (Hoet et al. 2013). However, the phenotypic and genotypic characterization of the MRSA isolates obtained from dogs is reported here to compare and establish relationships with the isolates found in the hospital environment.
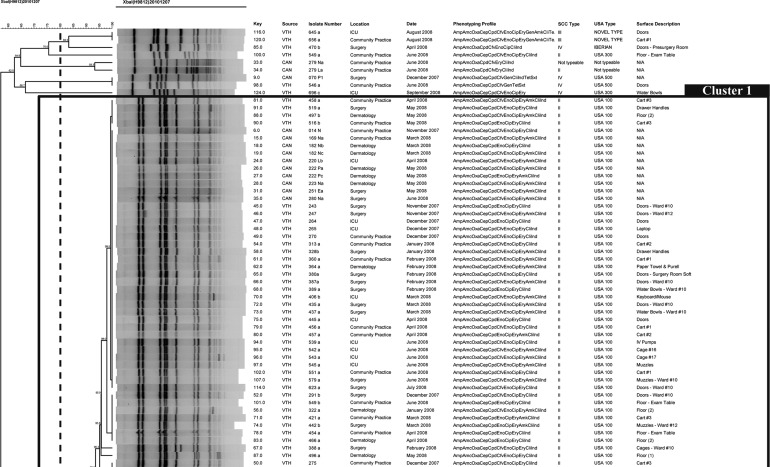
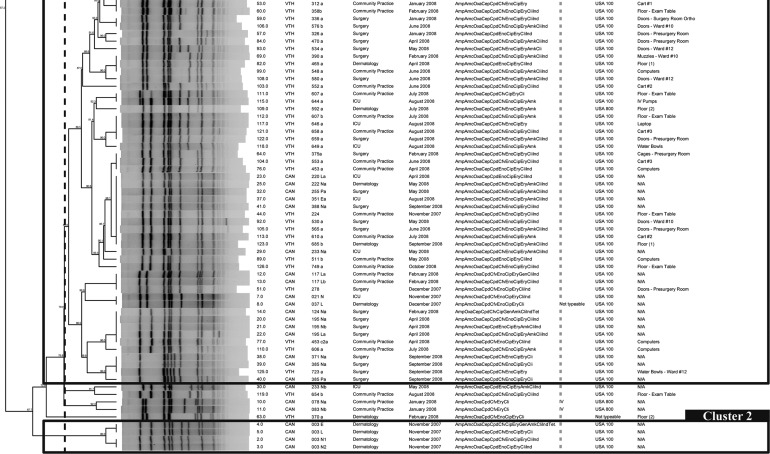
SCCmec typing and PFGE results are summarized in Table 5. USA100 was the most prevalent PFGE type with 86.5% (32/37) of the isolates. Other types found were USA500 in 2.7% (1/37) and USA800 in 5.4% (2/37) of the isolates. All USA100 isolates carried the SCCmec type II; in contrast, USA500 and USA800 isolates carried the SCCmec type IV. A dendrogram including only the canine isolates was built; 2 major clusters and 16 pulsotypes were identified, in which the majority of them (9/16) belong to 1 cluster. The most prevalent pulsotype in the environment, P10, was isolated from incoming dogs in November, March, April, May, and June. Moreover, a dendrogram built with canine and environmental isolates together (Fig. 4) showed that 1 pulsotype in particular that was never seen before in the hospital, P9, was apparently introduced by 2 different dogs on September 9th and 12th (11 months after the surveillance started). On September 15th, P9 was found for the first time in the environment (water bowls) of the service where the dogs were admitted, suggesting that dogs are capable of introducing and contaminating hospital surfaces during their visit (Fig. 4).
Table 5.
