Abstract
Wild species are essential hosts for maintaining Ixodes ticks and the tick-borne diseases. The aim of our study was to estimate the prevalence, the rate of co-infection with Babesia, Bartonella, and Anaplasma phagocytophilum, and the molecular diversity of tick-borne pathogens in roe deer in Poland. Almost half of the tested samples provided evidence of infection with at least 1 species. A. phagocytophilum (37.3%) was the most common and Bartonella (13.4%) the rarest infection. A total of 18.3% of all positive samples from roe deer were infected with at least 2 pathogens, and one-third of those were co-infected with A. phagocytophilum, Bartonella, and Babesia species. On the basis of multilocus molecular studies we conclude that: (1) Two different genetic variants of A. phagocytophilum, zoonotic and nonzoonotic, are widely distributed in Polish roe deer population; (2) the roe deer is the host for zoonotic Babesia (Bab. venatorum, Bab. divergens), closely related or identical with strains/species found in humans; (3) our Bab. capreoli and Bab. divergens isolates differed from reported genotypes at 2 conserved base positions, i.e., positions 631 and 663; and (4) this is the first description of Bart. schoenbuchensis infections in roe deer in Poland. We present 1 of the first complex epidemiological studies on the prevalence of Babesia, Bartonella, and A. phagocytophilum in naturally infected populations of roe deer. These game animals clearly have an important role as reservoir hosts of tick-borne pathogens, but the pathogenicity and zoonotic potential of the parasite genotypes hosted by roe deer requires further detailed investigation.
Key Words: Roe deer, Babesia, Anaplasma, Bartonella, Genetic diversity, Co-infection
Introduction
The transmission of infectious diseases between wild animals and humans is now an issue of major interest for scientists. It is believed that more than 70% of human emerging infectious diseases (EID) reported between 1940 and 2004 have their origin in wildlife, and vector-borne diseases are believed to have been responsible for almost 30% of EID events in the last decades (Jones et al. 2008). Therefore, it is crucial to identify the most important wild host species that serve as major reservoirs of infections acquired from, and transmitted by, ticks to humans and other animals.
In central Europe, the roe deer (Capreolus capreolus) is one of the most important hosts for adult Ixodes ricinus ticks, which act as vectors for several microbial pathogens (Kiffner et al. 2011). The local density of this vector species depends on many factors, including climatic conditions (mainly temperature and humidity), and also on the abundance of suitable warm-blooded vertebrate hosts (Randolph 2004). For example, the presence of wild ungulate species, such as roe deer, has been shown to be essential in maintaining and amplifying tick populations and, consequently, the tick-borne diseases vectored by these ticks (Carpi et al. 2008, Pugliese et al. 2008). Furthermore, both the numbers of infected ticks and the tick-borne encephalitis infections diagnosed in human patients are positively correlated with the density of local roe deer populations (Hudson et al. 2001, Rizzoli et al. 2009). The role of roe deer, as reservoir hosts of tick-borne pathogens and as the source of infections for I. ricinus nymphs and females, requires deeper investigation, because there is still a lack of complex epidemiological studies exploring the processes regulating the prevalence of tick-borne diseases in wild cervid populations.
It is already well established that roe deer constitute the main reservoir for the Anaplasma phagocytophilum, the agent of human anaplasmosis, and for Babesia venatorum (EU1) infection, previously recognized as an agent of human babesiosis in Europe (Herwaldt et al. 2003). Recently, the presence of Bab. divergens and a different Bab. Divergens–like organism was reported in wild cervids (roe deer and red deer) (Duh et al. 2005, Cancrini et al. 2008, Tampieri et al. 2008), but the exact systematic positions of these isolates are still being evaluated (Zintl et al. 2011).
A. phagocytophilum is not transmitted transovarially in ticks (Ogden et al. 1998), and therefore A. phagocytophilum is thought to be maintained mainly in reservoir hosts. Asymptomatic Babesia spp. infections in humans, especially infections with Bab. microti, may persist for months or even years and can lead to nonzoonotic transfer of cases of babesiosis during blood transfusion (Leiby 2011, Siński et al. 2011), posing a particular threat for immunocompromised individuals. Both in animals and humans, infections with A. phagocytophilum and Babesia spp. can manifest with a varied range of symptoms, from totally asymptomatic or very mild infections, through chronic and latent nonspecific manifestations, to acute life-threatening infections (Blanco and Oteo 2002, Vannier and Krause 2012). Humans are invariably accidental hosts in these cases, having acquired the pathogens through tick bites during inadvertent contact with these vectors in the natural environment, through blood transfusions, or by direct contact with deer blood, i.e., while hunting, especially during field dressing of game animals with bare hands.
Bacteria of the genus Bartonella are increasingly being recognized as important human pathogens and are responsible for a wide range of clinical manifestations, including Carrion's disease or trench fever. Lice and flies are thought to be their main vectors. Additionally I. ricinus may likely act as a vector of these bacteria, although the vector competence of ticks for Bartonella transmission is still discussed (Telford 3rd et al. 2010, Reis et al. 2011). Humans are believed to be competent hosts for only 2 species, Bart. quintana and Bart. bacilliformis (Dehio and Sander 1999), but the majority of human infections are due to zoonotic Bartonella species. These incidental infections may display various clinical manifestations, i.e., cat scratch disease (Bart. henselae, Bart. clarridgeiae), neuroretinitis (Bart. grahamii, isolated from rodents), endocarditis (Bart. vinsonii subsp. berkhoffi isolated from dogs) or myocarditis (Bart. washoensis from ground squirrels). Recently, 2 Bartonella species, Bart. schoenbuchensis and Bart. capreoli, have been detected in roe deer (Dehio et al. 2001, Bermond et al. 2002), but so far little is known about the risk of human infection with cervid-specific Bartonella species. No data exist on the prevalence of these bacteria in naturally infected host populations, nor on their genetic diversity and co-existence with other tick-borne disease pathogens.
In Poland, the distribution and public health relevance of tick-borne pathogen infections and co-infections in wild cervids has been relatively neglected when compared with other mammalian species (Siński et al. 2006, Welc-Falęciak et al. 2008, Welc-Falęciak et al. 2009) and remains to be fully defined. Relatively few studies have investigated the occurrence of tick-borne pathogens in roe deer, and little is known about the existence of co-infections and the genetic diversity of A. phagocytophilum, Babesia spp., and Bartonella spp. in these mammals (Rymaszewska 2008, Sawczuk et al. 2005, Michalik et al. 2012). It is worth noting that roe deer are actually the most widely distributed game species in Central Europe, and are hunted in large numbers. In Poland, roe deer are found throughout the country, and with approximately 830 000 individuals (data for 2011, Agricultural Property Agency, Directorate General of the State Forests and the Polish Hunting Association) represents the most numerous game mammals. In the hunting season, hunters and forestry workers are exposed to direct contact with animal blood as well as tick infestation, creating obvious opportunities for direct transmission of tick-borne disease pathogens. Therefore, in view of the relative paucity of information on the role of roe deer in the maintenance of tick-borne diseases, the main aim of our study was to assess the potential of these game animals as reservoirs of zoonotic tick-borne disease using complex multilocus molecular assays. Accordingly, in this paper we estimated the prevalence and rate of co-infection of Babesia spp., Bartonella spp., and A. phagocytophilum in roe deer and demonstrated the molecular diversity of tick-borne pathogens in this host species.