Molecular Characterization of MRSA Isolated from Canines Arriving to the Small Animal Hospital in The Ohio State University Veterinary Medical Center during the MRSA Active Surveillance
| |
By canine (total n=24)a |
By isolate (total n=37) |
||
|---|---|---|---|---|
| Canine | MRSA | % | MRSA | % |
| SCCmec typing | ||||
| Type II | 20/24 | 83.3% | 32/37 | 86.5% |
| Type IV | 3/24 | 12.5% | 3/37 | 8.1% |
| No type2 | 2/24 | 8.3% | 2/37 | 5.4% |
| PFGE | ||||
| USA 100 | 20/24 | 83.3% | 32/37 | 86.5% |
| USA 500 | 1/24 | 4.2% | 1/37 | 2.7% |
| USA 800 | 2/24 | 8.3% | 2/37 | 5.4% |
| No type3 | 1/24 | 4.2% | 2/37 | 5.4% |
Isolate from one canine was lost before phenotypic and genotypic characterization was performed
Not typeable following protocol by Milhierico et al. (2007).
Band pattern did not match with any USA type.
MRSA, methicillin-resistant Staphylococcus aureus; PFGE, pulsed-field gel electrophoresis.
FIG. 4.
Dendrogram based on the SmaI macrorestriction fragment profiles of 118 methicillin-resistant Staphylococcus aureus (MRSA) isolates obtained from environmental surfaces and canines admitted to the Small Animal Hospital in The Ohio State University Veterinary Medical Center. The percent similarity was calculated with Dice coefficients from the pulsed-field gel electrophoresis (PFGE) data. Band position tolerance and optimization were set at 1%.
Phenotypically, 11 different antimicrobial susceptibility profiles were found. All of the strains (100%) were classified as MDR MRSA. In addition to the expected resistance to β-lactams, 92.9% (26/28) of the canine MRSA strains were resistant to erythromycin, 82.1% (23/28) to ciprofloxacin, and 75.0% (21/28) to enrofloxacin. Also, inducible resistance to clindamycin was detected in 78.6% of the strains (22/28) obtained from the dogs. All of the strains (100%) were susceptible to doxycycline, chloramphenicol, and vancomycin.
Discussion
In this study, MRSA was detected in 13.5% of the sampled surfaces across different areas of the OSU VMC. Previous studies have focused on environmental contamination in the veterinary hospital setting during outbreaks, involved only 1 sampling time, or included repeated sampling but over a very short time interval (Weese et al. 2004, Loeffler et al. 2005, Heller et al. 2009, Murphy et al. 2010). Nevertheless, these studies reported the presence of MRSA on 1.4–10.0% of the surfaces sampled. Similarly, 1 cross-sectional study performed in the OSU VMC reported the presence of MRSA on 12% of the surfaces during a non-outbreak period, with 2 samplings performed only 1.5 months apart (Hoet et al. 2011). Collectively, it is clear that MRSA can be present and survive for an extended period of time on multiple surfaces in veterinary settings.
MRSA was isolated from multiple human- and animal-contact surfaces throughout the OSU VMC. Among the surfaces sampled, carts/gurneys, doors, examination tables, and floors were the most prevalent locations. Because MRSA is considered to be a primarily human pathogen (Morris et al. 2006), we did not expect to find similar levels of contamination on human- and animal-contact surfaces. Previous studies have also reported positive contamination on animal-contact surfaces (Weese et al. 2004, Loeffler et al. 2005, Heller et al. 2009, Hoet et al. 2011), which make them important targets for regular cleaning and disinfection in veterinary settings.
The high MRSA prevalence on the carts/gurneys is 1 of the most concerning findings of this study. At the OSU VMC, carts do not belong to any particular service, but they are used to move patients throughout the hospital and between services. These carts are contacted by multiple patients several times per day. If these patients have open wounds or are immunocompromised, this exposure could increase their chances of developing a nosocomial MRSA infection. Because these carts/gurneys are shared by all the services, they may also serve as a vehicle for dissemination of this pathogen throughout the hospital. This could explain the observed movement of pulsotypes among services with no shared staff or equipment. Finally, even though the carts/gurneys were categorized as an animal-contact surface, they are also touched by multiple veterinary personnel and students making them an important occupational health concern.
It was also concerning that the carts/gurneys were found to be MRSA positive with the same pulsotype over 3 consecutive months. There are 2 plausible explanations for the detection of the same MRSA strains on such surfaces. First, the lack of or improper application of cleaning and disinfection protocols allowed the maintenance and survival of MRSA for long periods of time. This is a critical aspect because previous studies have described how proper cleaning and disinfection of hospital surfaces contribute to the reduction of MRSA in the environment, even during outbreaks (Rampling et al. 2001, Schultsz et al. 2003). However, complete eradication of this pathogen from surfaces varies depending on the cleaning method used (Rutala et al. 1983, French et al. 2004, Jeanes et al. 2005, Otter et al. 2011). Second, the continuous detection of MRSA in these particular surfaces could be due to their recontamination either by the reintroduction of the same strain into the hospital environment (by animals and visitors) or by recurrent exposure to colonized or infected hospital staff. In any case, it is recommended that all personnel are trained to properly clean and disinfect carts/gurneys between patients. To prevent the long-term survival of MRSA on this type of mobile surface, protocols should also delegate a staff position or service responsible for ensuring that thorough disinfection of the carts/gurneys occurs routinely.
Across the hospital, 8 access doors from the different services were sampled every month. The majority (88%, 7/8) were contaminated with MRSA at least once during the year, with 1 maintaining the same pulsotype for 2 consecutive months on 2 separate occasions (November to December and February to March). This finding was not unexpected because door surfaces are contacted by multiple individuals several times per day and may not be regularly targeted for cleaning and disinfection. Other studies have reported the presence of MRSA on doors of both human and veterinary hospitals (Oie et al. 2002, Loeffler et al. 2005, Heller et al. 2009, Hoet et al. 2011). In 1 case, the same MRSA strain was found on the same door during 2 consecutive samplings 14 days apart (Heller et al. 2009). MRSA is capable of surviving on inanimate surfaces and/or objects (Kramer et al. 2006), especially when these surfaces are not cleaned appropriately, thereby increasing the probability of the environment serving as a reservoir for this nosocomial pathogen. In addition, the persistence of this pathogen on access doors could reflect a lack of or improper compliance with hand hygiene protocols by personnel (Farrington et al. 1990, Boyce and Pittet 2002, Boyce 2007, Otter et al. 2011) and/or the incorrect use of gloves (Boyce et al. 1997, Boyce 2007). In addition, the presence of MRSA on the doors could be a consequence of colonized or infected personnel working in those areas, who can cross-contaminate these surfaces (Farrington et al. 1990). To ensure that doors do not become a focal point for MRSA maintenance and dissemination in a veterinary hospital, they must be included in the standard operating procedures (SOPs) for regular cleaning and disinfection, and staff compliance with hand washing and proper glove use should be strictly enforced.
When evaluating the molecular epidemiology of the environmental isolates, over 90% were SCCmec type II and USA100. These findings demonstrate that there was little diversity in the MRSA isolates circulating within the hospital. MRSA strains with such molecular characteristics are frequently classified as hospital-acquired strains (HA-MRSA). This type of strain has been reported in the US general population, as well as in companion animals worldwide (Loeffler et al. 2005, Hanselman et al. 2006, Gorwitz et al. 2008, Kottler et al. 2010, Morris et al. 2010, Morris et al. 2012). Interestingly, the same strains present in the environment were also found in the incoming dogs, indicating that patients can also be a source of environmental contamination. This is further supported by our identification of a unique strain not seen before in the hospital arriving with two patients and then subsequently appearing in the environment on animal-contact surfaces. Because the personnel and dog owners were not screened, it is not possible to determine how much of the environmental contamination was due to the incoming patients versus other potential sources.