Materials And Methods
Blood samples
Blood samples were collected from 67 roe deer (52 females, 15 males) harvested during the seasonal cull in several provinces of Poland. The most representative samples (77%) were obtained during the fall 2010 (n=36) and the winter 2011 (n=16) in 3 districts (Murowana Goślina, Margonin, Sieraków) of the Wielkopolskie province, in west-central Poland. The remaining blood samples (n=15) were collected during the seasonal cull from east-central Poland (Lubuskie, Mazowieckie, and Warmińsko-Mazurskie provinces). From each animal, 3 mL of whole blood was collected into 0.001 M EDTA. Genomic DNA was extracted from whole blood using a DNAeasy Blood and Tissue Kit (Qiagen, Crawley, UK) and stored at −20°C.
PCR analysis
Primers and cycling conditions used in this study are listed in Table 1. Detection and genotyping of A. phagocytophilum and Bartonella spp. were performed by amplification and sequencing of 2 loci. For this, 2 sets of primers for the groESL heat shock operon and 16S rRNA gene for A. phagocytophilum or the β-subunit of the RNA polymerase gene (rpoB) and a fragment of the gene encoding the enzyme citrate synthase (gltA) for Bartonella spp. were applied. Babesia spp. were detected and identified using GR2/GF2 primers targeting the fragment of 18S rDNA. Genotyping of Babesia isolates from positive animals was done by amplification with CRYPTO R and CRYPTO F primers and sequencing of a long 18S rRNA gene fragment (Table 1). Reactions were performed in a final volume of 20 μL and contained 0.33 mM deoxyribonucleotide triphosphates (dNTPs; Eurobio, Lille, France), 2 mM MgCl2, 1× PCR buffer, 1 U Taq polymerase (Fermentas), 1 μM of each primer, and 5 μL of extracted DNA sample. Bab. divergens DNA extracted from a roe deer spleen, Bab. microti King College strain DNA isolated from BALB/c mice blood, and Bart. grahamii DNA obtained from bacterial cultures initiated with samples from free-living rodents were used as positive controls. A. phagocytophilum DNA isolated from blood of infected European bison (Bison bonasus) was used as positive control. Negative controls were performed in the absence of template DNA. Amplicons were visualized with Midori Green stain (Nippon Genetics Europe GmbH) following electrophoresis in 2% agarose gels. Amplicons were purified using the Axygen Clean-up purification kit (Axygen, USA) and sequenced by a private company (Genomed S.A., Poland) in both directions.
Table 1.
Nucleotide Sequences and Annealing Temperature of the Primers Used for Polymerase Chain Reaction
| Species | Gene | Primer | Sequence 5′→3′ | Fragment size (bp) | Reference |
|---|---|---|---|---|---|
| A. phagocytophilum | groESL | HS1 | TGGGCTGGTA(A/C)TGAAAT | 1350 | Sumner et al. (1997) |
| HS6 | CCICCIGGIACIA(C/T)ACCTTC | ||||
| HS43 (nested) | AT(A/T)GC(A/T)AA(G/A)GAAGCATAGTC | 480 | |||
| HS45 (nested) | ACTTCACG(C/T)(C/T)TCATAGAC | ||||
| 16S rRNA | ge3a | CACATGCAAGTCGAACGGATTATTC | 932 | Massung et al. (1998) | |
| ge10r | TTCCGTTAAGAAGGATCTAATCTCC | ||||
| ge9f | AACGGATTATTCTTTATAGCTTGCT | 546 | |||
| ge2 | GGCAGTATTAAAAGCAGCTCCAGG | ||||
| Bartonella | gltA | BhCS.781p | GGGGACCAGCTCATGGTGG | 380 | Norman et al. (1995) |
| BhCS.1137n | AATGCAAAAAGAACAGTAAACA | ||||
| rpoB | rpoR | CGCATTATGGTCGTATTTGTCC | 333 | Paziewska et al. (2011) | |
| rpoF | GCACGATT(C/T)GCATCATCATTTTCC | ||||
| Babesia | 18S rRNA | GR2 | CCAAAGACTTTGATTTCTCTC | 559 | Bonnet et al. (2007) |
| GF2 | G(C/T)(C/T)TTGTAATTGGAATGATGG | ||||
| 18S rRNA | CRYPTO R | GAATGATCCTTCCGCAGGTTCACCTAC | 1 727 | Herwaldt et al. (2003) | |
| CRYPTO F | AACCTGGTTGATCCTGCCAGTAGTCAT |
Phylogenetic analysis
DNA sequence alignments and phylogenetic analysis were conducted using MEGA version 5.0 (Tamura et al. 2011). Phylogenetic trees were created using alignments performed with the Kimura-2 parameter algorithm as a distance method and Neighbor Joining (NJ) as the tree construction method. For comparison, sequences of Babesia, Bartonella, and A. phagocytophilum species/strains obtained from GenBank (www.ncbi.nlm.nih.gov) were implemented in the sequence alignment. The stability of inferred phylogenies was assessed by bootstrap analysis of 1000 randomly generated sample trees.
Statistical analysis
The frequency distribution of infracommunity species richness was tested for goodness-of-fit to the positive binomial distribution (assumption of the null model is a regular distribution), the Poisson distribution (assumption of the null model is a random distribution), and the null model of Janovy et al. (1995) (assumption of the null model is that, in the absence of associations and interactions between species, the frequency distribution of infracommunity species richness is predicted by prevalence values). Goodness-of-fit in each case was tested by chi-squared analysis. The degree of aggregation in species richness was calculated by the index of dispersion (I; the variance to mean ratio) and the index of discrepancy (D) as described by Poulin (1993) (a value of 0 indicates an even distribution of counts across all hosts and a value of 1 indicates all pathogen genera aggregated in a single host).
Nucleotide sequences accession numbers
New nucleotide sequences were deposited in GenBank with the accession numbers JQ965530-31 and JQ955734-35 (16S rRNA and groESL genes of A. phagocytophilum, respectively), JQ929916 and JQ929918 (18S rRNA gene of Bab. divergens and Bab. capreoli, respectively), and JQ929915 and JQ955736 (gltA gene of Bart. capreoli and Bart. schoenbuchensis, respectively).
Results
In all, 67 roe deer were sampled. Overall, 6 species of tick-borne disease pathogens belonging to 3 genera were detected in blood samples, and half of them (35 out of 67) tested positively for at least 1 species. A. phagocytophilum was the most common infection and Bartonella spp. the rarest. The overall mean number of genera of pathogens (Anaplasma sp., Babesia sp., and Bartonella sp.) per host was 0.830±0.106 (standard error of the mean, SEM), with a variance to mean ratio of 1.11. Significant differences in overall prevalence of tick-borne disease pathogens between males and females roe deer were noted (females 63.5%, males 13.3%; Fisher exact test p=0.0002).
A. phagocytophilum infections
This was the most prevalent pathogen in our study; 37.3% (25/67) of blood samples tested positively for A. phagocytophilum. The 546-bp fragment of the 16S rRNA gene and the 480-bp fragment of the groESL heat shock operon were further analyzed for 20 isolates. The nucleotide identity/similarity of the sequenced 16S rDNA fragments was very high (99.8–100%). Twelve of 20 sequences were identical, representing genetic variant A. Only 8 isolates could be distinguished on the basis of substitution at position 175 (C→A) in a variable region near the 5′ end of the 16S rRNA gene, representing genetic variant B (Tables 2 and 3A). Isolates belonging to variant A were found in samples collected all over the area of Poland. Isolates of variant B were found only in blood samples from the Wielkopolskie district. Isolates from variants A and B showed 99.6–99.8% homology with the partial nucleotide sequence of A. phagocytophilum obtained from I. ricinus in Lithuana (JN181069) and Belarus (HQ629914) as well as roe deer from the Czech Republic (EU839847). Additionally, the 16S rRNA sequence of variant B differed from the human pathogenic strains isolated in Slovenia, Poland and Italy by only 2 nucleotides: G and A in positions 76 and 84, respectively (Table 3A).