All the isolates from both the environment and the dogs were MDR; some of them were resistant to 7 different classes of antimicrobial drugs. This observed resistance pattern agrees with the fact that HA-MRSA strains tend to be MDR (Pantosti and Venditti 2009). Finding that over 90% of the isolates detected in this study were HA-MRSA is an important issue, because treatment and management of this type of infection tends to be more complex and expensive (Chambers 2001, Etienne 2005).
A trend was observed where MRSA had a higher prevalence in the environment during the warm months of the year, peaking in June. Because there are no other studies reporting surveillance over long periods of time in veterinary hospitals, we cannot draw strong conclusions regarding seasonality. However, reports from human hospital surveillance suggest a seasonality of S. aureus and MRSA infections that occurred most frequently during the spring and summer (Kaier et al. 2010, Mermel et al. 2011).
Last, we acknowledge that our results are limited by our inability to screen hospital personnel (veterinarians, technicians, students, and staff) clients, or visitors during the surveillance. This limits our ability to determine the origin of the MRSA isolates contaminating the hospital environment. Further studies will enlighten our understanding of how, when, and where MRSA will contaminate the veterinary environment. Finally, we acknowledge that extrapolation of our results to other veterinary hospitals around the United States is not possible. However, our results may be useful in aiding veterinary hospitals in developing effective surveillance and monitoring programs, as well as biosecurity and biocontainment protocols that will limit the impact of this important nosocomial pathogen in veterinary settings.
Conclusion
Little is known about the ecology of MRSA contamination in veterinary hospital environments during non-outbreak periods. Our results suggest that MRSA is not only present on multiple human- and animal-contact surfaces throughout the hospital, but that it is also capable of surviving on these surfaces for long periods of time. Some MRSA isolates circulated across multiple surfaces and areas of the hospital for up to 9 months, whereas continuous introduction of new MRSA strains was observed. In addition, incoming patients carried and contaminated the hospital environment with this bacterium during their visits. Molecular epidemiological analysis revealed that the majority of the environmental and canine MRSA isolates were closely related HA-MRSA strains, showing little diversity. It is clear that environmental contamination plays an important role in the epidemiology and ecology of MRSA in veterinary hospitals, including the maintenance and transmission of this bacterium. These results have aided in the development of effective programs for the control of this nosocomial pathogen and prevention of its zoonotic transmission in The Ohio State University Veterinary Medical Center.
Acknowledgments
We wish to thank Duncan MacCannell, from the Centers for Disease Control and Prevention (CDC), for facilitating the database containing S. aureus strains with the most typical band patterns for each USA type for PFGE characterization. We also want to thank Dr. Herminia de Lencantre, from the Universidade Nova de Lisboa in Portugal, for providing MRSA controls isolates for the standardization of the SCCmec type multiplex PCR, and Amber Reed for her collaboration in the collection and processing of samples in the early stages of this study. We are grateful for the financial support provided for the development of this project by The Ohio State's Public Health Preparedness for Infectious Diseases (PHPID) research initiative, and the OSU Canine Research funds. Finally, we will like to thank the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program for providing several control strains. NARSA is supported under National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) contract number HHSN272200700055C.
Author Disclosure Statement
No competing financial interests exist.
References
- Abbott Y. Leggett B. Rossney A. Leonard F. Markey B. Isolation rates of methicillin-resistant Staphylococcus aureus in dogs, cats and horses in Ireland. Vet Rec. 2010;166:451–455. doi: 10.1136/vr.b4814. [DOI] [PubMed] [Google Scholar]
- Aklilu E. Zunita Z. Hassan L. Chen H. Phenotypic and genotypic characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolated from dogs and cats at University Veterinary Hospital, Universiti Putra Malaysia. Trop Biomed. 2010;27:483–492. [PubMed] [Google Scholar]
- Benedict K. Morley P. Van Metre D. Characteristics of biosecurity and infection control programs at veterinary teaching hospitals. J Am Vet Med Assoc. 2008;233:767–773. doi: 10.2460/javma.233.5.767. [DOI] [PubMed] [Google Scholar]
- Boost M. O'Donoghue M. James A. Prevalence of Staphylococcus aureus carriage among dogs and their owners. Epidemiol Infect. 2008;136:953–964. doi: 10.1017/S0950268807009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;18:622–627. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- Boyce J. Potter-Bynoe G. Chenevert C. King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–627. [PubMed] [Google Scholar]
- Boyce J. Pittet D. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Health-Care Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23:3–40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- CDC/Pulse-Net. Oxacillin-resistant Staphylococcus aureus on PulseNet (OPN): Laboratory Protocols for Molecular Typing of S. aureus by Pulsed-field Gel Electrophoresis (PFGE) www.cdc.gov/hai/pdfs/labsettings/ar_mras_pfge_s_aureus.pdf/ [Jan 17;2013 ]. www.cdc.gov/hai/pdfs/labsettings/ar_mras_pfge_s_aureus.pdf/
- Chambers H. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals; Approved Standard. M31-A3. Wayne, Pennsylvania, USA: Clinical and Laboratory Standards Institute (CLSI); 2008. [Google Scholar]
- Etienne J. Panton-Valentine Leukocidin: A marker of severity for Staphylococcus aureus infection? Clin Infect Dis. 2005;41:591–593. doi: 10.1086/432481. [DOI] [PubMed] [Google Scholar]
- Farrington M. Ling J. Ling T. French G. Outbreaks of infection with methicillin-resistant Staphylococcus aureus on neonatal and burns units of a new hospital. Epidemiol Infect. 1990;105:215–228. doi: 10.1017/s0950268800047828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn K. Crawford S. McElmeel M. Jorgensen J. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41:4740–4744. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G. Otter J. Shannon K. Adams N, et al. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gorwitz R. Kruszon-Moran D. McAllister S. McQuillan G, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- Hanselman B. Kruth S. Rousseau J. Low D, et al. Methicillin-resistant Staphylococcus aureus colonization in veterinary personnel. Emerg Infect Dis. 2006;12:1933–1938. doi: 10.3201/eid1212.060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. Armstrong S. Girvan E. Reid S, et al. Prevalence and distribution of methicillin-resistant Staphylococcus aureus within the environment and staff of a university veterinary clinic. J Small Anim Pract. 2009;50:168–173. doi: 10.1111/j.1748-5827.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- Hoet A. Johnson A. Nava-Hoet R. Bateman S, et al. Environmental methicillin-resistant Staphylococcus aureus in a veterinary teaching hospital during a nonoutbreak period. Vector Borne Zoonotic Dis. 2011;11:609–615. doi: 10.1089/vbz.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet A. Van Balen J. Nava-Hoet R. Bateman S, et al. Epidemiological profiling of MRSA positive dogs arriving at a veterinary teaching hospital. Vector Borne Zoonotic Dis. 2013 doi: 10.1089/vbz.2012.1089. in press. [DOI] [PubMed] [Google Scholar]
- Hota B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A. Rao G. Osman M. Merrick P. Erradication of persistent environmental MRSA. J Hosp Infect. 2005;61:85–86. doi: 10.1016/j.jhin.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Kaier K. Frank U. Conrad A. Meyer E. Seasonal and ascending trends in the incidence of carriage of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in 2 German hospitals. Infect Control Hosp Epidemiol. 2010;31:1154–1159. doi: 10.1086/656748. [DOI] [PubMed] [Google Scholar]