Table 2.
Heat Shock Operon groESL and 16S rRNA Gene Variants of A. phagocytophilum in Roe Deer from Different Districts of Poland
| Gene | No. of isolates | No. of samples | Site of study | Genetic variant |
|---|---|---|---|---|
| 16S rRNA | 10 | 12, 13, 17, 18, 33, 34, 41, 45, 48, 49 | North-central Poland (Wielkopolskie) | A |
| 2 | 61, 63 | East-central Poland (Mazowieckie, Podlaskie) | A | |
| 8 | 2, 27, 36, 38, 39, 44, 54, 55 | Wielkopolskie | B | |
| groESL | 9 | 12, 13, 17, 34, 41, 45, 48, 49, 55 | North-central Poland (Wielkopolskie) | C |
| 2 | 61, 63 | East-central Poland (Mazowieckie, Podlaskie) | C | |
| 9 | 2, 18, 27, 33, 36, 38, 39, 44, 54 | North-central Poland (Wielkopolskie) | D |
Isolates common for genetic variants A (16S rRNA) and C (groESL) or B (16S rRNA) and D (groESL) are shown in bold.
Table 3.
Table 3A.
Polymorphism in the Fragment of the 16S rRNA Gene (A) and groESL Heat Shock Operon
| A | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Nucleotide positions 5′→3′a |
|
|
|
|
||||
| Gene | Strain/genetic variant | No. of isolate | 76 | 84 | 165 | 175 | 328 | GenBank accession number | Host | Country | Reference |
| 16S rRNA | A | 12 | G | A | A | A | A | JQ965530 | Roe deer | Poland | This study |
| B | 8 | G | A | A | C | A | JQ965531 | Roe deer | Poland | This study | |
| A. phagocytophilum strains pathogenic for human | A | G | A | C | T | JN107802 | Human | Poland | Welc-Falęciak et al. (2010) | ||
| A | G | A | C | A | AY833407 | Human | Poland | Grzeszczuk et al. (2006) | |||
| A | G | A | C | A | GU236658 | Human | Slovenia | Scharf et al. (2011) | |||
| A | G | A | C | A | DQ029028 | Human | Italy | de la Fuente et al. (2005) | |||
| — | — | G | C | A | GU908492 | Human | China | Li et al. (2010)b | |||
| A | G | A | C | A | GU236664 | Human | USA | Scharf et al. (2011) | |||
The number corresponds to the positions of nucleotide substitutions relative to the sequence of the complete 16S rRNA gene of A. phagocytophilum strain HZ (NC_007797). Base substitutions are shown in bold.
Unpublished, sequence deposited in GenBank.
Table 3B.
Polymorphism in the Fragment of the 16S rRNA Gene (B) in A. phagocytophilum Isolates from Roe Deer and Human Pathogenic Strains (Sequences Published in GenBank)
| B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Nucleotide positions 5′→3′b |
|
|
|
|
||
| Gene | Strain/genetic variant | No. of isolates | 167 | 398 | 401 | GenBank accession number | Host | Country | Reference |
| groESL | C | 11 | G | G | A | JQ955735 | Roe deer | Poland | This study |
| D | 9 | G | A | A | JQ955734 | Roe deer | Poland | This study | |
| A. phagocytophilum strains pathogenic for human | A | A | A | AF033101 | Human | Slovenia | Petrovec et al. (1999) | ||
| G | A | G | JF494839 | Human | USA | Rejmanek et al. (2012) | |||
| G | A | G | EF473207 | Human | China | Zhang et al. (2007)b | |||
The number corresponds to the positions of nucleotide substitutions relative to the sequence of the groESL heat shock operon of the human pathogenic A. phagocytophilum strain (U96728). Base substitutions are shown in bold.
Unpublished, sequence deposited in GenBank.
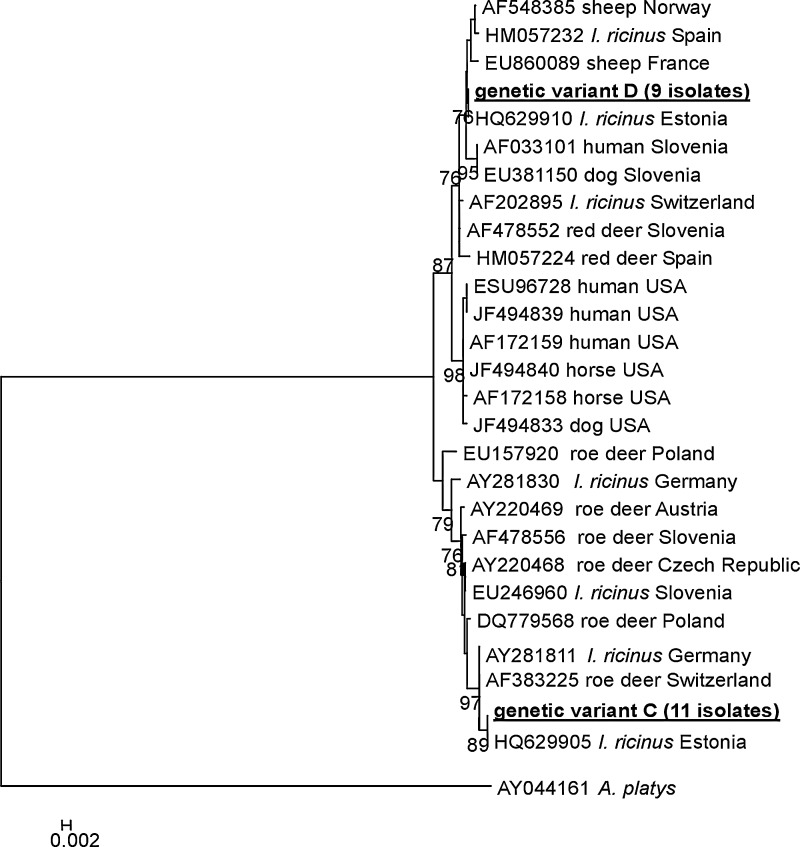
The partial groESL gene fragments (480 bp) were sequenced for all positive samples (n=20) because the 16S rRNA gene is too conserved for analysis of genetic heterogeneity. The level of homology between isolates was also very high (99.8–100%). Sequence analysis demonstrated nucleotide substitution at position 398 (G→A) (Table 3B) and allowed identification of 2 genetic variants. Genetic variant C included 11 isolates from samples collected in the Wielkopolskie, Podlaskie, and Mazowieckie districts (Table 2). Variant D was composed of 9 sequences found only in samples from the Wielkopolskie district. Scrutiny of the phylogenetic tree, based on the partial groESL operon sequences, showed that isolates from variant C were closely related to other European isolates, mainly from I. ricinus ticks and roe deer (Fig. 1). Isolates belonging to variant D clustered with A. phagocytophilum pathogenic for human and domestic animals in Europe, as well as in North America, and differed from them only by 1 nucleotide in positions 167 or 401 (Table 3).
FIG. 1.
Phylogenetic tree of the A. phagocytophilum isolates studied in the current work and chosen isolates from GenBank based on the fragment of the groESL heat shock operon. Numbers at the nodes of the tree indicate bootstrap values (1000 replicates, only bootstrap values >70% are shown). The nucleotide sequence of A. platys was used as an outgroup. Our isolates are marked in bold.
In spite of nucleotide substitutions in the groESL heat shock operon sequence, the deduced amino acid sequences did not change for any of the studied isolates. Combining the results of the molecular analysis of these 2 loci (groESL and 16S rRNA), 2 genogroups were obtained: (1) 10 isolates representing genetic variant A on the basis of 16S rRNA and variant C on basis of the groESL operon are probably nonzoonotic strains; and (2) 7 isolates representing variant B (16S rDNA) and variant D (groESL) are closely related to A. phagocythophilum strains pathogenic for human and domestic animals (Table 2, Fig. 1). Three isolates displayed contrasting results when genotyped at these 2 loci. The A. phagocytophilum isolate from roe deer no. 55 was classified as variant B accordingly to the 16S rRNA sequence, but represented variant C according to the groESL gene fragment (Table 2). Two isolates, nos. 18 and 33, were classified as variants A accordingly to 16S rRNA sequence but represented variant D accordingly to the groESL gene fragment (Table 2).
Babesia spp. infections
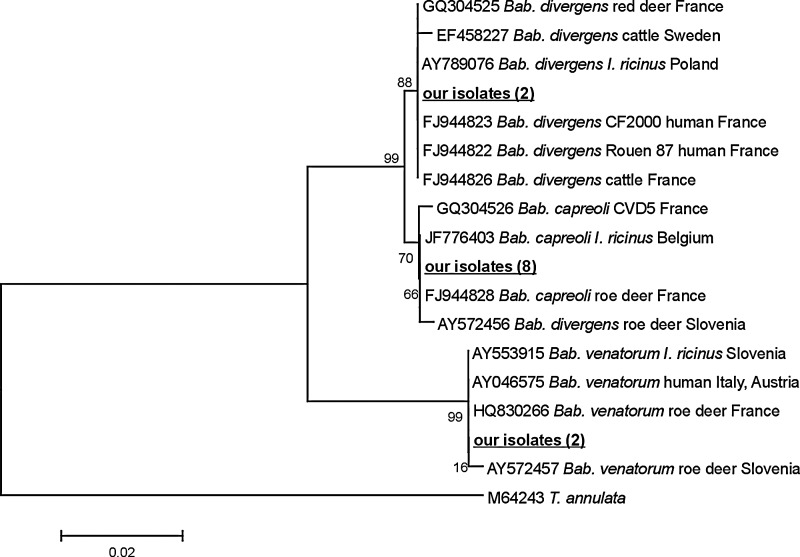
DNA from Babesia spp. was detected in 18 out of 67 animals (26.9%). Sequence analysis of the 1100-bp fragment of 18S rDNA obtained from 12 isolates showed the presence of 3 different Babesia species. The most prevalent species was Bab. capreoli (8/12, 66.7%) identical with Bab. capreoli F10 (FJ944828) originally isolated from roe deer in France (Fig. 2) and differing by 1 nucleotide (T→A) at position 620 from Bab. capreoli CVD5 (Table 4). Two out of 12 isolates (16.6%) showed 100% homology with Bab. venatorum (EU1) (HQ830266) derived from roe deer blood in France and were identical with Bab. venatorum (EU1) isolated from human in Europe (Fig. 2). The last 2 isolates were identified as Bab. divergens (2/12, 16.6%). These isolates showed 99.8% similarity to Bab. divergens (GQ304525) derived from red deer in France and clustered with other Bab. divergens isolates pathogenic for humans and cattle and differed from them only at 2 nucleotides positions, 54 and 148 (Table 4, Fig. 2). These 2 isolates differed from the Bab. capreoli and Bab. divergens–like isolates from Slovenia by 2 nucleotides at positions 631 and 663, supporting their identification as Bab. divergens (Table 4).
FIG. 2.
Phylogenetic tree of Babesia isolates studied in the current work and chosen isolates from GenBank based on the 18S rRNA gene fragment. Numbers at the nodes of the tree indicate bootstrap values (1000 replicates). The nucleotide sequence of T. annulata was used as anoutgroup. Our isolates are marked in bold.
Table 4.
Polymorphism in the Fragment of 18S rRNA Gene in Bab. divergens and Bab. capreoli Isolates from Roe Deer, Red Deer, Cattle, Ticks, and Human Pathogenic Strains (Sequences Published in GenBank)
| |
|
|
|
Nucleotide positions 5′→3′a |
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species/strain | Host | Country | GenBank accession number | 54 | 148 | 373 | 620 | 631 | 663 | 804 | 824 | Reference |
| Bab. divergens Rouen 87 clone 5 | Human | France | FJ944822 | C | C | A | T | A | A | G | G | Malandrin et al. (2010) |
| Bab. divergens RD54 | Roe deer | Poland | JQ929916 | T | T | A | T | A | A | G | G | This study |
| Bab. divergens CVD7 | Red deer | France | GQ304525 | C | C | A | T | A | A | G | G | Jouglin et al. (2009)b |
| Bab. divergens | I. ricinus | Poland | AY789076 | C | C | A | T | A | A | G | G | Pieniazek et al. (2006) |
| Bab. divergens B_di08 | Cattle | Sweden | EF458227 | C | C | A | T | A | A | G | A | Vogl et al. (2007)b |
| Bab. divergens C139 | Cattle | France | FJ944826 | C | C | A | T | A | A | G | G | Malandrin et al. (2010) |
| Bab. divergens CF2000 | Human | France | FJ944823 | C | C | A | T | A | A | G | G | Malandrin et al. (2010) |
| Bab. divergens-like | Roe deer | Slovenia | AY572456 | C | C | G | T | G | T | A | G | Duh et al. (2005) |
| Bab. capreoli RD33 | Roe deer | Poland | JQ929918 | C | C | A | T | G | T | G | G | This study |
| Bab. capreoli F10 | Roe deer | France | FJ944828 | C | C | A | T | G | T | G | G | Malandrin et al. (2010) |
| Bab. capreoli CVD5 | Roe deer | France | GQ304526 | C | C | A | A | G | T | G | G | Jouglin et al. (2009)b |
The number corresponds to the positions of nucleotide substitutions relative to the sequence of the18S rRNA gene of the human pathogenic Bab. divergens strain Rouen 87 clone 5. Base substitutions are shown in bold.
Unpublished, sequence deposited only in GenBank.
Bartonella infections
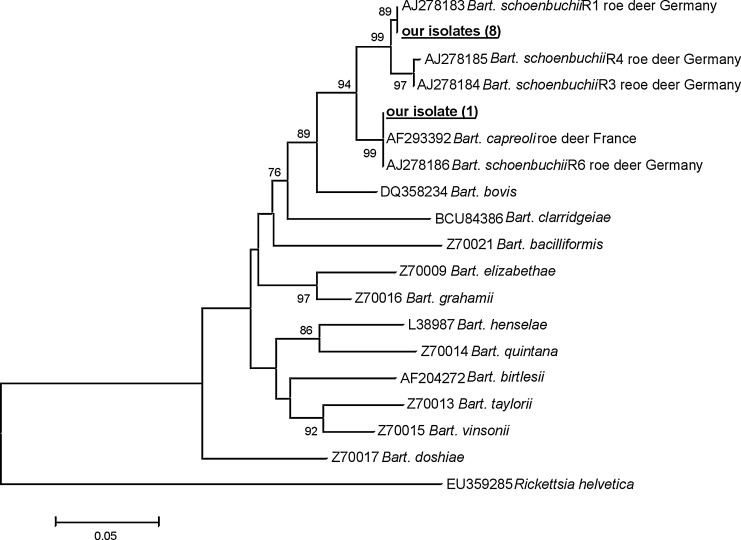
DNA from the genus Bartonella was found in 13.4% of the animals (9/67). Molecular analysis was based on the 333-bp fragment of the β-subunit of RNA polymerase (rpoB) and on the 380-bp fragment of the gene encoding the enzyme citrate synthase (gltA). Eight isolates were identical and showed a high level of rpoB and gltA sequence homology (>99%) with Bart. schoenbuchensis strain R1 from roe deer from Germany (nucleotide substitution at position 712 [T→C] in fragment of gltA gene; Table 5, Fig. 3). One isolate was closely related (>99% homology) to Bart. capreoli (isolated from a French roe deer) with a nucleotide substitution at position 736 (A→G) (Table 5, Fig. 3). The observed gltA sequence diversity affected the deduced amino acid sequences. Genetic diversity at position 712 or 736 in the gltA nucleotide sequences of Bart. schoenbuchensis or Bart. capreoli involved substitution of a Pro residue for Ser at positions 233 or a Val for Ile at position 241 in amino acid sequences of citrate synthase, respectively (the numbers correspond to the positions of the amino acids substituted relative to the corresponding amino acid sequences of Bart. schoenbuchensis strain R6, CAB95650).