- Kilic A. Li H. Stratton C. Tang Y. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of, as well as Panton-Valentine leukocidin occurrence among, methicillin-resistant Staphylococcus aureus isolates from children and adults in middle Tennessee. J Clin Microbiol. 2006;44:4436–4440. doi: 10.1128/JCM.01546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. Smith D. Laxminarayan R. Hospitalizations and deaths cause by methicillant resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottler S. Middleton J. Perry J. Weese J. Cohn L. Prevalence of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus carriage in three populations. J Vet Intern Med. 2010;24:132–139. doi: 10.1111/j.1939-1676.2009.0424.x. [DOI] [PubMed] [Google Scholar]
- Kramer A. Schwebke I. Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BCM Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard F. Abbott Y. Rossney A. Quinn P, et al. Methicillin-resistant Staphylococcus aureus isolated from a veterinary surgeon and five dogs in one practice. Vet Rec. 2006;158:155–159. doi: 10.1136/vr.158.5.155. [DOI] [PubMed] [Google Scholar]
- Loeffler A. Boag A. Sung J. Lindsay J, et al. Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J Antimicrob Chemother. 2005;56:692–697. doi: 10.1093/jac/dki312. [DOI] [PubMed] [Google Scholar]
- Loeffler A. Pfeiffer D. Lindsay J. Magalhães R, et al. Prevalence of and risk factors for MRSA carriage in companion animals: a survey of dogs, cats and horses. Epidemiol Infect. 2011;139:1019–1028. doi: 10.1017/S095026881000227X. [DOI] [PubMed] [Google Scholar]
- Manian F. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis. 2003;36:26–28. doi: 10.1086/344772. [DOI] [PubMed] [Google Scholar]
- McLean C. Ness M. Methicillin-resistant Staphylococcus aureus in a veterinary orthopedic referral hospital: Staff nasal colonization and incidence of clinical cases. J Small Anim Pract. 2008;49:170–177. doi: 10.1111/j.1748-5827.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Mermel L. Machan J. Parenteau S. Seasonality of MRSA infections. PLos One. 2011;6:e17925. doi: 10.1371/journal.pone.0017925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milheirico C. Oliveira D. de Lencastre H. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3374–3377. doi: 10.1128/AAC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley A. Stegger M. Bagcigil A. Baptiste K, et al. spa typing of methicillin-resistant Staphylococcus aureus isolated from domestic animals and veterinary staff in the UK and Ireland. J Antimicrob Chemother. 2006;58:1118–1123. doi: 10.1093/jac/dkl394. [DOI] [PubMed] [Google Scholar]
- Morris D. Rook K. Shofer F. Rankin S. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: A retrospective review of 749 isolates (2003–04) Vet Dermatol. 2006;17:332–337. doi: 10.1111/j.1365-3164.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- Morris D. Boston R. O'Shea K. Rankin S. The prevalence of carriage of methicillin-resistant staphylococci by veterinary dermatology practice staff and their respective pets. Vet Dermatol. 2010;21:400–407. doi: 10.1111/j.1365-3164.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- Morris D. Lautenbach E. Zaoutis T. Leckerman K, et al. Potential for pet animals to harbour methicillin-resistant Staphylococcus aureus when residing with human MRSA patients. Zoonoses Public Health. 2012;59:286–293. doi: 10.1111/j.1863-2378.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchan S. Kaufmann M. Deplano A. de Ryck R, et al. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003;41:1574–1585. doi: 10.1128/JCM.41.4.1574-1585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. Reid-Smith R. Boerlin P. Weese J, et al. Escherichia coli and selected veterinary and zoonotic pathogens isolated from environmental sites in companion animal veterinary hospitals in southern Ontario. Can Vet J. 2010;51:963–972. [PMC free article] [PubMed] [Google Scholar]
- Nienhoff U. Kadlec K. Chaberny I. Verspohl J, et al. Transmission of methicillin-resistant Staphylococcus aureus between humans and dogs: Two case reports. J Antimicrob Chemother. 2009;64:660–662. doi: 10.1093/jac/dkp243. [DOI] [PubMed] [Google Scholar]