Table 5.
Polymorphism in the Fragment of the gltA Gene in Bart. schoenbuchensis and Bart. capreoli Isolates from Roe Deer (Sequences Published in GenBank)
| |
|
|
|
Nucleotide positions 5′→3′a |
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species/strain | Host | Country | GenBank accession number | 681 | 711 | 712 | 732 | 736 | 807 | 837 | 859 | 861 | 900 | 915 | 916 | 930 | 948 | Reference |
| Bart. capreoli | Roe deer | France | AF293392 | A | C | T | A | A | T | C | C | C | A | T | C | C | C | Bermond et al. (2002) |
| Bart. capreoli RD27 | Roe deer | Poland | JQ929915 | A | C | T | A | G | T | C | C | C | A | T | C | C | C | This study |
| Bart. schoenbuchensis R1 | Roe deer | Germany | AJ278183 | A | T | T | G | A | C | C | C | A | G | C | T | C | T | Dehio et al. (2001) |
| Bart. schoenbuchensis R3 | Roe deer | Germany | AJ278184 | G | T | T | A | A | C | C | T | A | G | C | T | T | T | Dehio et al. (2001) |
| Bart. schoenbuchensis R4 | Roe deer | Germany | AJ278185 | G | T | T | A | A | C | T | T | A | G | C | T | T | T | Dehio et al. (2001) |
| Bart. schoenbuchensis R6 | Roe deer | Germany | AJ278186 | A | C | T | A | A | T | C | C | C | A | T | C | C | C | Dehio et al. (2001) |
| Bart. schoenbuchensis RD48 | Roe deer | Poland | JQ955736 | A | T | C | G | A | C | C | C | A | G | C | T | C | — | This study |
The number corresponds to the positions of nucleotide substitutions respect to the sequence gltA gene of Bart. schoenbuchensis strain R6. Base substitutions in sequences obtained in this study are shown in bold.
FIG. 3.
Phylogenetic tree of Bartonella isolates studied in the current work and chosen isolates from GenBank based on the gltA gene fragment. Numbers at the nodes of the tree indicate bootstrap values (1000 replicates; only bootstrap values >70% are shown). The nucleotide sequence of R. helvetica was used as an outgroup. Our isolates are marked in bold.
Infracommunity species richness and co-infection analysis
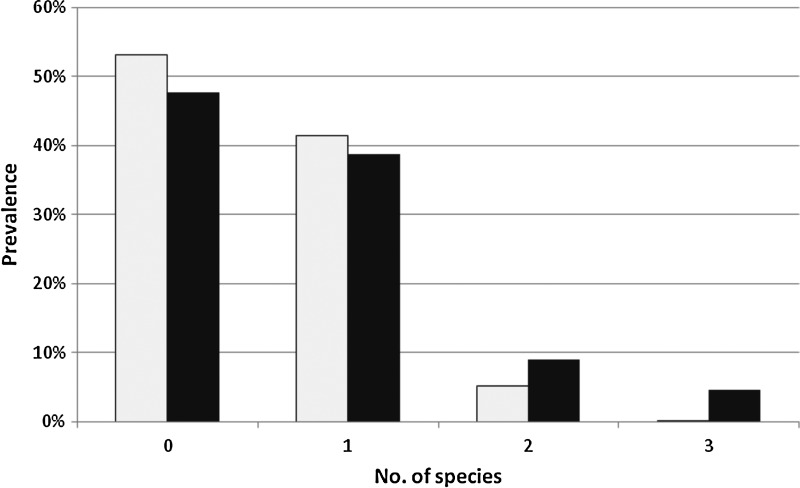
The analysis of co-infection was performed at the level of genera (Babesia, Bartonella, Anaplasma) because it was not possible to identify to the species level all positive samples. A total of 19.4% (13/67) of all positive roe deer yielded at least 2 pathogens, and one-third of those (4/13) were co-infected with A. phagocytophilum, Bartonella, and Babesia species. The genotyping results of the agents responsible for 10 co-infections are presented in Table 6. Among 10 combinations, only 1 case of co-infection with 2 potentially human pathogenic (zoonotic) species/strains was detected: A. phagocytophilum variant D and Bab. divergens infection (accompanied by B. schoenbuchensis). The observed distribution of infracommunity species richness did not conform to the normal, positive and negative binomial nor to the Poisson distributions (Fig. 4). It was significantly different (χ2=7.8, degrees of freedom [df]=3, p=0.05) from that predicted by the null model for interactions of parasite species in an assemblage (Janovy et al. 1995). Fewer single infections and significantly more cases of co-infection with 2 or 3 pathogens were observed in comparison to the values predicted by prevalence (Fig. 4).
Table 6.
Co-Infection with A. phagocytophilum, Babesia spp., and Bartonella spp. in Roe Deer
| |
|
Species/genetic variants of pathogen |
||
|---|---|---|---|---|
| No. of host | District | A. phagocytophilum/genetic variant (groESL) | Babesia | Bartonella |
| RD 2 | Wielkopolskie | A. phagocytophilum/D | Babesia sp. | — |
| RD 13 | Wielkopolskie | A. phagocytophilum/C | Bab. capreoli | — |
| RD 27 | Wielkopolskie | A. phagocytophilum/D | Bab. capreoli | Bart. schoenbuchensis |
| RD 29 | Wielkopolskie | A. phagocytophilum/nd | Bab. capreoli | Bart. schoenbuchensis |
| RD 33 | Wielkopolskie | A. phagocytophilum/D | Bab. capreoli | Bart. schoenbuchensis |
| RD 34 | Wielkopolskie | A. phagocytophilum/C | Bab. capreoli | — |
| RD 36 | Wielkopolskie | A. phagocytophilum/D | — | Bart. schoenbuchensis |
| RD 39 | Wielkopolskie | A. phagocytophilum/D | Babesia sp. | — |
| RD 45 | Wielkopolskie | A. phagocytophilum/C | Bab. capreoli | — |
| RD 48 | Wielkopolskie | A. phagocytophilum/C | — | Bart. schoenbuchensis |
| RD 49 | Wielkopolskie | A. phagocytophilum/C | — | Bart. schoenbuchensis |
| RD 54 | Wielkopolskie | A. phagocytophilum/D | Bab. divergens | Bart. schoenbuchensis |
| RD 61 | Mazowieckie | A. phagocytophilum/C | Bab. venatorum(EU1) | — |
Species or genetic variants pathogenic for human are shown in bold.
FIG. 4.
Frequency distribution of infracommunity species richness. The observed data are in the filled columns and those predicted by the null model of Janovy et al. (1995) are in the open columns. Full explanations and statistical analysis are given in the text.
Discussion
The results of our complex study, based on molecular analysis of several loci, revealed an interesting and unexpected diversity of tick-borne disease pathogens among roe deer populations from east-central and west-central Poland, providing the first detailed analysis of existing co-infections associated with this important European cervid species. Moreover, this is the first study, in which 3 genera of infectious agents were concurrently investigated in roe deer. Despite a limited number of tested animals, the high prevalence of pathogens enabled detailed molecular analysis based on several loci. Our genotyping showed that animals harbored more often the pathogen species/strains specific for this game animal (A. phagocytophilum variants A and C, Bab. capreoli, Bart. capreoli, Bart. schoenbuchensis) than the species/strains of public health significance (A. phagocythophilum variants B and D, Bab. divergens, Bab. venatorum [EU1]).
Previous molecular studies have shown a relatively high prevalence of tick-borne pathogens in naturally infected roe deer populations. The most common infection in the current study was A. phagocytophilum, with a prevalence of 37%. In Europe, it is believed that cervids are the main reservoir hosts of these bacteria, having been found commonly in roe deer in Spain (18%; de la Fuente et al. 2008), Switzerland (18%; Liz et al. 2002), Slovenia (85%; Petrovec et al. 2002), Slovakia (50%; Stefanidesova et al. 2008), and Austria (43%; Polin et al. 2004). In Poland, prevalence of this species has been reported to range from 9% to 38% in different regions of the country (Adamska and Skotarczak 2007, Michalik et al. 2009, Hapunik et al. 2011). In agreement with data published previously (von Loewenich et al. 2003), sequence analysis of the 2 loci in the current work revealed 2 distinct genetic lineages of A. phagocytophilum: (1) genetic variants detected in humans, ticks, dogs, horses, sheep, and red deer from Europe and the United States that are believed to be pathogenic; and (2) genetic variants isolated from ticks and roe deer in Europe that are probably nonzoonotic strains (Portillo et al. 2011, Katargina et al. 2012, Michalik et al. 2012). Recent studies have suggested that these different A. phagocytophilum variants have adopted different host tropisms (Petrovec et al. 2002, 2003) and that they show distinct but differing pathogenicity (Massung et al. 2003).
The results of previous studies support our finding of equal distribution of zoonotic and nonzoonotic A. phagocytophilum variants. For example, almost half of the genotyped A. phagocytophilum isolates from ticks in Germany were closely related to strains that caused granulocytic anaplasmosis/ehrlichiosis in human and animals in Europe or the United States (von Loewenich et al. 2003). The distribution of zoonotic and nonzoonotic variants in roe deer is very similar also in our studies (41% vs. 59%, respectively), but this issue certainly needs more scientific attention and further epidemiological studies. Additionally, we identified 3 A. phagocytophilum isolates that constituted contrasting variants, depending on the molecular locus used for genotyping. This may suggest the existence of recombination between zoonotic strains inducing human disease and nonzoonotic strains, as, for example, reported earlier in genotyping Bartonella from rodents (Paziewska et al. 2011). Overall genetic diversity in the groESL and 16S rRNA gene sequences is likely a result of bacterial population diversity within the marker region and probably does not affect the function/coded protein or mechanisms of pathogenesis among zoonotic isolates.
Roe and red deer have been shown to be hosts for 4 Babesia species, including zoonotic Bab. venatorum (EU1), Bab. divergens, and nonzoonotic Bab. capreoli and Bab. odocoilei-like organisms (Duh et al. 2005, Sawczuk et al. 2005, Bonnet et al. 2007, Cancrini et al. 2008, Hoby et al. 2009, Zintl et al. 2011). In Europe, the majority of human babesiosis cases have been caused by B. divergens, but several human cases of infection with B. venatorum have also been reported recently in Germany, Austria, Italy, and Poland (Herwaldt et al. 2003, Häselbarth et al. 2007, Welc-Falęciak et al. 2010). Only 1 case of human Bab. microti infection was reported in Europe (Hildebrandt et al. 2007). The prevalence of Babesia spp. infections in our study was almost 27%, which is in the range of the values reported for roe deer from other European countries (from 26% for Switzerland to 76% for Slovenia) (Duh et al. 2005, Hoby et al. 2009). On the basis of the 18S rRNA gene, we have identified 3 different Babesia species. Isolation of Bab. venatorum in our study confirmed that these animals are reservoir hosts of this relatively “new” pathogen also in Poland, and, to the best of our knowledge, this is the first report of the presence of Bab. venatorum in roe deer from Poland.
The presence of Bab. divergens in roe deer was confirmed in Slovenia (Duh et al. 2005), Italy (Cancrini et al. 2008, Tampieri et al. 2008), and also in Poland (Sawczuk et al. 2005), but molecular identification was based only on a short fragment (407 bp) of the nuclear small subunit rRNA gene. Differentiation of Bab. capreoli from Bab. divergens is possible only on the basis of molecular studies and its incapacity to infect gerbils or cattle under laboratory conditions (Gray et al. 1990, Herwaldt et al. 2003) because these species are morphologically indistinguishable from each other (Malandrin et al. 2010). Recent results indicate that Bab. capreoli and Bab. divergens can be distinguished on the basis of 3 conserved nucleotide differences at positions 631, 663, and 1637 in their 18S rRNA sequences (Table 4; Malandrin et al. 2010). Although Duh et al. (2005) showed a high level of similarity (99.6%) of their Babesia isolate obtained from roe deer to Bab. divergens species, molecular analysis of nucleotide substitutions suggests that this isolate is more closely related to Bab. capreoli (Table 4).
Identification of Babesia species in the present study was based on analysis of a 1100-bp fragment of the 18S rRNA gene that constitutes more than 60% of the complete gene (1728 bp). Nucleotide substitutions at positions 631 and 663 and phylogenetic relationship strongly suggest that in Poland roe deer are reservoir hosts for nonzoonotic Bab. capreoli and zoonotic Bab. divergens. Additionally, 2 of our isolates identified as Bab. divergens differed from strains known to be pathogenic in humans and cattle by only 2 nucleotides, which supports their potential public health significance. For example, in the case of Bab. venatorum, diversity among as many as 31 nucleotides in the 18S rDNA sequence did not affect its pathogenicity (Herwaldt et al. 2003), although we cannot exclude the possibility that 1 or other of the 2 nucleotide changes was critical. In our study, the prevalence of nonzoonotic Babesia species was twice as high (67% vs. 33%) as the prevalence of zoonotic ones. As in the case of A. phagocythophilum, the distribution of zoonotic/nonzoonotic species in reservoir hosts certainly needs more scientific attention and more extensive epidemiological studies.
In Europe, Bart. schoenbuchensis and Bart. capreoli have been identified recently and described in the blood of free-living roe deer, but so far there are few available data on the epidemiology of these 2 species in naturally infected hosts (Dehio et al. 2001, Bermond et al. 2002). Only 1 paper has reported on the prevalence of Bartonella in roe deer in Poland, in the area of Szczecin, where prevalence was higher than in our study (21% vs. 13%) (Skotarczak and Adamska 2005), but there are no more data available in the public domain. Our results confirmed the presence of Bart. capreoli and Bart. schoenbuchensis in roe deer, and of these the latter had a higher prevalence. Genetic diversity of the gltA nucleotide sequence was probably due to recombination among Bartonella strains, as was suggested by Paziewska et al. (2011). Because the observed gltA sequence diversity affected the deduced amino acid sequences of this gene product, there is a need for further study of the consequences of these changes for the structure and function of this protein.
This is the first report of Bart. schoenbuchensis in Poland. All of our isolates of Bart. schoenbuchensis were closely related to Bart. schoenbuchensis strain R1, that shows 86.6% sequence similarity (gltA) with Bart. bacilliformis (Dehio et al. 2001). Given its close relatedness to Bart. bacilliformis as well as its considerable heterogeneity, Dehio et al. (2001) speculated that Bart. schoenbuchensis may have not only the potential to cause zoonosis (i.e., in hunters and forestry personnel who are exposed to the blood of roe deer), but also may adapt to humans or eventually develop into a human-specific pathogen such as Bart. baciliformis.