- O'Mahony R. Abbott Y. Leonard F. Markey B, et al. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from animals and veterinary personnel in Ireland. Vet Microbiol. 2005;109:285–296. doi: 10.1016/j.vetmic.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Oie S. Hosokawa I. Kamiya A. Contamination of room door handles by methicillin-sensitive/methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2002;51:140–143. doi: 10.1053/jhin.2002.1221. [DOI] [PubMed] [Google Scholar]
- Otter J. Yezli S. French G. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- Pantosti A. Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front Microbiol. 2012;3:127. doi: 10.3389/fmicb.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantosti A. Venditti M. What is MRSA? Eur Respir J. 2009;34:1190–1196. doi: 10.1183/09031936.00007709. [DOI] [PubMed] [Google Scholar]
- Pinto J. Fowler V. Correa M. Lyman R, et al. Transmission of methicillin-resistant Staphylococcus aureus between human and hamster. J Clin Microbiol. 2011;49:1679–1680. doi: 10.1128/JCM.02469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampling A. Wiseman S. Davis L. Hyett A, et al. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2001;49:109–116. doi: 10.1053/jhin.2001.1013. [DOI] [PubMed] [Google Scholar]
- Rutala W. Katz E. Sherertz R. Sarubbi FJ. Environmental study of a methicillin-resistant Staphylococcus aureus epidemic in a burn unit. J Clin Microbiol. 1983;18:683–688. doi: 10.1128/jcm.18.3.683-688.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland B. Weese J. Bolin C. Au J, et al. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2009;15:1328–1330. doi: 10.3201/eid1508.081635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultsz C. Meester H. Kranenburg A. Savelkoul P, et al. Ultra-sonic nebulizers as a potential source of methicillin-resistant Staphylococcus aureus causing an outbreak in a university tertiary care hospital. J Hosp Infect. 2003;55:269–275. doi: 10.1016/s0195-6701(03)00263-9. [DOI] [PubMed] [Google Scholar]
- Seguin J. Walker R. Caron J. Kloos W, et al. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: Potential human-to-animal transmission. J Clin Microbiol. 1999;37:1459–1463. doi: 10.1128/jcm.37.5.1459-1463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T. Clarke P. O'Neill E. Dillane T, et al. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: Correlation with patient isolates and implications for hospital hygiene. J Hosp Infect. 2006;62:187–194. doi: 10.1016/j.jhin.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Singh A. Goering R. Simjee S. Foley S, et al. Application of molecular techniques to the study of hospital infection. Clin Microbiol Rev. 2006;19:512–530. doi: 10.1128/CMR.00025-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijkeren E. Wolfhagen M. Box A. Heck M, et al. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2004;10:2235–2237. doi: 10.3201/eid1012.040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijkeren E. Wolfhagen M. Heck M. Wannet W. Transmission of a Panton-Valentine leucocidin-positive, methicillin-resistant Staphylococcus aureus strain between humans and a dog. J Clin Microbiol. 2005;43:6209–6211. doi: 10.1128/JCM.43.12.6209-6211.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijkeren E. Moleman M. Sloet van Oldruitenborgh-Oosterbaan M. Multem J, et al. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: An investigation of several outbreaks. Vet Microbiol. 2010;141:96–102. doi: 10.1016/j.vetmic.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Weber D. Rutala W. Miller M. Huslage K, et al. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:25–33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- Weese J. DaCosta T. Button L. Goth K, et al. Isolation of methicillin-resistant Staphylococcus aureus from the environment in a veterinary teaching hospital. J Vet Intern Med. 2004;18:468–470. doi: 10.1892/0891-6640(2004)18<468:iomsaf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Weese J. Dick H. Willey B. McGeer A, et al. Suspected transmission of methicillin-resistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol. 2006;115:148–155. doi: 10.1016/j.vetmic.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Weese J. Faires M. Rousseau J. Bersenas A, et al. Cluster of methicillin-resistant Staphylococcus aureus colonization in a small animal intensive care unit. J Am Vet Med Assoc. 2007;231:1361–1364. doi: 10.2460/javma.231.9.1361. [DOI] [PubMed] [Google Scholar]