Recently, co-infections in tick vectors and reservoir hosts have generated a lot of attention because of the growing interest in how they interact with one another (Swanson et al. 2006, Telfer et al. 2010). Naturally occurring co-infections among ticks and free-living hosts are believed to constitute an additional and hitherto undervalued layer of public health risk (Telfer et al. 2010). Although 3 species co-infections were revealed in the current study, and even occurred more often than predicted by prevalence values of individual species, they did not seem to increase the risk of tick-borne diseases in humans, as nonzoonotic species/strains were involved in the great majority of cases. Our study clearly demonstrates that the precise identification of the species and strains involved in co-infection is crucially important for a full assessment of overall risk.
Conclusions
Applying a range of molecular tools, we have conducted 1 of the first epidemiological studies of the prevalence of Babesia, Bartonella, and A. phagocytophilum in naturally infected populations of roe deer. The relatively high prevalence of tick-borne disease pathogens encountered in our study and the significant proportion of zoonotic species/strains of A. phagocytophilum and Babesia that we identified underline the role of roe deer as a zoonotic reservoir of these pathogens in Europe. The genotyping results provided novel data on the genetic diversity of the pathogens, and this has been deposited in the GenBank database. Finally, our study of co-infection has emphasized the importance of detailed genotyping for meaningful and comprehensive assessment of the health risk arising from tick-borne diseases harbored by wild roe deer.
Acknowledgments
This study was supported in part by the Ministry of Science and Higher Education, Grant ‘Iuventus Plus’ no. IP2010 045470 and partially by Grant No. N N308 563840. We are grateful Dr. Justyna Dunaj and Prof. Grzegorz Karbowiak for the B. divergens and A. phagocytophilum DNA used as a positive controls.
Author Disclosure Statement
No competing financial interests exist.
References
- Adamska M. Skotarczak B. Wild game as a reservoir of Anaplasma phagocytophilum in north-western Poland. Wiad Parazytol. 2007;53:103–107. [PubMed] [Google Scholar]
- Bermond D. Boulouis HJ. Heller R. Van Laere G, et al. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol. 2002;52:383–390. doi: 10.1099/00207713-52-2-383. [DOI] [PubMed] [Google Scholar]
- Blanco JR. Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect. 2002;8:763–772. doi: 10.1046/j.1469-0691.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- Bonnet S. Jouglin M. L'Hostis M. Chauvin A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg Infect Dis. 2007;13:1208–1210. doi: 10.3201/eid1308.061560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancrini G. Gabrielli S. Lori A. Grifoni G, et al. Morphology and genetics of a Babesia isolate from Capreolus capreolus. J Wildl Dis. 2008;44:168–171. doi: 10.7589/0090-3558-44.1.168. [DOI] [PubMed] [Google Scholar]
- Carpi G. Cagnacci F. Neteler M. Rizzoli A. Tick infestation on roe deer in relation to geographic and remotely sensed climatic variables in a tick-borne encephalitis endemic area. Epidemiol Infect. 2008;136:1416–1424. doi: 10.1017/S0950268807000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J. Torina A. Naranjo V. Caracappa S, et al. Infection with Anaplasma phagocytophilum in a seronegative patient in Sicily, Italy: Case report. Ann Clin Microbiol Antimicrob. 2005;4:15. doi: 10.1186/1476-0711-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J. Ruiz-Fons F. Naranjo V. Torina A, et al. Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res Vet Sci. 2008;84:382–386. doi: 10.1016/j.rvsc.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Dehio C. Sander A. Bartonella as emerging pathogens. Trends Microbiol. 1999;7:226–228. doi: 10.1016/s0966-842x(99)01523-1. [DOI] [PubMed] [Google Scholar]
- Dehio C. Lanz C. Pohl R. Behrens P, et al. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int J Syst Evol Microbiol. 2001;51:1557–1565. doi: 10.1099/00207713-51-4-1557. [DOI] [PubMed] [Google Scholar]
- Duh D. Petrovec M. Bidovec A. Avsic-Zupanc T. Cervids as Babesiae hosts, Slovenia. Emerg Infect Dis. 2005;11:1121–1123. doi: 10.3201/eid1107.040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS. Murphy TM. Taylor SM. Blewett DA, et al. Comparative morphological and cross transmission studies with bovine and deer babesias in Ireland. Prev Vet Med. 1990;9:185–193. [Google Scholar]
- Grzeszczuk A. Ziarko S. Kovalchuk O. Stańczak J. Etiology of tick-borne febrile illnesses in adult residents of North-Eastern Poland: Report from a prospective clinical study. Int J Med Microbiol. 2006;S40:242–249. doi: 10.1016/j.ijmm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hapunik J. Víchová B. Karbowiak G. Wita I, et al. Wild and farm breeding cervids infections with Anaplasma phagocytophilum. Ann Agric Environ Med. 2011;18:73–77. [PubMed] [Google Scholar]
- Häselbarth K. Tenter AM. Brade V. Krieger G, et al. First case of human babesiosis in Germany—Clinical presentation and molecular characterization of the pathogen. Int J Med Microbiol. 2007;297:197–204. doi: 10.1016/j.ijmm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL. Cacciò S. Gherlinzoni F. Aspöck H, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–948. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A. Hunfeld KP. Baier M. Krumbholz A, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- Hoby S. Mathis A. Doherr MG. Robert N, et al. Babesia capreoli infections in alpine chamois (Rupicapra r. rupicapra), roe deer (Capreolus c. capreolus) and red deer (Cervus elaphus) from Switzerland. J Wildl Dis. 2009;45:748–753. doi: 10.7589/0090-3558-45.3.748. [DOI] [PubMed] [Google Scholar]
- Hudson PJ. Rizzoli A. Rosa R. Chemini C, et al. Tick-borne encephalitis virus in northern Italy: molecular analysis, relationships with density and seasonal dynamics of Ixodes ricinus. Med Vet Entomol. 2001;15:304–313. doi: 10.1046/j.0269-283x.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Janovy J., Jr Clopton RE. Clopton DA. Snyder SD, et al. Species density distributions as null models for ecologically significant interactions of parasite species in an assemblage. Ecol Model. 1995;77:189–196. [Google Scholar]
- Jones KE. Patel NG. Levy MA. Storeygard A, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katargina O. Geller J. Alekseev A. Dubinina H, et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin Microbiol Infect. 2012;18:40–46. doi: 10.1111/j.1469-0691.2010.03457.x. [DOI] [PubMed] [Google Scholar]
- Kiffner C. Lödige C. Alings M. Vor T. Rühe F. Attachment site selection of ticks on roe deer, Capreolus capreolus. Exp Appl Acarol. 2011;53:79–94. doi: 10.1007/s10493-010-9378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby DA. Transfusion-transmitted Babesia spp.: Bull's-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liz JS. Sumner JW. Pfister K. Brossard M. PCR detection and serological evidence of granulocytic ehrlichial infection in roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra) J Clin Microbiol. 2002;40:892–897. doi: 10.1128/JCM.40.3.892-897.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandrin L. Jouglin M. Sun Y. Brisseau N, et al. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): Isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. 2010;40:277–284. doi: 10.1016/j.ijpara.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Massung RF. Slater K. Owens JH. Nicholson WL, et al. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998;36:1090–1095. doi: 10.1128/jcm.36.4.1090-1095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF. Priestley RA. Levin ML. Route of transmission alters the infectivity of Anaplasma phagocytophila in mice. Ann NY Acad Sci. 2003;990:494–495. doi: 10.1111/j.1749-6632.2003.tb07416.x. [DOI] [PubMed] [Google Scholar]
- Michalik J. Stańczak J. Racewicz M. Cieniuch S, et al. Molecular evidence of Anaplasma phagocytophilum infection in wild cervids and feeding Ixodes ricinus ticks from west-central Poland. Clin Microbiol Infect. 2009;15(S2):81–83. doi: 10.1111/j.1469-0691.2008.02240.x. [DOI] [PubMed] [Google Scholar]
- Michalik J. Stańczak J. Cieniuch S. Racewicz M, et al. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg Infect Dis. 2012;18:998–1001. doi: 10.3201/eid1806.110997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AF. Regnery R. Jameson P. Greene C, et al. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH. Bown K. Horrocks BK. Woldehiwet Z, et al. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med Vet Entomol. 1998;12:423–429. doi: 10.1046/j.1365-2915.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Paziewska A. Harris PD. Zwolińska L. Bajer A, et al. Recombination within and between species of the alpha proteobacterium Bartonella infecting rodents. Microb Ecol. 2011;61:134–145. doi: 10.1007/s00248-010-9735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovec M. Sumner JW. Nicholson WL. Childs JE, et al. Identity of ehrlichial DNA sequences derived from Ixodes ricinus ticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J Clin Microbiol. 1999;37:209–210. doi: 10.1128/jcm.37.1.209-210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovec M. Bidovec A. Sumner JW. Nicholson WL, et al. Infection with Anaplasma phagocytophila in cervids from Slovenia: Evidence of two genotypic lineages. Wien Klin Wochenschr. 2002;114:641–647. [PubMed] [Google Scholar]
- Petrovec M. Sixl W. Schweiger R. Mikulasek S, et al. Infections of wild animals with Anaplasma phagocytophila in Austria and the Czech Republic. Ann NY Acad Sci. 2003;990:103–106. doi: 10.1111/j.1749-6632.2003.tb07345.x. [DOI] [PubMed] [Google Scholar]
- Pieniazek N. Sawczuk M. Skotarczak B. Molecular identification of Babesia parasites isolated from Ixodes ricinus ticks collected in northwestern Poland. J Parasitol. 2006;92:32–35. doi: 10.1645/GE-541R2.1. [DOI] [PubMed] [Google Scholar]
- Polin H. Hufnagl P. Haunschmid R. Gruber F, et al. Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J Clin Microbiol. 2004;42:2285–2286. doi: 10.1128/JCM.42.5.2285-2286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo A. Pérez-Martínez L. Santibáñez S. Santibáñez P, et al. Anaplasma spp. in wild mammals and Ixodes ricinus from the north of Spain. Vector Borne Zoonotic Dis. 2011;11:3–8. doi: 10.1089/vbz.2009.0214. [DOI] [PubMed] [Google Scholar]
- Poulin R. The disparity between observed and uniform distributions: A new look at parasite aggregation. Int J Parasitol. 1993;23:937–944. doi: 10.1016/0020-7519(93)90060-c. [DOI] [PubMed] [Google Scholar]
- Pugliese A. Rosà R. Effect of host populations on the intensity of ticks and the prevalence of tick-borne pathogens: How to interpret the results of deer exclosure experiments. Parasitology. 2008;135:1531–1544. doi: 10.1017/S003118200800036X. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Tick ecology: Processes and patterns behind the epidemiological risk posed by Ixodid ticks as vectors. Parasitology. 2004;129:S37–S65. doi: 10.1017/s0031182004004925. [DOI] [PubMed] [Google Scholar]
- Reis C. Cote M. Le Rhun D. Lecuelle B, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlessi. PLoS Negl Trop Dis. 2011;5:e1186. doi: 10.1371/journal.pntd.0001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejmanek D. Bradburd G. Foley J. Molecular characterization reveals distinct genospecies of Anaplasma phagocytophilum from diverse North American hosts. J Med Microbiol. 2012;61:204–212. doi: 10.1099/jmm.0.034702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli A. Hauffe HC. Tagliapietra V. Neteler M, et al. Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLoS One. 2009;4:e4336. doi: 10.1371/journal.pone.0004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymaszewska A. Divergence within the marker region of the groESL operon in Anaplasma phagocytophilum. Eur J Clin Microbiol Infect Dis. 2008;27:1025–1036. doi: 10.1007/s10096-008-0539-x. [DOI] [PubMed] [Google Scholar]
- Sawczuk M. Maciejewska A. Adamska M. Skotarczak B. Roe deer (Capreolus capreolus) and red deer (Cervus elaphus) as a reservoir of protozoans from Babesia and Theileria genus in north-western Poland. Wiad Parazytol. 2005;51:243–247. [PubMed] [Google Scholar]
- Scharf W. Schauer S. Freyburger F. Petrovec M, et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. 2011;49:790–796. doi: 10.1128/JCM.02051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siński E. Bajer A. Welc R. Pawełczyk A, et al. Babesia microti: Prevalence in wild rodents and Ixodes ricinus ticks from the Mazury Lakes District of North-Eastern Poland. Int J Med Microbiol. 2006;296:137–143. doi: 10.1016/j.ijmm.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Siński E. Welc-Faleciak R. Pogłód R. Babesia spp. infections transmitted through blood transfusion. Wiad Parazytol. 2011;57:77–81. [PubMed] [Google Scholar]
- Skotarczak B. Adamska M. Capreolus capreolus and Ixodes ricinus as a reservoir of Bartonella in north-western Poland. Wiad Parazytol. 2005;51:139–143. [PubMed] [Google Scholar]
- Stefanidesova K. Kocianova E. Boldis V. Kostanova Z, et al. Evidence of Anaplasma phagocytophilum and Rickettsia helvetica infection in free-ranging ungulates in central Slovakia. Eur J Wildl Res. 2008;54:519–524. [Google Scholar]
- Sumner JW. Nicholson WL. Massung RF. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson SJ. Neitzel D. Reed KD. Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampieri MP. Galuppi R. Bonoli C. Cancrini G, et al. Wild ungulates as Babesia hosts in northern and central Italy. Vector Borne Zoonotic Dis. 2008;8:667–674. doi: 10.1089/vbz.2008.0001. [DOI] [PubMed] [Google Scholar]
- Tamura K. Peterson D. Peterson N. Stecher G, et al. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S. Lambin X. Birtles R. Beldomenico P, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR., 3rd Wormser GP. Bartonella spp. transmission by ticks not established. Emerg Infect Dis. 2010;16:379–384. doi: 10.3201/eid1603.090443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier E. Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- von Loewenich FD. Baumgarten BU. Schröppel K. Geissdörfer W, et al. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. J Clin Microbiol. 2003;41:5033–5040. doi: 10.1128/JCM.41.11.5033-5040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welc-Falęciak R. Paziewska A. Bajer A. Behnke JM, et al. Bartonella spp. infection in rodents from different habitats in the Mazury Lake District, Northeast Poland. Vector Borne Zoonotic Dis. 2008;8:467–474. doi: 10.1089/vbz.2007.0217. [DOI] [PubMed] [Google Scholar]
- Welc-Falęciak R. Rodo A. Siński E. Bajer A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet Parasitol. 2009;166:191–198. doi: 10.1016/j.vetpar.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Welc-Falęciak R. Hildebrandt A. Siński E. Co-infection with Borrelia species and other tick-borne pathogens in humans: Two cases from Poland. Ann Agric Environ Med. 2010;17:309–313. [PubMed] [Google Scholar]
- Zintl A. Finnerty EJ. Murphy TM. de Waal T, et al. Babesias of red deer (Cervus elaphus) in Ireland. Vet Res. 2011;42:7. doi: 10.1186/1297-9716-42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]